Abstract
A pharmacological study was conducted to analyze the impact of inhibitors of Na+-H+ antiporters (amiloride 100 µM) and cation-chloride-cotransporters (bumetanide 200 µM) on two cultivars of the African rice species (Oryza glaberrima Steud) differing in salt resistance (TOG5307: salt-resistant and TOG5949: salt-sensitive) exposed to 75 mM NaCl during 3 days. Amiloride increased Na+ accumulation in roots and leaves to a higher extent in salt-resistant TOG5307 than in salt-sensitive TOG5949. Bumetanide reduced Cl− accumulation in both cultivars as well as K+ accumulation in TOG5307 and Na+ accumulation in TOG5949, suggesting that the cation-chloride-cotransporter in O. glaberrima does not necessarily strictly behave as a Na+:K+:2Cl− transporter. Inhibitors mainly acted on the absorption step but had low impact on root-to-shoot translocation process. The salt-resistant cultivar TOG5307 was able to efficiently regulate Na+ uptake and to cope with high concentration of accumulated toxic ions, as demonstrated by a higher cell viability index and a higher concentration of protein and photosynthetic pigments in NaCl-exposed plants comparatively to salt-sensitive TOG5949.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Limiting Na+ and Cl− accumulation within tissues is an important challenge for plants exposed to salinity. Sodium and chloride uptake by the roots of higher plants is controlled by a wide range of transporters (Teakle and Tyerman 2010; Zhang et al. 2010; Li et al. 2021). Na+/H+ antiporters assume key functions and were shown to extrude toxic Na+ from the cytosol to the apoplasm (as it is the case for the salt-overly-sensitive pathway, with SOS1 acting as a plasma membrane Na+/H+ antiporter (Guo et al. 2009; Deng et al. 2016)), or from the cytosol to the vacuoles, thus allowing Na+ compartmentation (such as some members of the NHX family (Wang et al. 2016)).
As an essential element, Cl− may be absorbed by numerous transporters including the cation-chloride-cotransporters (CCC) which are membrane-integral solute carriers that mediate electroneutral translocation of Cl−, coupled to K+ and/or Na+ (Henderson et al. 2018). A CCC-encoding gene has been identified in rice (Oryza sativa L.) (Kong et al. 2011) and is required for cell elongation and osmoregulation in the absence of salt (Chen et al. 2016). In the presence of high concentrations of NaCl, this transporter may contribute to long-distance Cl− transport and impact K+/Na+ ratio (Zhu et al. 2017).
The classical Asian rice species Oryza sativa is very sensitive to salinity (Solis et al. 2020), but the marginal African crop Oryza glaberrima Steud. recently received considerable attention in relation to its high level of resistance to a wide range of biotic and abiotic constraints (Veltlman et al. 2019). As far as salt is concerned, a recent screening performed among numerous cultivars allowed to identify a salt-resistant cultivar TOG5307 and a salt-sensitive genotype TOG5949 (Prodjinoto et al. 2018). The comparison between those cultivars suggests that an efficient regulation of Na+ absorption and Cl− translocation from the root to the shoot may be involved in salinity resistance in O. glaberrima (Prodjinoto et al. 2018; 2021).
Amiloride was shown to act as a powerful inhibitor of Na+/H+ antiporter (Taleisnik et al. 1991; Fukuda et al. 1998) while bumetanide is a cation-chloride-cotransporter inhibitor (Colmenero-Flores et al. 2007). In order to gain additional information regarding the involvement of these transporters in salinity resistance in Oryza glaberrima, a pharmacological approach was performed by exposing young seedlings from the two cultivars of African rice to 75 mM NaCl in the absence or in the presence of the inhibitors. Impacts of inhibitors were determined in relation to ion accumulation and to senescence-related parameters resulting from ion toxicity.
2 Material and Methods
2.1 Plant material and Growth Conditions
Seeds the two rice cultivars (Oryza glabberima Steud., cvs salt-resistant TOG TOG5307 (AccNumber WAB0021855) and salt-sensitive TOG5949 (AccNumber WAB0020144)) were dehusked and surface-sterilized with formol/hypochlorite. For each cultivar, 250 seeds germinated on two layers of filter paper Whatman N°2 moistened with 7 mL of half-strength Yoshida solution (Yoshida et al. 1976) in a growth chamber under 12-h day light photoperiod at 95 µmoles m−2 s−1 and a constant temperature of 25 °C. Ten-day-old seedlings were then acclimated to nutrient solution: 180 seedlings for each cultivar were distributed among 30 tanks containing 1.2 L of full-strength Yoshida nutrient solution. In each tank, 6 seedlings were placed on a plugged polystyrene plate floating at the top of the solution. The tanks were randomly distributed in the phytotron (temperature at 29 °C during the day and 26 °C during the night; 16 h day−1 photoperiod with a photon flux density of 300 µmoles m−2 s−1 provided by LED LumiGrow lights, 650 W, red-blue).
After 10 days of growth (plants at the 3-leaf stage), inhibitors were added to 20 tanks per cultivars: 10 tanks received 150 µM amiloride and 10 others received 200 µM bumetanide (both provided by Sigma-Aldrich chemical). The remaining 10 tanks did not receive inhibitors. One day after inhibitor application, NaCl was added to half of the tanks (including those which did not receive inhibitor) to reach a concentration of 75 mM which was maintained for 3 days until plant harvest. Electrical conductivity was 0.82 ± 0.07 mS cm−1 and 7.13 ± 0.24 mS cm−1 for control and salt solution, respectively; the presence of inhibitor had no impact on electrical conductivity.
2.2 Mineral Analysis
Roots and shoots of 5 plants for each treatment were separated; roots were thoroughly rinsed during 60 s in cold distilled water to remove ions from the free spaces. Organs were weighed and dried in an oven at 70 °C to constant weight. Dry matter was digested by HNO3 (68%) + HCl (1:3, v/v); Na+ and K+ were determined by flame emission using atomic absorption spectrometer (Thermo scientific S series model AAS4). Chloride was determined by liquid chromatography (HPLC-Dionex ICS2000, Dionex Corporation, Sunnyvale, California, USA) using a AS15/AG15 column/precolumn system and 20–38 mM KOH as eluant for 40 min.
Selective absorption capacity (SA) of the roots for K+ over Na+ was estimated according to Deng et al. (2016) as SA = (Na+/K+ in environment)/Na+/K+ in plant) while the selective transport (ST) of K+ was defined as ST = (K+/Na+ in shoots)/(K+/Na+ in roots).
2.3 Senescence-Related Parameters
Recorded senescence-related parameters mainly concern pigments and total soluble protein concentrations, cell membrane stability, and cell viability. Each parameter was quantified on five plants per treatment on leaf segments obtained from leaf nos. 2 and 3 (acropetal numbering). Chlorophyll and carotenoids were extracted with cold acetone 80%; absorbance of the extract was read at 445 and 663 nm and used to calculate pigment concentrations according to Lichtenthaler (1987).
Membrane permeability was assessed using two distinct methods. The electrolyte leakage technique was performed as detailed by Bajji et al. (2002). The leakage of UV-absorbing substances was quantified as a relative leakage ratio (RLR) according to Lutts et al. (1996).
Cell viability was determined on leaf segments (c.a. 50 mg FW) quickly rinsed in deionized water containing 0.05% Tween-20 and incubated in darkness at 30 °C in glass tubes containing 5 mL of 0.5% triphenyltetrazolium chloride (TTC; Sigma-Chemical) dissolved in 50 mM K2HPO4 at pH 7.0 during 15 h. Samples recovered after filtration were then incubated for 5 min in 3 mL glass vial containing 94% ethanol at 80 °C. The extracted formazan recovered after centrifugation at 5000 g was quantified spectrophotometrically at 487 nm, and viability index was defined as absorbance measured per g FW according to Lutts et al. (2004). Total soluble proteins were quantified on both roots and leaves according to Bradford (1976).
2.4 Statistical Analysis
The experiment used a completely randomized design. ANOVA 2 was performed considering cultivar and treatment as main factors. Analysis was performed on 5 biological replicates. Normality of the data was preliminary checked using the Shapiro–Wilk tests, before analysis and data were transformed when required. Statistical analysis was performed using SAS Entreprise Guide 6.1 (SAS 9.4 system for Windows).
3 Results
3.1 Mineral Nutrition
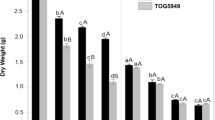
In control conditions (absence of NaCl and inhibitors), the two cultivars contained similar low concentrations of Na+ in roots (Fig. 1A) and shoots (Fig. 1B). The presence of NaCl increased Na+ concentration to a higher extent in TOG5949 than in TOG5307 for both organs. Amiloride had no impact on Na+ concentration in plants that were not exposed to NaCl, but it increased Na+ concentrations in roots and shoots of both cultivars in NaCl-treated plants: from a relative point of view, the recorded increase was higher in TOG5307 (47.3% in roots and 97.4% in leaves) than in TOG5949 (20.2% in roots and 27.7% in leaves). Bumetanide reduced Na+ concentration in the roots and leaves of TOG5949 while it had no significant effect on the Na+ content of TOG5307.
Concentrations of Na+ (A, B), Cl− (C, D) and K+ (E, F) in roots (A, C and E) and in shoots (B, D and F) in two cultivars of the African rice species Oryza glaberrima (TOG5307: salt-resistant and TOG5949: salt-sensitive). Plants were exposed during 72 h to 0 (control) or 75 mM NaCl in the presence or absence of amiloride (150 µM) or bumetanide (200 µM). Each value is the mean of 5 biological replicates and bars represent standard errors. Values with different letters are significantly different at P = 0.05 according to the Scheffé test
Control plants exposed to bumetanide presented deleterious symptoms of slight wilting: while the leaf water content was 90.3 ± 0.5% in the absence of the inhibitor, it dropped to 86.4 ± 0.8% in its presence. In the absence of bumetanide, TOG5949 accumulated higher amounts of Cl− in the leaves than TOG5307 (Fig. 1) while an inverse trend was recorded for the roots (Fig. 1C). Bumetanide strongly decreased Cl− content in the roots and leaves of the two cultivars, but the recorded decrease in the leaves was higher for TOG5949 than for TOG5307. Salinity decreased K+ concentration in the roots and shoots of both cvs, but K+ remained slightly higher in roots of TOG5307 than in TOG5949. Amiloride had no significant impact on K+ concentration. Conversely, bumetanide reduced the K+ concentration in TOG5307 exposed to NaCl comparatively to plants exposed to NaCl in the absence of inhibitor while this was not observed in TOG5949 (Fig. 1E and F ).
SA and ST values are provided in Table 1 for plants exposed to NaCl only, since only extremely low levels of Na+ were present in control plants. In the absence of inhibitors, selective K+ absorption in plants exposed to NaCl was higher in TOG5307 than in TOG5949, suggesting that salt resistance in TOG5307 was related to a better capacity of this cultivar to efficiently discriminate among monovalent cations. Amiloride reduced SA to a higher extent in TOG5307 than in TOG5949. Bumetanide increased SA in TOG5949. In contrast, differences between cultivars were quite less obvious for ST values, and this suggests that cultivars differed more for absorption selectivity of monovalent cations than for translocation selectivity. In NaCl-treated plants exposed to amiloride, ST was however higher in TOG5949 than in TOG5307.
3.2 Senescence-Related Parameters
Cell viability index and total soluble protein concentrations are provided in Fig. 2, for roots and for leaves of the two cultivars exposed to the various treatments. Amiloride in the absence of NaCl had only minor impact on these parameters while bumetanide decreased the recorded values for both of them. Salinity significantly decreased cell viability index (Fig. 2A and B ), but it remained higher in TOG5307 than in TOG5949. In the presence of NaCl, both amiloride and bumetanide increased cell viability in roots of TOG5307 but not in TOG5949. A contrasting behavior was observed at the shoot level, where bumetanide obviously increased cell viability in TOG5949 but not in TOG5307. Amiloride decreased protein content in NaCl-treated plants (Fig. 2C and D ) while bumetamide mitigated the deleterious impact of NaCl on protein content in the shoots of TOG5949.
Total viability index recorded by TTC transformation to formazan (ΔDO at 487 nm.g-1 FW; A, B) and total soluble protein concentration (mg.g−1 FW; C, D) in roots (A, C) and in shoots (B, D) of two cultivars of the African rice species Oryza glaberrima (TOG5307: salt-resistant and TOG5949: salt-sensitive). Plants were exposed during 72 h to 0 (control) or 75 mM NaCl in the presence or absence of amiloride (150 µM) or bumetanide (200 µM). Each value is the mean of 5 biological replicates, and bars represent standard errors. Values with different letters are significantly different at P = 0.05 according to the Scheffé test
In the absence of NaCl, bumetanide significantly decreased Chla and Chlb in the two cultivars (Table 2). In NaCl-treated plants, Chla, Chlb, and carotenoid concentrations were always higher in TOG5307 than in TOG5949. Amiloride decreased Chla and carotenoids in TOG5949 but not in TOG5307 while bumetanide increased Chla in the latter but not in the former. The two cultivars exhibited similar electrolyte leakage in the absence of NaCl (Table 2). Salt stress induced an obvious increase in EL values, and TOG5307 presented a higher EL value than TOG5949, the highest value being recorded in 75 mM NaCl + amiloride-treated plants. An inverse trend was observed for relative leakage ratio of UV-absorbing substance: it also increased in response to NaCl, but to a higher extent in TOG5949 than in TOG5307.
4 Discussion
Salinity resistance in plants is related to the restriction of Na+ and Cl− absorption, efficient compartmentation of toxic ions in vacuoles, restriction of Na+ and Cl− xylem loading, and osmoregulation allowing the plant to cope with the osmotic component of salt stress (van Zelm et al. 2020). The salt-overly-sensitive 1 (SOS1) is a Na+/H+ antiporter primarily expressed at the root tip epidermis, in parenchyma root cells and in xylem parenchyma at the xylem/symplast boundary (Shi et al. 2002; Guo et al. 2009). Other Na+/H+ antiporters NHX are located to the tonoplast and involved in vacuolar sequestration (Fukuda et al. 1998).
SOS1 inhibition by amiloride may at least partly explain the recorded increase in root Na+ content, considering that its presence in root meristem zone may contribute to Na+ extrusion to the surrounding medium (Shi et al. 2002). Amiloride-induced Na+ increase in the roots was higher in salt-tolerant TOG5307 than in salt-sensitive TOG5949, and this suggests that in NaCl-treated plants, SOS1 is more active in the former than in the latter. K+ and Na+ are similar in ionic radius and hydration energy (Zhang et al. 2010) and discrimination between these ions is an important component of salt tolerance in plants. The fact that amiloride reduced SA to a higher extent in TOG5307 than in TOG5949 reinforces the hypothesis that SOS1 is an efficient contributor to ion discrimination through its specific involvement in Na+ efflux, mainly in the salt-resistant cultivar.
At the xylem/symplast boundary, SOS1 mediates Na+ loading and thus contribute to Na+ delivery to the shoot (Shi et al. 2002; Zhu et al. 2017): blocking xylem loading by amiloride should contribute to reduce Na+ translocation to the shoots, but this was not supported by our data since amiloride increased rather than decreased Na+ accumulation in the leaves. Other transporters such as the high-affinity Na+/K+ permeable transporters from the HKT family are involved in Na+ retrieval from the xylem (Horie et al. 2009), and it could therefore not be excluded that salinity had a detrimental impact on these transporters which are not sensitive to amiloride.
Amiloride and its analogues are permeable weak base and may also interfere with vacuolar Na+/H+ antiport in root cells. Sodium compartmentation inside vacuoles is driven by the electrochemical gradient of protons generated by the V-type H+-ATPase and the H+-PPiase (Zhang et al., 2010) and is a wise strategy to avoid Na+-induced cytosolic damages. Reducing both SOS1 activity and vacuolar Na+ compartmentation may increase Na+ in the apoplast and its transport to the xylem by transpirational stream. This could partly explain the recorded increase in leaf Na+ occurring despite partial amiloride inhibition of SOS1. In amiloride-treated plants exposed to NaCl, sodium may also remain in the cytosol of cells and induce a wide range of metabolic disorders.
Until recently, chloride received less attention than Na+ in the plant response to NaCl. CCC belongs to cation-Cl− transporters and is essential for ion homeostasis. Bumetamide, which is a powerful inhibitor of CCC, had a detrimental impact on control plants, reducing water content, cell viability index, and protein content. This is in accordance with previous studies which reported that CCC is required in the absence of NaCl stress for efficient cell elongation and osmoregulation processes in O. sativa (Kong et al. 2011; Chen et al. 2016).
Although the studied cultivars did not differ in their response to bumetamide in the absence of NaCl, they displayed contrasting behavior when the inhibitor was added to NaCl-containing solution. CCC is present in root hairs and root epidermis, and it also retrieves Cl− from the root xylem (Colmenero-Flores et al. 2007). Bumetamide strongly decreased Cl− content in roots and leaves, but the recorded decrease was higher for TOG5949 than for TOG5307, which suggests that in salt stress conditions, CCC is a major contributor of Cl− uptake and transport in the salt-sensitive genotypes of O. glaberrima. The gene OsCCC1 from O. sativa was cloned, and its silencing led to lower K+ accumulation (Kong et al., 2011). CCC belongs to the NKCC cotransporter family and ensures Na+:K+:2Cl− transport (Han et al. 2020). In our study, however, we found that bumetamide reduced Na+ in TOG4949 but not in TOG5307, while it reduced K+ in TOG5307 but not in TOG5949. This is the first time, to the best of our knowledge, that cations involved in the symport mechanisms of CCC appeared different in salt-sensitive and salt-tolerant cultivars within a given species. According to Chen et al. (2016), affinity of OsCCC1 for Na+ is weak in O. sativa, but we suggest that it may differ depending on the cultivar in the closely related species O. glaberrima exposed to salinity.
Cell viability index, protein, and pigment concentrations remained less affected in the salt-tolerant TOG5307 than in the salt-sensitive TOG5949. Although it might be suggested that this is related to a lower level of Na+ accumulation (Fig. 1), this is not valid anymore for the NaCl + amiloride treatment: in this case, accumulation of Na+ was indeed higher in TOG5307 comparatively to TOG5949, but cell viability and protein concentration also remained higher in the former than in the latter. This suggests that TOG5307 displayed a better strategy of tolerance allowing it to cope more efficiently with accumulated Na+, and this could be partly due to a better maintenance of K+ nutrition and/or to higher accumulation of Na+ in the apoplasm comparatively to TOG5949. This hypothesis is supported by the data obtained for electrolyte leakage (Table 2): indeed, EL was the only senescence-related parameter which was higher in TOG5307 than in TOG5949, which is at first sight in contradiction with all other data. The possible explanation is that some of the ions leaking during the process and contributing to measured electrical conductivity in TOG5949 were in fact issued from apoplast and thus provided false information regarding cell membrane stability as previously demonstrated in Oryza sativa (Lutts et al., 1996). It is not demonstrated that significant amounts of inhibitors added to the nutrient solution may reach the aerial part of the plant (Taleisnik et al. 1991), but it cannot be excluded that the two considered cultivars differed for ion distribution independently of inhibitors. The data obtained with leakage of UV-absorbing substances necessarily issued from the symplast corroborated this view since RLR recorded in salt-treated plants was always lower in TOG5307 than in TOG5949.
Additional experiments are still required to precisely identify the impact of the studied transporters in plant response to salinity, especially considering that inhibitor like amiloride could also marginally impact other transport proteins such as CHX (Qu et al. 2021) and that some targets of the used inhibitors may also be localized in the endomembrane system (Henderson et al. 2015).
5 Conclusions
Inhibition of Na+-H+ antiporters in roots induced an increase in Na+ concentration in all organs, and a higher impact was recorded in the salt-resistant genotype suggesting that antiporters such as SOS1 play a major role in salinity resistance of African rice Oryza glaberrima. The CCC transporter is directly involved in Cl− accumulation, but the counterion involved in the symport was preferentially K+ in the salt-resistant cultivar and Na+ in the salt-sensitive one. The salt-resistant cultivar was able to regulate Na+ uptake and to cope with internal Na+ accumulation which might be related to a higher accumulation of toxic ions in the apoplast.
References
Bajji M, Kinet JM, Lutts S (2002) The use of electrolyte leakage method assessing cell membrane stability as a water stress tolerance test in durum wheat. Plant Growth Regul 36:61–70
Bradford MM (1976) A rapid sensitive method for quantification of microquantities of protein utilizing he principle of protein dye binding. Annal Biochem 16:559–566
Chen ZC, Yamaji N, Fujii-Kashino M, Ma JF (2016) A cation-chloride-cotransporter gene is required for cell elongation and osmoregulation in rice. Plant Physiol 171:494–507
Colmenero-Flores JM, Martínez G, Gamba G, Vázquez N, Igleisias DJ, Brumós J, Talón M (2007) Identification and functional characterization of cation-chloride-cotransporters in plants. Plant J 50:278–292
Deng YQ, Bao J, Yuan F, Liang X, Feng ZT, Wang BS (2016) Exogenous hydrogen sulfide alleviates salt stress in wheat seedlings by decreasing Na+ content. Plant Growth Regul 79:391–399
Fukuda A, Yazaki Y, Ishikawa T, Koike S, Tanaka Y (1998) Na+/H+ antiporter in tonoplast vesicles from rice roots. Plant Cell Physiol 39:196–201
Guo KM, Babourina O, Rengel Z (2009) Na+/H+ antiporter activity of the SOS1 gene: lifetime imaging analysis and electrophysiological studies on Arabidopsis seedlings. Physiol Plant 137:155–165
Han B, Jiang Y, Cui G, Mi J, Rob M et al (2020) Cation-chloride co-transporter 1 (CCC1) mediates plant resistance against Pseudomonas syringae. Plant Physiol 182:1052–1065
Henderson SW, Wege S, Qiu J, Blackmore DH, Walker AR, Tyrman SD, Walker RR, Gilligham M (2015) Grapevine and Arabidopsis cation-chloride-cotransporters localize to the Golgi and trans-Golgi network and indirectly influence long-distance ion transport and plant salt tolerance. Plant Physiol 169:2215–2229
Henderson SW, Wege S, Gilliham M (2018) Plant-cation-chloride-cotransporters (CCC): evolutionary origins and functional insights. Int J Mol Sci 19:492
Horie T, Hauser F, Schroeder JI (2009) HKT transporter-mediated salinity resistance mechanisms in Arabidopsis and monocot crop plants. Trends Plant Sci 14:660–668
Kong XQ, Gao XH, Sun W, An J, Zhao YX, Zhang H (2011) Cloning and functional characterization of a cation-chloride-cotransporter gene OsCCC1. Plant Mol Biol 75:567–578
Li P, Luo T, Pu X, Zhou Y, Yu J, Liu L (2021) Plant transporters: roles in stress responses and effects on growth and development. Plant Growth Regul 93:253–266
Lichtenthaler HK (1987) Chlorophylls and carotenoids: pigments of photosynthetic biomembranes. Meth Enzymol 148:350–382
Lutts S, Kinet JM, Bouharmont J (1996) NaCl-induced senescence in leaves of rice (Oryza sativa L.) cultivars differing in salinity resistance. Ann Bot 78:389–398
Lutts S, Almansouri M, Kinet JM (2004) Salinity and water stress have contrasting effects on the relationship between growth and cell viability during and after stress exposure in durum wheat callus. Plant Sci 67:9–18
Prodjinoto H, Gandonou C, Lutts S (2018) Screening for salinity tolerance of Oryza glaberrima Steud. seedlings. Afr J Agric Res 133:561–583
Prodjinoto H, Irakoze W, Gandonou C, Lepoint G, Lutts S (2021) Discriminating the impact of Na+ and Cl− in the deleterious effects of salt stress on the African rice species (Oryza glaberrima Steud.). Plant Growth Regul 94:201–219
Qu Y, Guan R, Bose J, Henderson SW, Wege S, Qiu L, Gilliham M (2021) Soybean CHX-type ion transport protein GmSALT3 confers leaf Na+ exclusion via a root derived mechanism, and Cl− exclusion via a shoot derived process. Plant Cell Environ 44:856–869
Shi HZ, Quintero FJ, Pardo JM, Zhu JK (2002) The putative plasma membrane Na+/H+ antiporter SOS1 controls long-distance Na+ transport in plants. Plant Cell 14:465–477
Solis CA, Yong MT, Vinarao R, Jena K, Holford P, Shabala L, Zhou M, Shabala S, Chen ZH (2020) Back to the wild: on quest for donors towards salinity tolerant rice. Front Plant Sci 11:323
Taleisnik E, Grunberg K, Lino A (1991) Effects of amiloride on sodium accumulation in intact Lycopersicum esculentum plants. J Plant Physiol 138:634–639
Teakle NL, Tyerman SD (2010) Mechanisms of Cl− transport contributing to salt tolerance. Plant Cell Environ 33:566–589
Van Zelm E, Zhang Y, Testerink C (2020) Salt tolerance mechanisms of plants. Ann Rev Plant Biol 71:403–433
Veltlman MA, Flowers JM, van Andel TR, Schranz ME (2019) Origins and geographic diversification of African rice (Oryza glaberrima). PLoS One 14:e0203508
Wang B, Zhai H, He S, Zhang H, Ren Z, Zhang D, Liu Q (2016) A vacuolar Na+/H+ antiporter gene, INHX2, enhances salt and drought tolerance in transgenic sweet potato. Scien Hort 201:153–166
Yoshida S, Forno DA, Cock JH, Gomez KA (1976) Laboratory manual for physiological studies of rice, 3rd edn. International Rice Research Institute, Manila, Philippines
Zhang JL, Flowers TJ, Wang SM (2010) Mechanisms of sodium uptake by root of higher plants. Plant Soil 326:45–60
Zhu M, Zhou M, Shabala L, Shabala S (2017) Physiological and molecular mechanisms mediating xylem Na+ loading in barley in the context of salinity stress tolerance. Plant Cell Environ 40:1009–1020
Acknowledgements
The authors are grateful to Mrs. Brigitte Van Pee and to Mr. Baudouin Capelle for the efficient technical assistance
Funding
CAI (Comité d’Action Internationale) from the Université catholique de Louvain (UCLouvain) provided the research grant of H. Prodjinoto.
Author information
Authors and Affiliations
Contributions
HP performed the whole experiment, SL and CG managed the project, SL conceived the experimental design, WI conducted the statistical treatment of the data, SL and HP wrote the first draft, and all the authors prepared the final version and approved submission.
Corresponding author
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Prodjinoto, H., Irakoze, W., Gandonou, C. et al. Inhibitors of Na/H Antiporter and Cation-Chloride-Cotransporters Have Contrasting Effects on Two Cultivars of Oryza glaberrima Steud. Differing in Salinity Resistance. J Soil Sci Plant Nutr 21, 3247–3253 (2021). https://doi.org/10.1007/s42729-021-00603-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42729-021-00603-z