Abstract
Main conclusion
Arabidopsis plants in NaCl suffering half growth inhibition do not suffer osmotic stress and seldom shoot Na + toxicity; overaccumulation of Na + plus K + might trigger the inhibition.
It is widely assumed that salinity inhibits plant growth by osmotic stress and shoot Na+ toxicity. This study aims to examine the growth inhibition of Arabidopsis thaliana by NaCl concentrations that allow the completion of the life cycle. Unaffected Col-0 wild-type plants were used to define nontoxic Na+ contents; Na+ toxicities in shoots and roots were analyzed in hkt1 and sos1 mutants, respectively. The growth inhibition of Col-0 plants at 40 mM Na+ was mild and equivalent to that produced by 8 and 4 mM Na+ in hkt1 and sos1 plants, respectively. Therefore, these mutants allowed to study the toxicity of Na+ in the absence of an osmotic challenge. Col-0 and Ts-1 accessions showed very different Na+ contents but similar growth inhibitions; Ts-1 plants showed very high leaf Na+ contents but no symptoms of Na+ toxicity. Ak-1, C24, and Fei-0 plants were highly affected by NaCl showing evident symptoms of shoot Na+ toxicity. Increasing K+ in isotonic NaCl/KCl combinations dramatically decreased the Na+ content in all Arabidopsis accessions and eliminated the signs of Na+ toxicity in most of them but did not relieve growth inhibition. This suggested that the dominant inhibition in these conditions was either osmotic or of an ionic nature unspecific for Na+ or K+. Col-0 and Ts-1 plants growing in sorbitol showed a clear osmotic stress characterized by a notable decrease of their water content, but this response did not occur in NaCl. Overaccumulation of Na+ plus K+ might trigger growth reduction in NaCl-treated plants.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Soil salinization by NaCl is a worldwide problem that affects food security. This problem stems from the high sensitivity of most plants to Na+, which is an abundant cation in the Earth’s crust that accumulates in farming lands through varied mechanisms (Hasegawa 2013). NaCl decreases the osmotic potential of soil water and Cl− can be toxic (Teakle and Tyerman 2010) but many deleterious effects of NaCl in plants are currently explained by the cellular accumulation of Na+ (Blumwald et al. 2000; Hasegawa et al. 2000; Munns and Tester 2008), which occurs through processes that have been widely studied but not thoroughly characterized (Amtmann and Sanders 1998; Hasegawa 2013). Some plants, known as halophytes, tolerate high Na+ concentrations, but most crop plants belong to a much larger group of plants, known as glycophytes, which are Na+-intolerant (Flowers et al. 2010; Rozema and Schat 2013). This intolerance occurs at rather low Na+ concentrations and is related to a high Na+ accumulation that occurs in plants but that is a rare characteristic among other living organisms. For example, at 145 mM Na+ in the extracellular medium, the intracellular Na+ content of mammalian cells is 5–15 mM (Alberts et al. 2007), while in contrasts, in the root cells of Arabidopsis and Thellungiella, Na+ concentrations in this range are attained at 5 mM external Na+, approximately (Alemán et al. 2009).
In contrast with the tendency of plants to accumulate Na+ when this cation is present in the external medium, the currently accepted paradigm establishes that K+ is the most abundant cation in plant cells and that the substitution of Na+ for K+ is toxic and causes the inhibition of plant growth (Maathuis and Amtmann 1999; Blumwald et al. 2000; Horie et al. 2008; Munns and Tester 2008; Hauser and Horie 2010). However, this notion of toxicity associated with the cytoplasmic presence of Na+ originated from studies with animal cells (Steinbach 1962 and references therein), which evolved in Na+ environments and are efficient Na+ excluders. It was later extended to plant cells (Evans and Sorger 1966) with increasing emphasis over time, paying insufficient attention to the specific characteristics that plants acquire during their adaptation to the environments where they evolved. In fact, the emergence of terrestrial life in the Cambrian era and plant evolution from bryophytes to angiosperms occurred in oligotrophic environments where K+ was released from rocks by a slow process of weathering (Algeo and Scheckler 1998) and where Na+ could partially substitute for K+ in both fungi and plants (Rodríguez-Navarro and Rubio 2006; Benito et al. 2011). This scenario would explain the existence of high-affinity Na+ transporters that can accumulate Na+ when K+ limitation slows growth (Benito et al. 2004, 2012; Haro et al. 2010; Schulze et al. 2012). The existence of Na+-specific transporters questions the toxicity of Na+ in fungal and plant cells, because they would not provide a real physiological advantage if Na+ could not substitute for a significant proportion of the cellular K+. Furthermore, Na+ is used also as a “cheap osmoticum” by plant cell (e.g., Pardo and Quintero 2002; Flowers et al. 2015). In line with these general notions many observations question the imperative exclusion of Na+ from the cytosol as a plant cell dogma (Cheeseman 2013; Kronzucker et al. 2013).
Consistent with the economic importance and physiological relevance of salt intolerance in plants, the research performed on this subject has been very extensive; an indicator of this activity is the number of reviews, which was more than 200 between 2000 and 2012 (Cheeseman 2013). However, a clear model that explains salt tolerance, salt stress, and Na+ toxicity is still pending (Cheeseman 2013; Kronzucker et al. 2013). The main reason for the apparent contradiction that exists between the large amount of information and the absence of concrete models is probably due to variability in the strategies that different plants have independently developed to adapt to salinity (Flowers et al. 2010; Bennett et al. 2013; Rozema and Schat 2013). Another reason might be an excessive focus on short-term shock treatments (Cheeseman 2013), which might have failed to provide crucial data to describe salt effects.
Even assuming a large diversity of salt adaptations, a parallel approach to studies of many plant species is to intensify the study of a few model species, to construct comprehensive models that later can be adapted to the characteristics of other plants. Arabidopsis is one of these model plants (Zhu 2000; Moller and Tester 2007) for two main reasons: the extensive genetic resources that are available and the large natural variability in NaCl tolerance among accessions (Labidi et al. 2002; Rus et al. 2006; Baxter et al. 2010; Katori et al. 2010). In the currently accepted model, Arabidopsis responds to toxic levels of Na+ as a typical glycophyte, over-accumulating Na+ in leaves and suffering premature leaf senescence (Horie et al. 2009; Hauser and Horie 2010). However, as already mentioned, this model is questioned and, in fact, it does not integrate tissue tolerance and osmotic effects, which are important components of the plant response to NaCl (Moller and Tester 2007; Jha et al. 2010; Rahnama et al. 2010; Roy et al. 2014; Flowers et al. 2015). Therefore, before extensive genetic studies are tackled, Na+ toxicity in Arabidopsis requires a more extensive characterization. This conclusion also applies to osmotic and other effects that might not be specific of Na+.
In addition to natural variability, the inhibitory effect of Na+ is greatly increased in some Arabidopsis mutants, in which Na+ transport is affected. This occurs because independently of its specific toxicity, the leaf Na+ content depends on the Na+ taken up from the soil, its accumulation in the root symplast, and subsequent long-distance transport to leaves. This transport occurs via two well-characterized processes that operate in opposite directions, one charging Na+ from the root symplast into the xylem and the other retrieving Na+ from the xylem to the root symplast. The former is mediated by the SOS1 (salt-overly sensitive) antiporter, which also mediates the efflux of cytosolic Na+ to the soil solution, and the latter, by the HKT1 (high-affinity K+ transporter) Na+ transporter. In Arabidopsis, the genes that encode these two transporters have been cloned and the corresponding knockout mutant lines have been identified and used to study the function of the SOS1 and HKT1 transporters on the distribution of Na+ in the plant (Davenport et al. 2007). Therefore, these mutants are useful tools to study Na+ toxicity. The characterization of other effects might be a more complex task, but is achievable.
This study aimed to define further the response of Arabidopsis to Na+, at NaCl concentrations that plants can tolerate without interrupting their life cycle, mimicking farming in NaCl-contaminated soils, where crops are harvested, albeit with a lower productivity. Our first goal was to determine the detrimental effects of Na+ toxicity on both roots and shoots in the absence of osmotic stress. For this purpose we used the sos1 and hkt1 mutants. The second goal was to establish the causes by which NaCl inhibits the growth of Arabidopsis, integrating Na+ toxicity and the osmotic response.
Materials and methods
Plant material
Seeds of Arabidopsis thaliana (L.) Heynh. accessions were obtained from Carlos Alonso-Blanco and Rhonda Meyer. Seeds of gl1, gl1 sos1-1 (Wu et al. 1996) and gl1 hkt1-4 (Rus et al. 2004) were obtained from José Manuel Pardo.
Hydroponics
Seeds were surface-sterilized according to standard protocols, stratified for 48 h at 4 °C in water in the dark and sown on 0.6 % agarose in Araponics seedholders (Araponics NV, Liege, Belgium). The seedholders were then placed into hydroponic boxes with a culture medium, which was based on that used in previous studies (Alemán et al. 2009), consisting of 1.4 mM Ca(NO3)2, 0.2 mM MgSO4, 0.2 mM H3PO4, 1.0 mM KCl and the following micronutrients: 25 µM H3BO3, 20 µM Fe-EDTA (from H4EDTA), 2 µM MnSO4, 2 µM ZnSO4, 0.5 CuSO4, 0.5 µM (NH4)6Mo7O24. The pH was adjusted to 5.7 with Ca(OH)2. The Na+ content of this medium was from 2 to 3 µM. Plants were grown in Araponics boxes with from 8 to 12 plants (see, for example, Fig. 3), and the medium was replaced every week. The plants were grown under a 16/8 h light/dark cycle at 23 °C with an irradiance of 110 µmol m−2 s−1 in a CONVIRON MTR30 growth chamber (Conviron E15; Controlled Environments Ltd., Winnipeg, Manitoba, Canada). In many assays, plants were grown beyond the flowering stage because the uneven distribution of Na+ in Arabidopsis shoots (Berthomieu et al. 2003) might imply changes in the relationship of Arabidopsis plants with Na+ during the life cycle. In our conditions, 3-week-old Col-0 plants showed a small inflorescence (<10 % rosette weight) in a small number of plants. Four-week-old plants showed an actively growing green rosette that had not initiated senescence; the flowering stem weighed approximately the same as the rosette and the first siliques of the main stalk were almost full-length but were still completely green.
Testing approach
To decrease size variability, we grew the plants for 2 weeks at 1.0 mM K+ and 0.2 mM Na+ and for two additional weeks at the tested Na+ concentrations (Supplemental Fig. S1a). We always maintained a small concentration of Na+ in the culture medium because the growth of Arabidopsis plants might be slightly stimulated by Na+ in hydroponic cultures; the optimal Na+ concentration in the Col-0 accession might be in the 5–10 mM range (at 1 mM K+). However, our control plants were grown at 0.2 mM Na+ to ensure that hkt1 and sos1 plants were not Na+ inhibited in control conditions; preliminary experiments showed that this Na+ concentration was suitable for our purposes. Plants were sampled after one and 2 weeks in Na+ (Supplemental Fig. S1a) to avoid that transitory growth arrests or delays (Munns 1993) mislead the conclusions. It is worth noting that by weighing the plants several days following exposure to NaCl and comparing with control plants, a transitory arrest of growth followed by growth restoration cannot be distinguished from a permanent low growth rate. Similarly, if growth inhibition started 3 or 4 days after the addition of Na+, its quantification by weighing the plants after 5 days in Na+ would be erroneous. In our conditions, these transitory effects lasted less than 1 week and the increase in weight during the second week in Na+ should reflect the actual growth rate of the plants at the tested Na+ concentration. We describe the tested plants as 1-week- or 2-weeks-in-Na+ plants. For simplicity, we also use these names for control plants in 0.2 mM Na+, which should be properly named 3- and 4-week-old plants. Basic data in this study were obtained with the hkt1-4 and sos1-1 mutants, which also carry the gl1 mutation. Although preliminary experiments showed that the gl1 mutation did not affect the Na+ tolerance of Col-0 plants (not shown), experiments with hkt1-4 and sos1-1 plants were performed in parallel with gl1 plants, which were taken as controls.
Plants germinated in Na+
Following the approach described above, plants showed little size variability within the same experiment, which allowed more robust statistical analyses with lower number of plants. Preliminary experiments showed that this approach did not modify either growth inhibition or plant Na+ contents in comparison to plants that had been grown continuously in Na+. To confirm this finding we studied plants that were permanently grown at 40 mM NaCl. For this purpose, after stratification, the seedholders were transferred to the hydroponic medium supplemented with 40 mM NaCl. Afterwards, plants were handled as described above.
Vertical plates
Sterilized seeds were placed on square plates with half-strength Murashige & Skoog (MS) medium (Duchefa Biochemie B.V., Haarlem, The Netherlands). This medium was supplemented with 1.0 % sucrose and 0.8 % agarose. Seeds were stratified for 48 h at 4 °C in the dark and then the plates were positioned vertically in a growth chamber under a 16/8 h light/dark cycle at 21 °C. Five-day-old seedlings were transferred to plates with the hydroponic medium described above supplemented with 1.0 % sucrose, 0.8 % agarose, and the required amount of NaCl, as stated in each case. The Na+ concentration in the agarose base medium was from 9 to 11 µM.
Culture vessels
Sterilized seeds were placed on ECO2boxes (Duchefa Biochemie B.V., Haarlem, The Netherlands) containing 180 ml of 0.25x strength MS medium supplemented with 1 % sucrose and 0.4 % agarose. This medium was further supplemented with the required concentration of cations (the base medium contained 5 mM K+ and 0.1 mM Na+) or sorbitol. In some experiments the hydroponic medium described above substituted for the 0.25× strength MS medium. The seeds were stratified for 48 h at 4 °C in the dark and then the vessels were transferred to a growth chamber under a 16/8 h light/dark cycle at 21 °C. The shoots of 2-week-old seedlings were collected in groups of five to seven plants of similar size. The seedlings obtained by this procedure grew slowly and showed large size variability. Therefore, we did not make growth rate comparisons between treatments in this approach. In contrast, fresh weight and K+ and Na+ contents referred to dry weight showed a low variability, which allowed comparisons between treatments that were statistically highly significant.
Cation contents
The roots of hydroponic plants were washed for 20 min in pH 6.5, 10 mM Mes-Ca2+ buffer (the time course of K+ and Na+ losses in small roots demonstrated that a washing time of 5 min is sufficient to eliminate the external cations but that a significant loss of these ions from the root symplast did not occur in 30 min). Shoots and roots were then separated, dried at 65 °C for 2 days, weighed, and digested with 1 M HNO3 for 2 days at room temperature. The K+ and Na+ concentrations were determined by atomic emission spectrophotometry and the K+ and Na+ contents referred to the dry weight of the tissue. The Na+/K+ molar ratios were obtained directly from the cation concentrations in the nitric extracts without considering the weight of the plant material. The roots of seedlings from vertical plates were washed in distilled water and shoots and roots were digested with 1 M HNO3 for 2 days at room temperature. In these seedlings, only the Na+/K+ molar ratios were determined. Although Na+ content could be similarly expressed in terms of dry weight or Na+/K+ molar ratio, we used the Na+/K+ molar ratio as a practical indicator of the Na+ content of the organs of the plant. This ratio has two advantages over expressing the Na+ content by dry weight: it takes into account the K+ content, which in the other case is not considered and can be determined even when roots are small and cannot be accurately weighed, which occurred frequently in Na+ inhibited roots (see below a more extensive discussion).
Anthocyanin analysis
Leaf tissue from plants grown in the selected conditions were ground in liquid nitrogen with mortar and pestle; samples of approximately 100 mg were processed immediately or maintained frozen at −80 °C. Samples of ground tissue were suspended in 0.6 mL 1 % HCl in ethanol, vortexed and incubated at 4 °C for 2 h, centrifuged twice, and the absorption determined at 530 nm; the contents were expressed in A530 g−1 fresh weight (Ronchi et al. 1997).
Statistical analyses
The results were obtained during a period of one and a half year; the time between two repetitions of the same treatment was many weeks or months. In repetitions of the same treatment the weight of plants showed notable variability, but the variability was smaller when the repetitions were performed in parallel in boxes handled simultaneously using the same batch of seeds. Each experiment included a box of plants at 0.2 mM Na+ as a reference for all treatments in that experiment. Weights of tested and control plants at 0.2 mM Na+ were always proportional. In contrast to the variability in weight, the K+ and Na+ contents referred to either dry weight or Na+/K+ molar ratios were fairly constant even in repetitions in which the 0.2 mM Na+ controls showed maximum or minimum weight values. The only exception was the Na+/K+ molar ratio in the rosettes of 2-weeks-in-Na+ Col-0 plants because it depended on the size of the inflorescence (see below). In some treatments the roots of 1-week-in-Na+ plants weighed 1 mg or less and their Na+ content expressed with reference to dry weight had a low reliability. In these cases, the Na+/K+ ratio was highly consistent across repetitions of the same experiment and, therefore, we report only this ratio. The gl1, sos1, and hkt1 mutations did not apparently affect the growth of Arabidopsis plants under control conditions. The weight of plants obtained from high quality seeds of Col-0, gl1, gl1 sos1-1, and gl1 hkt1-4 lines germinated and grown in parallel at 0.2 mM Na+ were not significantly different in experiments with 6–10 plants.
All treatments were repeated at least three times, using eight plants per treatment, but many treatments were repeated more times because they were included in several sets of treatments. The results reported in figures or tables correspond to one experiment including all treatments in parallel. All repetitions led to exactly the same conclusions although the weights of plants were different.
Figures and tables show the mean and standard deviation of the data. Significant differences between means were determined by the Student’s t test; statistical significance was set at P < 0.01.
Results
Organ distribution and temporal evolution of Na+ in Col-0 plants
Attending to the inhibitory effect of NaCl on Col-0 plants (see below), we fixed 80 and 20 mM NaCl concentrations as the upper and lower limits of our study. Within this range of concentrations, inflorescences were the organs with the lowest Na+ content and rosettes showed the highest content (Table 1; Supplemental Tables S1 and S2). Regarding temporal evolution, the comparison between 1-week- and 2-weeks-in-Na+ provided two interesting observations: the Na+/K+ ratio in roots was practically constant, whereas that in rosettes increased dramatically after bolting. This increase was the consequence of the Na+ excluded from the inflorescence while the Na+ content of the shoot (rosette and inflorescence) remained constant with reference to its dry weight (Supplemental Fig. S1b summarizes these changes in terms of the Na+/K+ ratio in plants growing at 40 mM NaCl). This temporal evolution could be confounded with a permanent Na+ increase as leaves age (Tester and Davenport 2003), which might complicate the analysis of the toxic effects of Na+. However, we found that this permanent increase did not occur in Col-0 plants, which reached an almost constant Na+ content approximately 5 days after being exposed to 40 and 60 mM NaCl; only at 80 mM NaCl there was a statistically significant 35 % increase of the shoot Na+ content during the second week in Na+ (the increase of the Na+ content during the second week in NaCl can be calculated by comparing 1-week- and 2-weeks-in-Na+ plants in Supplemental Tables S1 and S2).
To confirm the absence of a permanent increase of the Na+ content of shoots, we performed an additional experiment in which Na+ was added to the plants 1 week earlier than in the standard procedure. After 2 weeks in Na+ these plants did not bolt and the mean Na+/K+ ratio in the rosettes (2.0 ± 0.4) was not significantly different from the corresponding mean found in our standard 1-week-in-Na+ plants (1.8 ± 0.3 in Table 1). This result demonstrated that, in our standard conditions, the increase of the Na+ content of Col-0 rosettes during the second week in Na+ was due to the growth of the inflorescence.
Na+ content and growth of Col-0 plants
In 2-weeks-in-Na+ plants, 20 mM NaCl either did not affect growth, in most repetitions, or reduced the shoot weight (rosette plus inflorescence) by less than 10 % as compared to control plants. At 60 and 80 mM NaCl the weight of shoots was reduced to approximately one-half and one-third, respectively (Fig. 1; Supplemental Tables S1 and S2). In roots, the response followed the same pattern but was more difficult to quantify because of their small size in 1-week-in-Na+ plants. All these responses were similar to those reported previously (Labidi et al. 2002; Alemán et al. 2009; Kaddour et al. 2010) for Col-0 plants at 50 mM NaCl; this is relevant because the testing conditions differed between studies. Performing the experiments as described here, the bolting time was not changed by the NaCl treatments. The inflorescence/rosette weight ratio might be slightly affected at high Na+ concentrations, but this was not quantified. In plants growing at 60 and 80 mM NaCl, many leaves were yellowish (see below); this response was also observed at 40 mM Na+ but was less severe and occurred during the second week.
To determine the Na+ levels that were or were not toxic in Col-0 plants, the growth of the inflorescence created a problem because it produced a continuous increase of the rosette Na+ content. Moreover, it was obvious that the reduction of the growth rate by a toxic Na+ level might not occur immediately. Therefore, the toxicity of Na+/K+ molar ratios was assessed in 1-week-in-Na+ plants by considering whether 2-weeks-in-Na+ plants were normal or growth inhibited. As described above, at 20 mM NaCl, the weight of 2-weeks-in-Na+ Col-0 plants was not affected with reference to control plants (Fig. 1). This implied that the Na+/K+ molar ratios in roots and rosettes in 1-week-in-Na+ plants, 0.32 and 0.66, respectively (Table 1), were perfectly tolerated by the plants. A similar analysis for 40 mM NaCl plants, which were growth inhibited, suggested that Na+/K+ ratios of 0.78 in roots or 1.8 in shoots might be associated to Na+ toxicity (Fig. 1; 1-week-in-Na+ plants in Table 1). However, this toxicity was only a possibility because it was unknown whether the inhibition at 40 mM NaCl was due to Na+ toxicity in roots or shoots or produced by the osmotic stress while Na+ was innocuous. In other words, healthy plants at 20 mM NaCl suffered neither Na+ toxicity nor other type of inhibition but growth inhibition at higher NaCl concentrations might result from multiple causes.
Next, we addressed Na+-specific effects at low Na+ concentrations (<20 mM NaCl) using the hkt1 and sos1 mutants.
Na+ toxicity in the shoots of hkt1 plants
It is well established that hkt1 plants have a defective retrieval of Na+ from the xylem sap and show an exaggerated delivery of Na+ to the shoot (Mäser et al. 2002; Berthomieu et al. 2003; Rus et al. 2004, 2006; Sunarpi et al. 2005; Davenport et al. 2007). Consequently, we found higher shoot Na+ content in hkt1-4 versus gl1 plants (Table 2; Supplemental Tables S3 and S4) and a high Na+ sensitivity of hkt1-4 plants (Fig. 2). The higher shoot Na+ content was evident even in control plants at 0.2 mM Na+, but was more evident at higher Na+ concentrations. A convenient parameter to characterize hkt1-4 plants was the rosette-Na+/root-Na+ ratio in 1-week-in-Na+ plants, which had only two compartments: shoots and roots (Supplemental Fig. S1b). In gl1 plants the rosette-Na+/root-Na+ ratio amounted to from 2 to 3 at all NaCl concentrations that we tested, whereas it amounted to 8 and 69 in hkt1-4 plants at 0.2 and 4.0 mM Na+, respectively. At 8.0 mM Na+, where hkt1-4 plants were mildly inhibited (see below), the rosette-Na+/root-Na+ ratio amounted to 91 (Table 2). In terms of inflorescence/rosette Na+ distribution hkt1-4 plants were similar but not identical to wild-type plants (compare 20 mM Na+ in Table 1 with 4.0 mM Na+ hkt1-4 plants in Table 2; a more complete comparison can be made in Supplemental Tables S2 and S4). These observations are consistent with previous descriptions (Berthomieu et al. 2003).
At 8.0 mM NaCl the shoot growth of hkt1-4 plants was reduced by 25 % (Fig. 2), which was equivalent to that produced by 40 mM Na+ in Col-0 plants. The root Na+/K+ ratio of 8.0 mM Na+ hkt1-4 plants was much lower than those of 8.0 mM Na+ gl1 and 20 mM Na+ Col-0 plants, which were not inhibited (0.02 versus 0.1 and 0.3, respectively). These data demonstrated root Na+ toxicity was not the cause of growth inhibition in 8.0 mM Na+ hkt1-4 plants. Considering that hkt1-4 plants in 4 mM Na+ were unaffected and in 8 mM Na+ were inhibited (Fig. 2), the rosettes of these plants provided the Na+/K+ ratios that were associated with Na+ leaf toxicity in Col-0 plants: a ratio around 1.0 was not toxic whereas a ratio of 2.0 was toxic (Table 2). Shoot Na+ toxicity has been associated with senescent-like yellow leaves (e.g., Rajendran et al. 2009); in 8.0 mM Na+ hkt1-4 plants, the first yellow leaves appeared after 10 days in Na+ when growth had clearly decreased. After 2 weeks, the first pair of leaves was affected in most but not in all plants. This suggests that senescent-like yellow leaves were a clear symptom of shoot Na+ toxicity in Col-0 plants but of late onset.
Despite their low Na+ content the roots of Na+-inhibited hkt1-4 plants were smaller than those of control plants (Supplemental Table S4), which suggested that their slow growth rate was induced by the inhibited shoots.
Na+ toxicity in the roots of sos1 plants
Next we tested sos1-1 plants, for which a defective Na+ efflux from root cells to the external medium and a defective loading of Na+ from the root symplast into the xylem have been described (Davenport et al. 2007; Qiu et al. 2003; Shi et al. 2000, 2002). Consistent with these defects, sos1-1 plants had an excess of Na+ in the roots, which was clearly detected even in control plants at 0.2 mM Na+. The rosette-Na+/root-Na+ ratio in sos1-1 plants was 1.0–1.2 (Table 2).
sos1-1 plants tolerated approximately ten-fold less Na+ than Col-0 or gl1 plants (Fig. 2). At 2.0 mM Na+, sos1-1 plants showed weak inhibition of rosette growth (preliminary experiments showed that 0.2 and 1.0 mM Na+ sos1-1 plants were identical; the real limit of Na+ tolerance in sos1-1 plants is probably slightly less than 2.0 mM). At higher NaCl concentrations, there were no equivalent inhibitions in sos1-1 and Col-0 plants when both roots and shoots were considered, because of the remarkable root growth inhibition in sos1-1 plants, which was almost total by as little as 4.0 mM Na+ (Fig. 2). In terms of the inhibition of shoot growth, sos1-1 plants growing at 4.0 mM Na+ could be roughly compared with 40 mM Na+ Col-0 plants. The shoot Na+/K+ ratio of 4.0 mM Na+ sos1-1 plants was lower than those of 20 mM Na+ Col-0 and 4.0 mM Na+ hkt1-4 plants, which were not inhibited (0.54 versus 0.66 and 0.97, respectively, in 1-week-in-Na+ plants). Therefore, 4.0 mM sos1-1 plants were not inhibited by shoot Na+ toxicity.
The highly inhibited root growth in 4.0 mM Na+ sos1-1 plants (Fig. 2) took place when the root Na+/K+ ratio was 0.56 (1-week-in-Na+ plants in Table 2), which indicated that this ratio was associated to root Na+ toxicity. Unfortunately, the continually increasing Na+ content in sos1-1 roots made it difficult to determine the limit of the non-toxic Na+ content in roots. However, we can establish that a Na+/K+ ratio of 0.32 was not toxic (1-week-in-20 mM Na+ Col-0 plants; Table 1).
Although the shoot Na+ content of sos1-1 plants in 4.0 mM Na+ was lower than the toxic limit, shoot growth was inhibited, which indicated that shoot inhibition occurred as a consequence of root inhibition.
Symptoms of shoot and root Na+ toxicities
The experiments described above with hkt1-4 and sos1-1 plants addressed Na+ toxicity in shoots and roots at mild inhibitions. To investigate the differences between shoot and root toxicities in more detail, we used more drastic inhibitions, growing hkt1-4 and sos1-1 plants in parallel at 16 and 5.0 mM Na+, respectively. We selected these concentrations because they produced similar growth inhibitions of the shoots of both plant lines (approximately 60 %), but the plants survived and produced flowers.
In these tests, the transfer of 2-week-old plants to the selected Na+ concentrations did not produce immediate effects. The fourth day, Na+ inhibited and control plants did not show appreciable size differences, but 1 week after the transfer, the differences were clear (Fig. 3a). At this time, some of the oldest leaves of 16 mM Na+ hkt1-4 plants were slightly yellowish and the leaves of 5.0 mM Na+ sos1-1 plants were slightly darker green. After 2 weeks in Na+, the size difference between Na+-inhibited and control plants (0.2 and 16 mM Na+ gl1, 0.2 mM Na+ hkt1-4 and sos1-1 plants) was notable (Fig. 3b). In addition, hkt1-4 rosettes showed many senescent-like yellow leaves and many leaves of sos1-1 plants were purple and darker green, resembling a mild dwarf defect (Clouse and Langford 1996). The roots of hkt1-4 and sos1-1 plants were also smaller than those of control plants and sos1-1 roots were much smaller than hkt1-4 roots (Fig. 3b). Consistent with the aforementioned causes of the inhibitions in hkt1-4 and sos1-1 plants, the Na+/K+ ratios revealed the expected shoot and root Na+ toxicities (in 1-week-in-Na+ plants the Na+/K+ ratios were: 16 mM Na+ hkt1-4 plants, root = 0.04 ± 0.01, rosette = 12 ± 1; 5.0 mM Na+ sos1-1 plants, root = 2.2 ± 0.3, rosette = 1.4 ± 0.1).
Physical appearance of hkt1-4 and sos1-1 plants growing at 16 and 5 mM Na+, respectively, in comparison to controls: 0.2 mM Na+ gl1, hkt1-4, and sos1-1 plants, and 5 and 16 mM Na+ gl1 plants. Plants were grown for 2 weeks at 0.2 mM Na+ and transferred to the indicated Na+ concentrations. a and b, 1-week- and 2-weeks-in-Na+ plants, respectively
The shoot anthocyanin content was an excellent indicator of root Na+ toxicity in our experiments. In 1-week-in-Na+ plants, 16 mM Na+ hkt1-4 and 5.0 mM Na+ sos1-1 plants had similar shoot anthocyanin contents that were approximately four times higher than in all control plants (0.2 and 16 mM Na+ gl1, 0.2 mM Na+ hkt1-4 and sos1-1 plants; A530 g−1 FW = 1.0 ± 0.4). In 2-weeks-in-Na+ plants, the differences increased notably because whereas the anthocyanin content of 16 mM Na+ hkt1-4 and control plants did not increase appreciably during the second week in Na+, that in 5.0 mM Na+ sos1-1 increased more than six times (A530 g−1 FW = 26.1 ± 0.8; Supplemental Fig. S2).
In summary, although the inhibition by Na+ in 16 mM Na+ hkt1-4 and 5.0 mM Na+ sos1-1 plants was originated in shoots and roots, respectively, shoot observation alone was not sufficient to distinguish both types of inhibition. A distinction was possible in terms of the senescent-like yellow leaves of hkt1-4 plants and the darker green and purple color of sos1-1 plants but only after many days of exposure to Na+ in plants that were highly inhibited.
Alternative tests for Na+ toxicity evaluation
To rule out specific effects of our tests of Na+ tolerance, we compared our results to those obtained using two alternative methods for testing Na+ tolerance: germinating and growing the plants in Na+ and testing only root growth in agar plates.
Two-week-old Col-0 plants germinated and grown at 40 mM Na+ showed high size variability and were classified into three groups, large, medium, and small. Plants of these three groups grew at similar rates during the last week in Na+ (same weight ratio between 4- and 3-week-old plants in both cases) and the corresponding Na+ contents in roots, shoots, and inflorescences were not significantly different. Comparing these plants with those germinated at 0.2 mM Na+ and transferred to 40 mM we found that the two types of plants were very similar in growth rate during the last week in Na+ and Na+ content. The only notable difference was in the inflorescence, which was smaller and showed a higher variability in size in the plants that were permanently grown in Na+.
The nondestructive method of measuring root growth in agar plates has been widely used to characterize responses to NaCl (Liu and Zhu 1997; Zhang et al. 2011; Shelden et al. 2013) and is an attractive method for genetic studies. Therefore, we compared this and the hydroponic method in the characterization of the effect of NaCl in hkt1-4 and sos1-1 plants. For this purpose, five-day-old seedlings grown in half-strength MS medium were transferred to the medium that was used in the hydroponic cultures supplemented with sucrose, agarose, and increasing NaCl concentrations from 0.2 to 100 mM. After 48 h of adaptation to this new medium, root length growth was monitored for seven additional days. Although root growth was not linear with time, the mean growth rate in this period expressed as growth in 24 h was sufficiently precise for our purposes. The root growth test revealed the sensitivity of sos1-1 plants to Na+ but did not detect the Na+ sensitivity of hkt1-4 plants (Fig. 4).
NaCl inhibition in Col-0 and Ts-1 plants is not dominated by Na+ toxicity
The second goal of this study was to determine the basis of growth inhibition of Arabidopsis by NaCl. We have already estimated the Na+/K+ ratios that were associated to Na+ toxicity in rosettes and roots in the absence of osmotic stress. The comparison of these ratios with those in Col-0 plants growing in increasing NaCl concentrations (Table 1) predicted that Na+ intoxication in rosettes and roots was mild at 40 mM and severe at 60–80 mM NaCl. Considering this comparison, it was evident that Na+ toxicity in either shoots or roots might be the cause of the growth inhibition of Col-0 plants by NaCl (Fig. 1). However, this coincidence neither demonstrated this causality nor ruled out other types of inhibitions. Therefore, our next step was to investigate other causes of inhibitions and, for this purpose, we decided to use an additional accession, Ts-1. This accession carries a hypomorphic HKT1 gene that leads to high Na+ content in shoots and, remarkably, to increased Na+ tolerance (Rus et al. 2006; Baxter et al. 2010).
Experiments with Ts-1 plants (Fig. 5; Table 3; Supplemental Table S2) similar to those performed with Col-0 plants (Fig. 1; Table 1) revealed that the response of these two accessions to NaCl was similar in terms of growth and very different in terms of Na+ content and rosette-Na+/root-Na+ ratio (in Col-0 plants this ratio should be calculated in 1-week-Na+-plants, before bolting, but this requirement does not apply to Ts-1 plants because they did not bolt in 4 weeks). For example, in 2-weeks-in 60 mM NaCl Ts-1 plants, the shoot and root Na+/K+ ratios were 8.8 and 0.28, respectively, to be compared with 2.8 and 1.3 in 1-week-in-Na+ Col-0 plants. Despite the high Na+ in shoots in Ts-1 plants in 60 or 80 mM NaCl, only the cotyledons and occasionally the first two leaves showed a senescence-like yellowing, which was shown by many leaves of Col-0 plants at these NaCl concentrations.
The similarities and contrasts between the responses of Col-0 and Ts-1 plants to NaCl suggested that Na+ toxicity in either shoot or roots did not dominate the observed growth inhibitions. To demonstrate this conjecture we used isotonic combinations of KCl and NaCl (Lauter et al. 1988; Benlloch et al. 1994; Kinraide 1999) that together amounted to 60 mM. By this procedure the Na+/K+ ratio in roots and shoots can be changed dramatically and the effect of this change on the growth of the plant can be easily tested. An excess of KCl might lead to an excess of K+ in leaves and K+ toxicity but combinations of KCl and NaCl are less toxic than any of these salts alone (Benlloch et al. 1994; Kinraide 1999). In any case, our tests were performed at K+ concentrations that were below toxic levels. In our cultural conditions, KCl was not toxic up to 20 mM; K+ concentrations of 5 and 10 mM are equivalent to 0.25x and 0.5 × strength MS medium (Murashige and Skoog 1962). Above 20 mM, KCl was much more toxic than NaCl.
In these experiments, 2-week-old Col-0 and Ts-1 plants were grown for two additional weeks in several combinations of KCl and NaCl (mM): 5–55, 10–50, and 20–40, in parallel with our control (0.2 mM Na+; 1–0.2 in Fig. 6) and inhibited (60 mM NaCl; 1–60 in Fig. 6) plants. As a result of the increase of the external K+, both Col-0 and Ts-1 plants decreased dramatically their shoot Na+/K+ ratio and to a lesser extent their root Na+/K+ ratio (Fig. 6a; Supplemental Table S5). In contrast with these changes, the weight of shoots was scarcely affected in Col-0 and completely unaffected in Ts-1 plants (Fig. 6b). In roots the decrease of the Na+/K+ ratio produced growth stimulation (at 5–55 and 10–50 in Col-0 and at the three combinations in Ts-1). Although we did not obtained statistical significance, this stimulation was probably only partial, insufficient to recover the weight observed in control plants (Fig. 6b; Supplemental Table S5). In parallel with the decrease of the shoot Na+/K+ molar ratio, the senescent-like yellow leaves that appeared in 60 mM NaCl Col-0 plants disappeared in the 5–55 combination (Fig. 6c) and higher K+ concentrations.
Response of the growth of Col-0 and Ts-1 plants to the increase of K+ in 60 mM isotonic combinations of KCl and NaCl in comparison with 0.2 (control 1–0.2) and 60 (inhibited 1–60) mM Na+ plants. Na+/K+ molar ratio (a) and weights (b) of roots, rosettes, and inflorescences (only in Col-0). c Physical appearance of 2-weeks-in-Na+ Col-0 and Ts-1 plants; senescence-like leaves in 60 mM NaCl Col-0 plants disappeared when K+ was increased to 5 mM (c plants in 0.2 mM Na+ were bigger than the other plants and are shown at a smaller scale; the inflorescences of Col-0 plants were removed for a better observation of the rosettes). a and b, same letter in the same organ of plants in different conditions indicates that the means are not statistically different; b Col-0 plants, statistical differences have been calculated for the weight of rosette plus inflorescence
In summary, the symptoms of shoot Na+ toxicity disappeared as a function of the decrease of the Na+ content in Col-0 plants while growth inhibition was insensitive to this decrease of Na+ in both Col-0 and Ts-1 plants. This latter observation is not new (Lauter et al. 1988) and strongly suggested that shoot growth inhibition of Col-0 and Ts-1 plants by 1–60, 5–55, 10–50, and 20–40 mM KCl-NaCl was not due to Na+ toxicity.
NaCl does not inhibit Col-0 and Ts-1 plants by an osmotic effect
According to current dogma, salinity inhibits plant growth by osmotic and ionic effects (Munns and Tester 2008). We have shown that Col-0 plants exposed to 60 mM NaCl suffered Na+ toxicity but that this toxicity was not the cause of the observed growth inhibition and that Ts-1 plants did not show symptoms of Na+ toxicity. These observations suggested that Col-0 and Ts-1 plants at 60 mM NaCl or KCl–NaCl combinations were inhibited by an osmotic effect, but this assumption needed to be verified. However, a clear test for osmotic inhibition does not exist because most compounds that are added to the roots of plants at a high concentration may be toxic or induce the growth of bacteria. Therefore, to overcome this problem at least partially, we designed experiments in sterility using sorbitol to decrease the water potential and measuring the fresh weight/dry weight ratio as an indicator of the osmotic stress (Handa et al. 1983). The results were clear, Col-0 and Ts-1 plants growing in 120 mM sorbitol decreased their water content by approximately 50 % with reference to control plants while this decreased did not occurred in 10–50 KCl–NaCl plants (Fig. 7a). To further support these results we performed a series of experiments with Col-0 plants using culture vessels or vertical plates with 0.25× strength MS or hydroponic medium in the four combinations. The results of these experiments were remarkable coincident; considering all the data, the wet weight/dry weight ratio for control and 10–50 plants was 12.7 ± 0.9 and 13.3 ± 1.6, respectively, while it was 7.2 ± 0.9 for 120 mM sorbitol plants. Regarding the cation content by dry weight, the K+ plus Na+ content increased in 10–50 KCl–NaCl plants and decreased in sorbitol plants (Fig. 7b).
Osmotic response and adjustment of Arabidopsis seedlings growing in the hydroponic medium containing 1 mM K+ and 0.2 mM Na+ (control plants), 10 mM KCl plus 50 mM NaCl, or 120 mM sorbitol in control medium. Tests in vessels in sterile conditions: fresh weight/dry weight ratio (a) and K+ plus Na+ content (b). Two-week-old plants germinated and grown in the tested conditions
The simplest interpretation of these results was that the high increase in the K+ plus Na+ content of 10–50 mM KCl–NaCl plants provided their osmotic adjustment to the more negative water potential of this medium. Similar increases in hydroponic tests (e.g., Supplemental Table S5) suggested that hydroponic plants were also osmotically adjusted. To test this conjecture, we measured the wet weight/dry weight ratio in hydroponic cultures of Col-0 and Ts-1 plants in control conditions and growing at 60 mM NaCl and 10-50 mM KCl-NaCl for 1 week (Col-0 plants did not bolt in these tests). We found that under the two challenged conditions the wet weight/dry weight ratio did not change in comparison with control plants and that the responses of Col-0 and Ts-1 plants were indistinguishable (control = 11.8 ± 0.2 and 11.2 ± 0.6; 60 mM NaCl = 10.9 ± 0.6 and 11.6 ± 0.7; 10–50 mM KCl–NaCl = 11.3 ± 0.5 and 11.0 ± 0.3; for Col-0 and Ts-1 plants, respectively).
These results strongly suggest that salinized plants were osmotically adjusted independently from the culture conditions.
Salinity response across Arabidopsis accessions
It is widely accepted that the high sensitivity of most plants to NaCl is caused by the toxic effects of Na+ in leaves (Maathuis and Amtmann 1999; Blumwald et al. 2000; Horie et al. 2008; Munns and Tester 2008; Hauser and Horie 2010) and also that the increase of the HKT1 activity decreases the shoot Na+ content and increases Na+ tolerance (Farquharson 2009; Moller et al. 2009; Plett et al. 2010). However, we found that when the growth of Col-0 and Ts-1 plants was inhibited by NaCl, leaf Na+ toxicity was neither observed in Ts-1 plants nor the dominant inhibitory effect in Col-0 plants. This contradiction raised the question of whether the Col-0 and Ts-1 accessions were exceptions in Arabidopsis. Therefore, we tested the Na+ tolerance and Na+ contents in Arabidopsis accessions (to be published elsewhere) and tried to identify accessions that mimicked the response of the Col-0 hkt1 mutant to NaCl, which was our model of shoot Na+ toxicity. In this search, we identified three accessions, Ak-1, C24, and Fei-0, which did not survive in 60 mM NaCl and showed the symptoms of the hkt1 mutant. To further investigate this response, we tested the growth at 15–45 mM KCl-NaCl, finding that the symptoms of leaf Na+ toxicity disappeared or were highly improved but that plant growth remained inhibited, although to a lesser extent (Fig. 8 show the results for these three accessions in parallel with other three accessions that did not show symptoms of Na+ toxicity). As shown above for Col-0 and Ts-1, all tested accessions showed similar growth inhibition at 15–45 mM KCl-NaCl but very different Na+/K+ molar ratio in roots and shoots. At 30 mM Na+ (1 mM K+), where all accessions grew (Fig. 8), the differences in Na+/K+ molar ratios between accessions were also detectable (Table 4). All these data suggest that although leaf Na+ toxicity seems to take place in some Arabidopsis accessions, NaCl inhibits the growth of many Arabidopsis accessions by a mechanism that is independent from leaf Na+ toxicity.
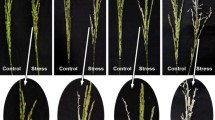
Diversity of the responses of Arabidopsis accessions in 1–0.2, 1–30, 1–60, 15–45 mM KCl-NaCl. Senescent-like yellow leaves in Ak-1, C24, and Fei-0 accessions resemble leaf Na+ toxicity in hkt1 plants. Physical appearance of 2-weeks-in-Na+ plants in hydroponic cultures. Exceptionally, the picture of Ak-1 plants at 60 mM NaCl (frame) corresponds to 1-week-in-Na+ plants because this accession is very sensitive to NaCl
Next, we tested the osmotic adjustment under salinity in several accessions: Fei-0, C24, Tsu-1, Ll-0, and Cvi. For this purpose we measured the wet weight/dry weight ratio in 1-week-in-Na+ plants in control conditions and 60 mM NaCl (in these conditions no accession bolted and the symptoms of Na+ toxicity did not exist or were minimal). The measured ratios were indistinguishable from those reported above for Col-0 and Ts-1 plants (data not shown).
Discussion
This study aimed to describe the responses of Arabidopsis to Na+, at NaCl concentrations that reduce plant growth without interrupting the life cycle, mimicking the growth of crops in salinized soils that impose yield reductions but not the death of plants—rice farming in some tidal wetlands being a good example. Especially, we aimed to separate toxic and osmotic effects.
Prior to a discussion of the main results, two observations deserve to be commented. The first refers to a plant response that modifies Na+ toxicity, because Na+ distribution in the plant was linked to plant development. At rosette stage, Na+ was distributed between the two classical compartments: roots and shoots, but after bolting, the inflorescence forms a third compartment that can be distinguished from the rosette by its low Na+ content. Thus, in parallel with the growth of the inflorescence, the Na+ content of the rosette increased notably, which had a strong effect on rosette Na+ toxicity. The second refers to our use of the Na+/K+ molar ratio as an indicator of the Na+ status of the organs of the plant. Although our conclusions do not depend on the use of this indicator, other indicators of the Na+ content that do not take into account the K+ content (e.g., based on dry weight) were less convenient than the Na+/K+ ratio to describe plant responses. The main reason for this is that the Na+ plus K+ content by dry weight is not constant when the external NaCl increases, which occurs because the Na+ content increases more than the K+ content decreases (Supplemental Tables S1 and S2). A demonstration of the convenience of the use of the Na+/K+ ratio can be obtained comparing 2-weeks-in-Na+ Col-0 plants at 40 mM Na+ (Supplemental Table S2) and 5–55 mM K+-Na+ (Supplemental Table S5). In both cases, the rosette Na+ content was 1500 nmol mg−1 but while the plants at 40 mM Na+ showed many senescent-like yellow leaves; all the leaves were green in 5–55 plants (Fig. 7). Thus, rosette Na+ toxicity could not be related to the Na+ content expressed in terms of dry weight. In contrast, the Na+/K+ ratios in these two cases amounted to 5.2 and 2.6, respectively, which were values more in consonance with the symptoms. It is worth noting that the Na+/K+ molar ratio was a convenient indicator to predict the response of the same accession in different conditions but not to predict the response of different accessions in the same condition.
Na+ toxicity in shoots and roots
Leaf Na+ toxicity is widely considered as the main cause of growth inhibition in salinized plants (Maathuis and Amtmann 1999; Blumwald et al. 2000; Horie et al. 2008; Munns and Tester 2008; Hauser and Horie 2010), but our results suggest more complex causes.
Col-0 plants growing at 20 mM NaCl were at their limit of Na+ tolerance. The corresponding limits for hkt1-4 and sos1-1 plants were 4.0 and 2.0 mM Na+, respectively, which were too low to produce osmotic inhibition. Taking the shoot and root Na+ contents of 20 mM Col-0 plants as nontoxic, we concluded that the growth inhibition by Na+ of hkt1-4 plants was not due to a toxic root Na+ content and that the inhibition of sos1-1 plants was not due to a toxic shoot Na+ content (Table 2). In consequence, Na+ inhibited hkt1-4 and sos1-1 plants suffered shoot and root Na+ toxicity, respectively. Remarkably, by only comparing the size of the shoots of these mutant plants, shoot and root Na+ toxicity could not be distinguished (Fig. 3); other observations are necessary to identify the origin of the inhibition. This lack of size-based differences implies that the coordinated growth of shoots and roots produces smaller roots in plants in which only the shoots are affected by Na+ and smaller shoots in plants in which only the roots are affected. These responses might be due to long-range signaling, which occurs when plants suffer stress conditions, including a lack of nutrients or biological attacks (Liu et al. 2009; Sparks et al. 2013). In consequence, shoot and root inhibition by Na+ in Arabidopsis and possibly in other species have similar consequences on the size of the plants and the two types of inhibition might be confused. In this study, shoot Na+ toxicity was revealed by senescent-like yellow leaves and root Na+ toxicity by a high shoot anthocyanin content. However, in both cases symptoms were clear only when growth inhibitions were important; the assessment of Na+ tissue tolerance based on the senescing leaf area (Rajendran et al. 2009) would be misleading in Arabidopsis. Regarding the anthocyanin content, it is worth noting that the connection between anthocyanin accumulation, abiotic stress, and the SOS pathway (Van Oosten et al. 2013) suggests that conclusions about anthocyanin content obtained in a sos1 mutant must be taken with caution.
We concluded above that, in Col-0, Na+/K+ ratios of 1.0 and 0.3 were not toxic in rosettes and roots, respectively, but modest increasing of these ratios might be toxic. These low ratios suggest a high sensitivity that cannot be taken as a general characteristic of Arabidopsis. In fact, in the Ts-1 accession, a rosette Na+/K+ ratio of 3.1 was not toxic in 20 mM NaCl plants. Moreover, we did not observe symptoms of leaf Na+ toxicity in Ts-1 plants at 40 or 60 mM NaCl (Fig. 6c) and only mild symptoms at 80 mM NaCl; in the rosettes of these plants the Na+/K+ ratio amounted to 5.8, 8.8, and 19.2, respectively. The toxicity of Na+ in the cytosol of non-animal cells has been widely discussed (see introduction) but, in contrast with animal cells, there are no convincing data that indicate a high toxicity due to biochemical reasons in non-animal cells (Cheeseman 2013; Kronzucker et al. 2013). In fact, Na+ cannot be considered toxic in fungal cells because a cytosolic Na+/K+ ratio of 2 is tolerated by the fungus Ustilago maydis (Benito et al. 2009). Therefore, assuming the large volume of vacuoles in many plant cells and their capacity to accumulate Na+ (Shabala and Cuin 2007; Munns and Tester 2008; Hasegawa 2013; Roy et al. 2014), it can be expected that high Na+/K+ ratios in the whole tissue can be well tolerated in plant tissues. This reasoning would explain the high shoot Na+/K+ ratios of Ts-1 plants that are apparently normal. Accordingly, Na+ toxicity at low tissue Na+/K+ ratios probably reflects a low capacity to accumulate Na+ in vacuoles. This might apply to Col-0 shoots and Col-0 and Ts-1 roots. However, atypically high cytosolic Na+ sensitivity cannot currently be ruled out in these cases.
In summary, growth inhibition in Arabidopsis may be produced by either shoot or root Na+ toxicity but the distinction between these two causes might be difficult to establish. For this purpose, the Na+ content is not a reliable benchmark for toxicity when different accessions are studied.
Na+ toxicity and water deficit are not the dominant causes of growth inhibition by NaCl
The drastic inhibition of Ak-1, C24, and Fei-0 plants by 60 mM NaCl was probably the result of leaf Na+ toxicity. Firstly, because the physical aspect of these plants was very similar to that of hkt1 plants growing at 16 mM Na+ and, secondly, because the symptoms of Na+ toxicity were completely or partially relieved by increasing the K+ concentration of the medium. However, in these accessions, when Na+ toxicity was not apparent or minimal in isotonic KCl–NaCl combinations, growth inhibition was only partially relieved (Fig. 8), which suggests that the remaining inhibition was not due to Na+ toxicity. Similarly, it seems unlikely that NaCl inhibition in Col-0 and Ts-1 plants is due to Na+ toxicity. If this were the case, this growth inhibition should have been relieved with the increase of the external K+ in isotonic KCl-NaCl combinations, which effectively decreased the shoot and root Na+ contents and eliminated the senescent-like yellow leaves in Col-0 plants (Fig. 6; Supplemental Table S5). A similar conclusion applies to Ler-0, Tsu-1, and Ll-0 plants (Fig. 8). In summary, all these findings suggest leaf Na+ toxicity is not a cause of growth inhibition by NaCl in many Arabidopsis accessions. Furthermore, it seems that in all accessions the dominant inhibition at 60 mM NaCl or isotonic combinations of KCl and NaCl is not due to leaf Na+ toxicity and operates independently of this toxicity.
In a classic description of NaCl inhibition based on osmotic stress and shoot Na+ toxicity (e.g., Munns 2002), the absence of Na+ toxicity would suggest that osmotic stress produces the inhibition. Certainly, NaCl reduces the water potential of the external medium but the existence of osmotic inhibition in adapted plants growing in NaCl would imply an incomplete osmotic adjustment and the existence of a water deficit (Morgan 1984). In our experiments, Col-0 and Ts-1 seedlings in sterility showed a clear water deficit at 120 mM sorbitol but not at 60 mM NaCl or 10–50 mM KCl-NaCl (Fig. 7). In the last two conditions there was a large increase of the K+ plus Na+ content, which could be the basis of the osmotic adjustment that prevents water deficit. Both the absence of water deficit and the large increase of the K+ plus Na+ content also occurred in hydroponic cultures in all Arabidopsis accessions that we tested. In all these cases, the increase in the Na+ content was the dominant response, which is consistent with previous studies (Pardo and Quintero 2002; Flowers et al. 2015).
The notion that salinized Arabidopsis plants maintain a normal water content while sorbitol-treated plants cannot maintain it is consistent with previously described results using cultured tomato cells treated with polyethylene glycol (Handa et al. 1983) and cultured tobacco cells treated with NaCl, dextran, or polyethylene glycol (Heyser and Nabors 1981a, b). The notion is also consistent with the responses of tomato plants treated with mannitol and NaCl (Slayter 1961) and with those of cotton and pepper plants under salinity and drought treatments (Shalhevet and Hsiao 1986). In addition to these comparative studies using plant cells or plants under different treatments, there is a large amount of studies demonstrating that many plants growing in NaCl are osmotically adjusted and unaffected in their water content or turgor (Boyer 1965; Robinson et al. 1983; Salim 1989; Maggio et al. 2007; Díaz-López et al. 2012). All this suggests that the observed osmotic adjustment and absence of water deficit in Arabidopsis plants at 60 mM NaCl can be considered a normal response. The same conclusion can be drawn from a previous study with Arabidopsis plants growing at 100 mM NaCl (Jha et al. 2010).
If neither Na+ toxicity nor water deficit is the dominant cause of growth inhibition by NaCl, the causes are not obvious. Although these causes have not been investigated in this study, it seems reasonable to assume that they originate inside the plant, not outside of it, and that the overaccumulation of K+ plus Na+ might trigger the inhibition. It might occur by interfering with stomatal conductance (Maggio et al. 2007) or be produced by the osmolyte (Na+ plus K+) overaccumulation (Maggio et al. 2002). However, the most likely possibility is a stress response that restrains growth to avoid the risk of growing prolifically under sub-optimal conditions (Dolferus 2014). This response is mediated by DELLA proteins and modulated by several phytohormones (Achard et al. 2006).
The Arabidopsis model in plant salt tolerance
The wide use of Arabidopsis as a model plant and its large resources for genetic and molecular studies raises the question of whether Na+ tolerance in Arabidopsis can be used as a model for crop plants (Zhu 2000; Moller and Tester 2007). This question is clearly relevant, but before discussing it, the term model must be defined regarding extension and exact meaning. The extension is important because if the model includes many details, the number of plants that respond to the Arabidopsis model might be low. The adaptation to soils with a high Na+ content was probably essential in the late evolution of many land plants and occurred independently in different plants (Flowers et al. 2010; Bennett et al. 2013; Rozema and Schat 2013). Therefore, the use of Arabidopsis as a model should refer to general effects and movements of Na+ but not to every detail of the processes involved.
A second issue refers to the concept of salt tolerance and the tests to quantify it. Currently, many different tests have been used in Arabidopsis: time of survival at killing NaCl concentrations of either plants (Rus et al. 2006; Katori et al. 2010) or seedlings (Katori et al. 2010), weight increase after either a few (Moller et al. 2009; Jha et al. 2010) or many (Labidi et al. 2002; Alemán et al. 2009; Kaddour et al. 2010) days in NaCl, germination at high NaCl concentrations (Quesada et al. 2002), and probably others. It is not clear that all the different concepts of Na+ tolerance that these approaches involve are coincident with the concept of Na+ tolerance used in crop plant breeding. Moreover, they do not produce the same results. For example, depending on the approach, Ler is much (Jha et al. 2010) or slightly (Labidi et al. 2002) more Na+ tolerant than Col-0, and Ts-1 more (Rus et al. 2006) or less Na+ tolerant (Katori et al. 2010) than Col-0. Our results give at least a partial explanation to these contradictions because all accessions might eventually be inhibited by shoot and root Na+ toxicity and K+ plus Na+ overaccumulation, and the dominant inhibition and extension of its effect might depend on the testing conditions.
Another interesting issue regarding the usefulness of Arabidopsis as a model plant for salt tolerance research refers to the diversity of responses that exist across accessions. Previous (Attia et al. 2008; Jha et al. 2010; Kaddour et al. 2010; Katori et al. 2010; Labidi et al. 2002) and present results lead to the conclusion that this diversity is very high regarding Na+ accumulation and toxicity. In contrast, diversity seems to be low regarding the growth inhibition that is independent from Na+ toxicity and inexistent regarding osmoregulation.
What Arabidopsis suggests for improving crop salt tolerance
Crop salt tolerance has posed many difficulties to plant breeders because it shows the characteristics of a multigenic trait that reflects its physiological complexity (Flowers 2004). As already discussed, this physiological complexity can be described as root and shoot Na+ toxicities, and the growth restraint produced by the stressing conditions, which operate by independent mechanisms; the complexity becomes higher considering that shoot and root Na+ toxicities depend on several traits, such as total Na+ uptake, shoot/root distribution, and intrinsic cellular tolerance. Therefore, treating salt tolerance as a single trait and measuring plant growth or seed production to evaluate the response of the plant to the salt challenge might make it very difficult to identify the individual genes that are involved in the complex response. For example, at 60 mM NaCl, Col-0 plants show shoot and root Na+ toxicity, and the growth inhibition response; alleviating only one of these causes of inhibition would have little effect on the growth performance of these plants. If Arabidopsis can be taken as a model, plant salt tolerance must be improved particularizing the genetic strategies depending on the dominant inhibitory effect of NaCl, which should be determined previously in each case.
Author contribution statement
RA-A, RH, and BB conducted experiments. AR-N wrote the manuscript. All authors contributed to conceive and design research, and read and approved the manuscript.
References
Achard P, Cheng H, De Grauwe L et al (2006) Integration of plant responses to environmentally activated phytohormonal signals. Science 311:91–94
Alberts B, Johnson A, Lewis J, Raff M, Roberts K, Walter P (2007) Molecular biology of the cell, 5th edn. Garland Science, New York
Alemán F, Nieves-Cordones M, Martínez V, Rubio F (2009) Potassium/sodium steady-state homeostasis in Thelungiella halophila and Arabidopsis thaliana under long-term salinity conditions. Plant Sci 176:768–774
Algeo TJ, Scheckler SE (1998) Terrestrial-marine teleconnections in the Devonian: links between the evolution of land plants, weathering process, and marine anoxic events. Phil Trans R Soc Lond B 353:113–130
Amtmann A, Sanders D (1998) Mechanisms of Na+ uptake by plant cells. Adv Bot Res 29:75–112
Attia H, Arnaud N, Karray N, Lachaâl M (2008) Long-term effects of mild salt stress on growth, ion accumulation and superoxide dismutase expression of Arabidopsis rosette leaves. Physiol Plant 132:293–305
Baxter I, Brazelton JN, Yu D et al (2010) A coastal cline in sodium accumulation in Arabidopsis thaliana is driven by natural variation of the sodium transporter AtHKT1;1. PLoS Genet 6(11):e1001193
Benito B, Garciadeblás B, Schreier P, Rodríguez-Navarro A (2004) Novel P-type ATPases mediate high-affinity potassium or sodium uptake in fungi. Eukaryot Cell 3:359–368
Benito B, Garciadeblás B, Pérez-Martín J, Rodríguez-Navarro A (2009) Growth at high pH and sodium and potassium tolerance in media above the cytoplasmic pH depend on ENA ATPases in Ustilago maydis. Eukaryot Cell 8:821–829
Benito B, Gaciadeblás B, Fraile-Escanciano A, Rodríguez-Navarro A (2011) Potassium and sodium uptake in fungi. The transporter diversity in Magnaporthe oryzae. Fungal Genet Biol 48:812–822
Benito B, Garciadeblás B, Rodríguez-Navarro A (2012) Hak transporters from Physcomitrella patens and Yarrowia lipolytica mediate sodium uptake. Plant Cell Physiol 53:1117–1123
Benlloch M, Ojeda MA, Ramos J, Rodriguez-Navarro A (1994) Salt sensitivity and low discrimination between potassium and sodium in bean plants. Plant Soil 166:117–123
Bennett TH, Flowers TJ, Bromham L (2013) Repeated evolution of salt-tolerance in grasses. Biol Lett 9:20130029
Berthomieu P, Conéjéro G, Nublat A et al (2003) Functional analysis of AtHKT1 in Arabidopsis shows that Na+ recirculation by the phloem is crucial for salt tolerance. EMBO J 22:2004–2014
Blumwald E, Aharon GS, Apse MP (2000) Sodium transport in plant cells. Biochim Biophys Acta 1465:140–151
Boyer JS (1965) Effects of osmotic water stress on metabolic rates of cotton plants with open stomata. Plant Physiol 40:229–234
Cheeseman JM (2013) The integration of activity in saline environments: problems and perspectives. Funct Plant Biol 40:759–774
Clouse SD, Langford M (1996) Brassinosteroid-insensitive mutant in Arabidopsis thaliana exhibits multiple defects in growth and development. Plant Physiol 111:671–678
Davenport RJ, Muñoz-Mayor A, Jha D, Essah PA, Rus A, Tester M (2007) The Na+ transporter AtHKT1;1 controls retrieval of Na+ from the xylem in Arabidosis. Plant Cell Environ 30:497–507
Díaz-López L, Gimeno V, Lidón V, Simón I, Martínez V, García-Sánchez F (2012) The tolerance of Jatropha curcas seedlings to NaCl: an ecophysiological analysis. Plant Physiol Biochem 54:34–42
Dolferus R (2014) To grow or not to grow: a stressful decision for plants. Plant Sci 229:247–261
Evans HJ, Sorger G (1966) Role of mineral elements with emphasis on the univalent cations. Annu Rev Plant Physiol 17:47–76
Farquharson KL (2009) Targeted overexpression of a sodium transporter in the root stele increases salinity tolerance. Plant Cell 21:1875
Flowers TJ (2004) Improving crop salt tolerance. J Exp Bot 55:307–319
Flowers TJ, Galal HK, Bromham L (2010) Evolution of halophytes: multiple origins of salt tolerance in land plants. Funct Plant Biol 37:604–612
Flowers TJ, Munns R, Colmer TD (2015) Sodium chloride toxicity and the cellular basis of salt tolerance in halophytes. Ann Bot-London 115:419–431
Handa S, Bressan RA, Handa AK, Carpita NC, Hasegawa PM (1983) Solutes contributing to osmotic adjustment in cultured plant cells adapted to water stress. Plant Physiol 73:834–843
Haro R, Bañuelos MA, Rodríguez-Navarro A (2010) High-affinity sodium uptake in land plants. Plant Cell Physiol 51:68–79
Hasegawa PM (2013) Sodium (Na+) homeostasis and salt tolerance of plants. Environ Exp Bot 92:19–31
Hasegawa PM, Bressan RA, Zhu J-K, Bohnert HJ (2000) Plant cellular and molecular responses to high salinity. Annu Rev Plant Physiol Plant Mol Biol 51:463–499
Hauser F, Horie T (2010) A conserved primary salt tolerance mechanism mediated by HKT transporters: a mechanism for sodium exclusion and maintenance of high K+/Na+ ratio in leaves during salinity stress. Plant Cell Environ 33:552–565
Heyser JW, Nabors MW (1981a) Growth, water content, and solute accumulation of two tobacco cell lines cultured on sodium chloride, dextran, and polyethylene glycol. Plant Physiol 68:1454–1459
Heyser JW, Nabors MW (1981b) Osmotic adjustment of cultured tobacco cells (Nicotiana tabacum var. Samsum) grown on sodium chloride. Plant Physiol 67:720–727
Horie T, Sugawara M, Okunou K, Nakayama H, Schroeder JI, Shinmyo A, Yoshida K (2008) Functions of HKT transporters in sodium transport in roots and in protecting leaves from salinity stress. Plant Biotechnol 25:233–239
Horie T, Hauser F, Schroeder JI (2009) HKT transporter-mediated salinity resistance mechanism in Arabidopsis and monocot plants. Trends Plant Sci 14:660–668
Jha D, Shirley N, Tester M, Roy SJ (2010) Variation in salinity tolerance and shoot sodium accumulation in Arabidopsis ecotypes linked to differences in the natural expression levels of transporters involved in sodium transport. Plant Cell Environ 33:793–804
Kaddour R, M’rah S, Karray-Bouraoui N, Lambert C, Berthomieu P, Lahaâl M (2010) Physiological and molecular characterization of salt response of Arabidopsis thaliana NOK2 ecotype. Acta Physiol Plant 32:503–510
Katori T, Ikeda A, Iuchi S et al (2010) Dissecting the genetic control of natural variation in salt tolerance of Arabidopsis thaliana accessions. J Exp Bot 61:1125–1138
Kinraide TB (1999) Interactions among Ca2+, Na+ and K+ in salinity toxicity: quantitative resolution of multiple toxic and ameliorative effects. J Exp Bot 50:1495–1505
Kronzucker HJ, Coskun D, Schulze LM, Wong JR, Britto DT (2013) Sodium as nutrient and toxicant. Plant Soil 369:1–23
Labidi N, Lachaâl M, Chibani F, Grignon C, Hajji M (2002) Variability of the response to sodium chloride of eight ecotypes of Arabidopsis thaliana. J Plant Nut 25:2627–2638
Lauter DJ, Meiri A, Shuali M (1988) Isoosmotic regulation of cotton and peanut at saline concentrations of K and Na. Plant Physiol 87:911–916
Liu J, Zhu J-K (1997) An Arabidopsis mutant that requires increased calcium for potassium nutrition and salt tolerance. Proc Natl Acad Sci USA 94:14960–14964
Liu T-Y, Chang C-Y, Chiou T-J (2009) The long-distance signaling of mineral macronutrients. Curr Opin Plant Biol 12:312–319
Maathuis FJM, Amtmann A (1999) K+ nutrition and Na+ toxicity: the basis of cellular K+/Na+ ratios. Ann Bot 84:123–133
Maggio A, Miyazaki S, Veronese P et al (2002) Does proline accumulation play an active role in stress-induced growth reduction? Plant J 31:699–712
Maggio A, Raimondi G, Martino A, Pascale SD (2007) Salt stress response in tomato beyond the salinity tolerance threshold. Environ Exp Bot 59:276–282
Mäser P, Eckelman B, Vaidyanathan R et al (2002) Altered shoot/root Na+ distribution and bifurcating salt sensitivity in Arabidopsis by genetic disruption of the Na+ transporter AtHKT1. FEBS Lett 531:157–161
Moller IS, Tester M (2007) Salinity tolerance of Arabidospsis: a good model for cereals? Trends Plant Sci 12:534–540
Moller IS, Gilliham M, Jha D et al (2009) Shoot Na+ exclusion and increased salinity tolerance engineered by cell type-specific alteration of Na+ transport in Arabidopsis. Plant Cell 21:2163–2178
Morgan JM (1984) Osmoregulation and water stress in higher plants. Annu Rev Plant Physiol 35:299–319
Munns R (1993) Physiological processes limiting plant growth in saline soils: some dogmas and hypotheses. Plant Cell Environ 16:15–24
Munns R (2002) Comparative physiology of salt and water stress. Plant Cell Environ 25:239–250
Munns R, Tester M (2008) Mechanisms of salinity tolerance. Annu Rev Plant Biol 59:651–681
Murashige T, Skoog F (1962) A revised medium for rapid growth and bio-assays with tobacco tissue cultures. Physiol Plant 15:473–479
Pardo JM, Quintero FJ (2002) Plants and sodium ions: keeping company with the enemy. Genome Biol 3:1017.1011–1017.1013
Plett D, Safwat G, Moller IS et al (2010) Improved salinity tolerance of rice through cell type-specific expression of AtHKT1;1. PLoS ONE 5(9):e12571
Qiu QS, Barkla BJ, Vera-Estrella R, Zhu JK, Schumaker KS (2003) Na+/H+ exchange activity in the plasma membrane of Arabidopsis. Plant Physiol 132:1041–1052
Quesada V, García-Martínez S, Piqueras P, Ponce MR, Micol JL (2002) Genetic architecture of NaCl tolerance in Arabidopsis. Plant Physiol 130:951–963
Rahnama A, James RA, Poustini K, Munns R (2010) Stomatal conductance as a screen for osmotic stress tolerance in durum wheat growing in saline soil. Funct Plant Biol 37:255–263
Rajendran K, Tester M, Roy SJ (2009) Quantifying the three main components of salinity tolerance in cereals. Plant Cell Environ 32:237–249
Robinson SP, Downton WJS, Millhouse JA (1983) Photosynthesis and ion content of leaves and isolated chloroplasts of salt-treated spinach. Plant Physiol 73:238–242
Rodríguez-Navarro A, Rubio F (2006) High-affinity potassium and sodium transport systems in plants J Exp Bot 57:1149–1160
Ronchi A, Farina G, Gozzo F, Tonelli C (1997) Effects of a triazolic fungicide on maize plant metabolism: modifications of transcript abundance in resistance-related pathways. Plant Sci 130:51–62
Roy SJ, Negrao S, Tester M (2014) Salt resistant crop plants. Curr Opin Biotech 26:115–124
Rozema J, Schat H (2013) Salt tolerance of halophytes, research questions reviewed in the perspective of saline agriculture. Environ Exp Bot 92:83–95
Rus A, Lee BH, Munoz-Mayor A et al (2004) AtHKT1 facilitates Na+ homeostasis and K+ nutrition in planta. Plant Physiol 136:2500–2511
Rus A, Baxter I, Muthukumar B, Gustin J, Lahner B, Yakubova E, Salt DE (2006) Natural variants of AtHKT1 enhance Na+ accumulation in two wild populations of Arabidopsis. PLoS Genet 2(12):e210
Salim M (1989) Effects of salinity and relative humidity on growth and ionic relations of plants. New Phytol 113:13–20
Schulze LM, Britto DT, Li M, Kronzucker HJ (2012) A pharmacological analysis of high-affinity sodium transport in barley (Hordeum vulgare L.): a 24Na+/42K+ study. J Exp Bot 63:2479–2489
Shabala S, Cuin TA (2007) Potassium transport and plant salt tolerance. Physiol Plant 133:651–669
Shalhevet J, Hsiao TC (1986) Salinity and drought. A comparison of their effects on osmotic adjustment, assimilation, transpiration and growth. Irrig Sci 7:249–264
Shelden MC, Roessner U, Sharp RE, Tester M, Bacic A (2013) Genetic variation in the root growth response of barley genotypes to salinity stress. Funct Plant Biol 40:516–530
Shi H, Ishitani M, Kim C, Zhu J-K (2000) The Arabidopsis thaliana salt tolerance gene SOS1 encodes a putative Na+/H+ antiporter. Proc Natl Acad Sci USA 97:6896–6901
Shi H, Quintero FJ, Pardo JM, Zhu J-K (2002) The putative plasma membrane Na+/H+ antiporter SOS1 controls long-distance Na+ transport in plants. Plant Cell 14:465–477
Slayter RO (1961) Effects of several osmotic substrates on the water relationships of tomato. Aust J Biol Sci 14:519–540
Sparks E, Wachsman G, Benfey PN (2013) Spatiotemporal signalling in plant development. Nature Rev Genet 14:631–644
Steinbach HB (1962) The prevalence of K. Perspect Biol Med 69:2015–2026
Sunarpi Horie T, Motoda J et al (2005) Enhanced salt tolerance mediated by AtHKT1 transporter-induced Na+ unloading from xylem vessels to xylem parenchyma cells. Plant J 44:928–938
Teakle NL, Tyerman SD (2010) Mechanisms of Cl− transport contributing to salt tolerance. Plant Cell Environ 33:566–589
Tester M, Davenport R (2003) Na+ tolerance and Na+ transport in higher plants. Ann Bot 91:503–527
Van Oosten MJ, Sharkhuu A, Batelli G, Bressan RA, Maggio A (2013) The Arabidopsis thaliana mutant air1 implicates SOS3 in the regulation of anthocynins under salt stress. Plant Mol Biol 83:405–415
Wu S-J, Ding L, Zhu J-K (1996) SOS1, a genetic locus essential for salt tolerance and potassium acquisition. Plant Cell 8:617–627
Zhang Z, Zhang S, Zhang Y et al (2011) Arabidopsis floral initiator SKB1 confers high salt tolerance by regulating transcription and pre-mRNA splicing through altering histone H4R3 and small nuclear ribonucleoprotein LSM4 methylation. Plant Cell 23:396–411
Zhu J-K (2000) Genetic analysis of plant salt tolerance using Arabidopsis. Plant Physiol 124:941–948
Acknowledgments
We thank Carlos Alonso-Blanco, Rhonda Meyer, and José Manuel Pardo for kindly providing seeds of the Arabidopsis accession, and gl1, hkt1-4, and sos1-1 mutants. This work was supported by the Spanish Ministerio de Economía y Competitividad, Grant number AGL2012-36174 and fellowship to RA-A.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Álvarez-Aragón, R., Haro, R., Benito, B. et al. Salt intolerance in Arabidopsis: shoot and root sodium toxicity, and inhibition by sodium-plus-potassium overaccumulation. Planta 243, 97–114 (2016). https://doi.org/10.1007/s00425-015-2400-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00425-015-2400-7