Abstract
Main conclusion
Chemical analyses and glycome profiling demonstrate differences in the structures of the xyloglucan, galactomannan, glucuronoxylan, and rhamnogalacturonan I isolated from soybean ( Glycine max ) roots and root hair cell walls.
The root hair is a plant cell that extends only at its tip. All other root cells have the ability to grow in different directions (diffuse growth). Although both growth modes require controlled expansion of the cell wall, the types and structures of polysaccharides in the walls of diffuse and tip-growing cells from the same plant have not been determined. Soybean (Glycine max) is one of the few plants whose root hairs can be isolated in amounts sufficient for cell wall chemical characterization. Here, we describe the structural features of rhamnogalacturonan I, rhamnogalacturonan II, xyloglucan, glucomannan, and 4-O-methyl glucuronoxylan present in the cell walls of soybean root hairs and roots stripped of root hairs. Irrespective of cell type, rhamnogalacturonan II exists as a dimer that is cross-linked by a borate ester. Root hair rhamnogalacturonan I contains more neutral oligosaccharide side chains than its root counterpart. At least 90 % of the glucuronic acid is 4-O-methylated in root glucuronoxylan. Only 50 % of this glycose is 4-O-methylated in the root hair counterpart. Mono O-acetylated fucose-containing subunits account for at least 60 % of the neutral xyloglucan from root and root hair walls. By contrast, a galacturonic acid-containing xyloglucan was detected only in root hair cell walls. Soybean homologs of the Arabidopsis xyloglucan-specific galacturonosyltransferase are highly expressed only in root hairs. A mannose-rich polysaccharide was also detected only in root hair cell walls. Our data demonstrate that the walls of tip-growing root hairs cells have structural features that distinguish them from the walls of other roots cells.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The plant root is the principal organ responsible for the absorption of water and dissolved nutrients. The root anchors and supports the plant body and may also store the products of photosynthesis and other nutrients (Raven et al. 2005). Roots contain anatomically and developmentally diverse cells from epidermal, endodermal, pericycle, cortex and vascular tissues (Raven et al. 2005). These cells are capable of diffuse growth and thus have the ability to expand in different directions (Cosgrove 2005). By contrast, root hairs, which develop from a single epidermal cell (trichoblast) extend by tip growth (Bibikova and Gilroy 2003; Nielsen 2009). These elongated cells greatly increase the surface area of the root and thereby increase the efficiency of nutrient and water uptake (Jungk 2001). Root hairs may also have specialized functions in some plants. For example, legume root hairs are the initial site of attachment for symbiotic bacteria that then invade the root cortex and form nitrogen-fixing nodules (Schubert 1986; Oldroyd and Downie 2008).
There are several reports that describe the types of polysaccharides present in root cell walls (Nevins et al. 1967; Matoh et al. 1993; Freshour et al. 1996). One study reported the glycosyl residue compositions of isolated root hair cell walls, although no wall polysaccharides were isolated and structurally characterized (Mort and Grover 1988). Nevertheless, these authors concluded that dicot root hair walls are pectin-rich and thus similar to the primary walls of growing cells in other dicot tissues.
There is increasing evidence that root hair development is affected in plants carrying mutations that affect the synthesis of cell wall polysaccharides. For example, disrupting the cellulose synthase-like gene, CSLD3, alters the normal organization of cellulose and xyloglucan in the root hair cell wall and leads to abnormal tip growth and hair cell rupture (Galway et al. 2011). CSLD3 has been proposed to encode a 1, 4-linked β-glucan synthase that is localized to the apical plasma-membrane domain in root hairs (Park et al. 2011). There is also evidence that other members of the CSLD family of genes are required for wall synthesis in tip growing cells (Bernal et al. 2008) and that some of these genes may be involved in the synthesis of mannan-like polysaccharides (Yin et al. 2011). Plants carrying loss-of-function mutations in two genes encoding different xyloglucan-specific c(XXT1 and XXT2) have short and thick root hairs that often bulge at their tips (Zabotina et al. 2012), indicating that xyloglucan is required for normal expansion in tip-growing root hairs. A β-GalA-containing xyloglucan was reported to be exclusively present in Arabidopsis thaliana root hairs (Peña et al. 2012). Mutants that lack a functional copy of the gene (XUT1), which encodes the xyloglucan-specific GalA transferase, have shorter root hairs than wild-type plants (Peña et al. 2012). Interestingly, plants carrying loss of function mutations in XXT1, XXT2, or XUT1 have roots and aerial portions that are indistinguishable from wild-type. Together these results suggest that xyloglucan has a critical function in tip growing cells that is not essential in cells that expand by diffuse growth.
Here, we report the partial structural characterization of rhamnogalacturonan I (RG-I), rhamnogalacturonan II (RG-II), xyloglucan, and 4-O-methyl glucuronoxylan that are present in the cell walls of soybean root hairs and the walls of roots stripped of root hairs. We provide evidence that an acidic xyloglucan and a mannose-rich polysaccharide are present exclusively in root hair cell walls. These data, together with glycome profiling using monoclonal antibodies (mAbs) that recognize epitopes of diverse cell wall polysaccharides (Pattathil et al. 2010), show that the RG-I, xyloglucan, and 4-O-methyl glucuronoxylan present in root hair walls have structural features that distinguish them from their root wall counterparts.
Materials and methods
Isolation of soybean root hairs and roots stripped of root hairs
Soybean root hairs were isolated as previously described (Brechenmacher et al. 2009). Briefly, soybean seeds (Glycine max ‘Williams 82'; Missouri foundation seed stocks, Columbia, MO, USA) were surface sterilized twice for 10 min each with aq 20 % (v/v) clorox, rinsed five times with sterile water, soaked for 10 min in 0.1 N HCl, rinsed five more times in sterile water, and then air-dried for 20 min. Sterilized seeds were germinated and grown in the absence of light for 3 days at 25 °C and 80 % humidity on nitrogen-free B and D agar medium (Broughton and Dilworth 1971). Approximately 1500 soybean roots were harvested, frozen in liquid nitrogen and gently stirred for 20 min to break the root hairs off of the roots. Root hairs were separated from roots by filtering through a wire mesh and kept at −80 °C. This procedure generates root hairs that are largely free of any other root cells and stripped roots that are devoid of root hairs (Wan et al. 2005).
Growth of soybeans in the presence and absence of Bradyrhizobium japonicum
Bradyrhizobium japonicum USDA110 was grown for 4 days at 30 °C in RDY medium (So et al. 1987) containing chloramphenicol (40 μg/mL). The culture was centrifuged at 2000g for 10 min and the resulting pellet washed with sterile water and then suspended in sterile water to an OD600nm of ~0.9. Four-day-old etiolated soybean seedlings were sprayed with the suspension of B. japonicum cells or with water until the seedlings were evenly wet. The treated seedlings were then grown for an additional 48 h in the absence of light. Root hairs and stripped roots were prepared from these plants.
Isolation and fractionation of soybean cell walls
Cell walls were prepared as the alcohol-insoluble residues (AIR) as described (Hoffman et al. 2005). Briefly, batches of stripped roots from plants grown in the presence (494, 367, 692, and 655 mg) and absence (536, 401, 574, and 683 mg) of B. japonicum, and batches of root hairs from plants grown in the presence (160, 177, 144, and 205 mg) and absence (153, 192, 179, and 169 mg) of B. japonicum, were suspended in liquid nitrogen and ground to a fine powder with a mortar and pestle. The powder was then homogenized in aq. 80 % (v/v) EtOH. The suspensions were centrifuged (2500g for 10 min) and the pellet washed with absolute EtOH. The pellet was then suspended in MeOH-CHCl3 (1:1, v/v). After 30 min the suspension was filtered using Whatman No 1 paper, and the retentate washed with acetone and vacuum dried.
Suspensions of the AIR in 50 mM potassium phosphate, pH 6.8, were treated for 24 h at 22 °C with α-amylase (11 units per mg AIR; Sigma-Aldrich, St. Louis, MO, USA). The suspension was filtered using a nylon filter (0.2 µm pore size) and the filtrate then dialyzed (3500 molecular weight cut-off) against deionized water and freeze dried. The α-amylase-insoluble material was suspended in 50 mM ammonium formate, pH 5, and treated twice with an homogeneous endopolygalacturonase II (EPG, 10 units) that had been purified from the growth media of Aspergillus niger (Bussink et al. 1990) by ion-exchange chromatography as described (Clay et al. 1997) and an homogeneous A. niger pectin methyl esterase (PME, 10 units) purified as described (Warren et al. 2002). The EPG/PME solubilized material was dialyzed and freeze dried. The EPG/PME-insoluble material was then treated for 16 h at 22 C with a xyloglucan-specific endoglucanase (XEG; 2 units, Novozymes, Bagsvaerd, Denmark) as described (Pauly et al. 1999). The suspension was filtered using a nylon filter (0.2 µm pore size). The filtrate was repeatedly freeze dried to remove the ammonium formate. The retentate was treated sequentially with 2 M imidazole–HCl, pH 7, 1 M KOH containing 0.5 % (w/v) NaBH4 and then with 4 M KOH containing 0.5 % (w/v) NaBH4 (16 h at 22 C for each treatment). The soluble fractions were neutralized if required, dialyzed (3500 MWCO) against deionized water and freeze dried. The final KOH-insoluble material was also dialyzed and freeze dried.
Glycosyl residue composition analyses
Total carbohydrate was determined colorimetrically using the phenol–sulfuric acid method (York et al. 1986). Galacturonic acid was quantified colorimetrically using authentic galacturonic acid as standard (Blumenkrantz and Asboe-Hansen 1973). Glycosyl residue compositions were determined by analyses of the trimethylsilyl methyl glycoside and the alditol acetate derivatives using gas chromatography combined with electron-impact mass spectrometry (GC–EI–MS) with myo-inositol as standard (York et al. 1986).
Glycome profiling using monoclonal antibodies
Glycome profiling of the cell wall extracts was performed using mAbs as described (Pattathil et al. 2010, 2012; DeMartini et al. 2011). All mAbs (CCRC, JIM and MAC series, see Supplementary Table S1) were obtained from laboratory stocks at the Complex Carbohydrate Research Center (http://www.carbosource.net ).
Data analyses
Standard statistical analysis (two-Sample z-TEST) and data mining algorithms (Haykin 2008; Aguiar et al. 2012; Barbosa et al. 2014) were used to compare glycosyl-residue compositions and glycome profiling data from different tissues and different treatments.
Immunocytochemistry
Soybean (G. max, cultivar Williams 82) seeds were surface sterilized 3 times for 10 min in aq 30 % (v/v) clorox. The seeds were then washed 3 times with sterile water, treated for 10 min with 10 mM HCl and then rinsed five times with sterile water. Sterile seeds were drained, allowed to dry for 30 min and then transferred to Petri dishes containing solid legume plant growth medium (Broughton and Dilworth 1971). Seeds were grown for 3 days at 25 °C in the absence of light (80 % relative humidity). 72 h-old seedlings were sprayed with a suspension of B. japonicum USDA 110 cells (A600nm 0.8) or with sterile water. The seedlings were then grown for additional 48 h in the dark prior to harvesting for microscopy studies.
Five-day-old soybean roots were fixed and processed for immunolabeling as described (Avci et al. 2012). Semi-thin sections (250 nm) were cut using a Leica EM UC6 microtome (Leica Microsystems, Wetzlar, Germany) and mounted on glass slides (colorfrost/plus, Fisher Scientific, Pittsburgh, PA, USA). Sections were blocked for 45 min with 3 % (w/v) non-fat dry milk in 10 mM potassium phosphate, pH 7.1, containing 0.5 M NaCl (KPBS), and then were washed for 5 min with KPBS. Undiluted hybridoma supernatant of the monoclonal antibodies was applied to the sections and allowed to react for 60–90 min. Sections were then washed three times for 5 min with KPBS. Goat anti-mouse conjugated to Alexa-fluor 488 (Invitrogen, Carlsbad, CA, USA) diluted 1:100 in KPBS was applied and reacted for 60-90 min. Sections were then washed for 5 min with KPBS, and then for 5 min with distilled water. Prior to applying a cover slip, CITIFLUOR antifadant mounting medium AF1 (Electron Microscopy Sciences, Hatfield, PA, USA) was applied. Imaging was carried out on an Eclipse 80i microscope equipped with epifluorescence optics, a Nikon DS-Ri1 camera and NIS-Elements Basic Research software (Nikon, Melville, NY, USA). Images were assembled using Adobe Photoshop (Adobe Systems, San Jose, CA, USA).
Cell wall polysaccharide analyses
Rhamnogalacturonans I and II
Solutions of the EPG/PME-released materials (~5 mg) in 50 mM ammonium formate (500 μL), pH 5, were fractionated on a Superdex HR-75 column (1 × 30 cm) eluted with 50 mM ammonium formate at 0.5 mL/min using a Dionex Ultimate 3000 liquid chromatograph and a Showdex RI-101 refractive index detector (Thermo Scientific, Sunnyvale, CA, USA). Fractions corresponding to RG-I and RG-II were collected manually and repeatedly freeze dried to remove ammonium formate.
Xyloglucan
Solutions of the 4 M KOH-soluble materials (1–2 mg) in 50 mM ammonium formate (500 uL), pH 5, were treated for 16 h at 22 °C with XEG (1 unit) as described (Pauly et al. 1999). Ethanol was then added to 70 % (v/v) and the mixture kept for 24 h at 4 °C. The precipitate that formed was removed by centrifugation and the soluble fraction concentrated to dryness under a flow of air. The residue was dissolved in water and repeatedly freeze dried to remove ammonium formate. The residue as well as the material released by XEG treatment of the cell walls was separately dissolved in water (~1 mg/mL) and a portion (2 μL) mixed with an equal volume of 10 mM NaCl. A portion (1 μL) of this mixture was then added to the dihydroxybenzoic acid matrix (1 μL, 10 mg/mL in aq 50 % (v/v) acetonitrile) on the stainless steel target plate. The solutions were evaporated to dryness with a flow of warm air. Positive ion matrix-assisted laser-desorption time-of-flight mass spectrometry (MALDI-TOF–MS) was performed with a Bruker Microflex LT mass spectrometer (Bruker Daltonik, Bremen, Germany). At least 200 spectra were collected and averaged.
Xylan
Solutions of the 1 M KOH-soluble materials (1–2 mg) in 50 mM ammonium formate (500 μL), pH 5, were treated for 16 h at 22 °C with endoxylanase (M1, 1 unit, Megazyme, Co. Wicklow, Ireland). Ethanol was then added to 70 % (v/v) and the mixture kept for 24 h at 4 °C. The precipitate that formed was removed by centrifugation and the soluble fraction concentrated to dryness under a flow of air. The residue was dissolved in water and repeatedly freeze dried to remove residual ammonium formate. The residue was then dissolved in water (~1 mg/mL) and a portion analyzed by MALDI-TOF–MS.
Glucomannan
Solutions of the 1 M KOH-soluble materials (1–2 mg) in 50 mM ammonium formate (500 μL), pH 4, were treated for 16 h at 37 °C with endo-mannanase (E-BMANN, 1 unit, Megazyme). Ethanol was then added to 70 % and the mixture kept for 24 h at 4 C. The precipitate that formed was removed by centrifugation and the soluble fraction concentrated to dryness under a flow of air. Solutions of the materials (~1 mg) in 50 mM ammonium formate (500 uL), pH 5, were fractionated on a Superdex HR-75 column (1 × 30 cm) eluted with 50 mM ammonium formate at 0.5 mL/min using a Dionex Ultimate 3000 liquid chromatograph and a Showdex RI-101 refractive index detector. Fractions eluting in the region for oligosaccharides (32–34 and 35–37 min) were collected manually and repeatedly freeze dried to remove ammonium formate. The residue was then dissolved in water (~1 mg/mL) and a portion analyzed by MALDI-TOF–MS.
Comparison of cell wall-related gene expression
Soybean homologs of Arabidopsis cell wall-related genes were identified by sequence comparison to the Department of Energy Phytozome/soybean database (http://www.phytozome.net/soybean.php). The tissue-specific expression patterns of these genes were then extracted from the soybean gene expression transcriptomics atlas (Libault et al. 2010). These publicly available transcriptomics data (soyKB database, http://www.soykb.org) were produced by Solexa sequencing of three replicates of each tissue (Joshi et al. 2012).
Results
The polysaccharides solubilized by enzymatic and alkali treatment of root hair and stripped root cell walls do not have identical glycosyl-residue compositions
Root hairs and the corresponding roots that had been stripped of their root hairs were generated from plants grown in the absence and presence of B. japonicum, a nitrogen-fixing symbiont of soybean. Cell walls were then prepared as their AIRs. These walls were then treated with a combination of enzymes and aqueous alkali to generate a series of fractions enriched in pectic and hemicellulosic polysaccharides. The amounts of each fraction obtained by these treatments are shown in Supplementary Table S2.
The polysaccharides solubilized from stripped root and root hair cell walls from plants grown in the absence of B. japonicum had broadly similar but not identical glycosyl residue compositions (Table 1). There were also discernible differences in the glycome profiles of these wall extracts (Fig. 1). Growing soybean plants in the presence of B. japonicum had little if any discernible effect on the glycosyl-residue compositions of the root and root hair wall polysaccharides (Supplementary Table S3) or the glycome profiles of the wall extracts (Supplementary Fig. S1). This may reflect the fact that only a small fraction of root hairs respond to inoculation with the bacteria (Calvert et al. 1984).
Glycome profiles of the material solubilized from the cell walls of roots stripped of their root hairs and root hairs from soybean plants grown in the absence of B. japonicum. a The yields of the material solubilized by enzymic and base treatments. b The glycome profiles of root and root hair wall extracts. c The difference map generated by subtracting the value for root binding from the value for root hair binding
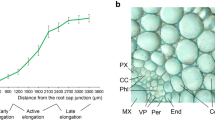
The walls of all root cells, including root hairs, were labeled with mAbs that recognize the arabinogalactan side chains of RG-I or arabinogalactan protein (CCRC-M7), 1, 5-linked α-arabinan (LM6) and methyl-esterified pectin (CCRC-M38) (Fig. 2). All walls, except those of root hairs and xylem, were labeled with LM5, a mAb that recognizes 1, 4-linked β-galactan (Fig. 2). CCRC-M1, which recognizes fucosylated epitopes of xyloglucan, mainly labeled the walls of root hairs and phloem. CCRC-M84, which is believed to recognize mono and di-fucosylated epitopes of xyloglucan (Tuomivaara, Hahn, and York, unpublished data) mainly labeled the walls of root hairs and suggest that the fucosylation status of xyloglucan in root and root hair cell walls is not identical. Somewhat less intense labeling was observed with LM15, a mAb that recognizes xylosylated epitopes of xyloglucan. The xylem walls of the soybean roots were strongly labeled with LM10 and LM11, two mAbs that recognize xylan (Fig. 2), a result that is consistent with the glycome profiling data (Fig. 1). Such results are not unexpected as xylem contains cells with secondary cell walls that are rich in glucuronoxylan (Cosgrove and Jarvis 2012). The walls of the remaining cells, including root hairs, showed little if any labeling with these xylan-specific mAbs. Together the results of glycosyl residue composition analyses, glycome profiling and immunolabeling reveal small but discernible, differences in the amounts and types of polysaccharides in the cell walls of root hairs compared to the cell walls of roots stripped of their root hairs.
The major structural features of selected pectic and hemicellulosic polysaccharides present in the cell walls of root hair and roots stripped of hairs
We next compared the major structural features of two pectic polysaccharides (RG-I and RG-II) and three hemicellulosic polysaccharides (glucuronoxylan, glucomannan and xyloglucan) to more fully understand the differences in the c (Table 1) and glycome profiles (Fig. 1) of the enzymatic and alkali fractions from stripped root and root hair walls. Structural data is provided for selected pectic and hemicellulosic polysaccharides released from the cell walls of root hairs and roots stripped of their hairs from soybean plants grown in the absence of B. japonicum. No discernible differences were observed in the structures of these polysaccharides when soybean plants were inoculated with B. japonicum (Supplementary Figs. S1, S2, S3, S4, S5 and Supplementary Tables S2, S3, S4, S5).
RG-II structure is conserved in the cell walls of root hairs and roots stripped of hairs
Three major peaks corresponding to RG-I, RG-II, and oligogalacturonides were obtained when the EPG-soluble materials from root hairs and stripped roots were fractionated on a Superdex SD-75 size-exclusion column (Fig. 3). Virtually all of the RG-II exists as the borate ester cross-linked dimer irrespective of tissue source. Moreover, the glycosyl residue compositions of the RG-II from roots and hairs were comparable (Table 2). By contrast, the glycosyl residue compositions of the RG-I-enriched fraction from root hair and roots were not identical (Table 2). The ratios of Rha to Ara + Gal suggest that either the number or length of the Gal-rich side chains is greater in root hair RG-I than in root RG-I.
Rhamnogalacturonans enriched in Ara and Gal have been solubilized from soybean cotyledons and soybean meal with water and aqueous buffers containing calcium-chelators (Yamaguchi et al. 1996; Huisman et al. 2001; Nakamura et al. 2002). These pectic polysaccharides and the phosphate buffer-soluble pectin from root and root hair walls (Table 1) have comparable ratios of Rha to Gal + Ara, consistent with the occurrence of long Ara- and Gal-containing side chains attached to the rhamnogalacturonan backbone (Fransen et al. 2000; Nakamura et al. 2002). The Rha to Gal + Ara ratio is much lower in the RG-I released by EPG/PME treatment of root and root hair hair walls (Table 2). Highly purified EPG and PME were used to release RG-I from the soybean root and root hair cell walls. Nevertheless, we cannot discount the possibility that these enzymes contain residual glycanase activities that generate root RG-Is with shorter side chains than their buffer-soluble counterparts. Further detailed studies are required to determine if the fine structures of the rhamnogalacturonans present in the walls of soybean cotyledons, roots and root hairs differ substantially.
Soybean root hair cell walls contain small amounts of glucuronoxylan
The 1 M KOH-soluble materials from stripped roots and root hair cell walls both contain substantial amounts of xylose (Table 1). These fractions were treated with an endoxylanase to determine if any of the xylose originates from 1, 4-linked β-d-xylan. MALDI-TOF MS analyses of the products generated from roots (Fig. 4a) gave a series of oligosaccharides comprising 4 to 7 Xyl residues and one glucuronic acid (GlcA). At least 90 % of the GlcA residues are methyl-etherified at O-4 (MeGlcA). Our immunolabeling data (Fig. 2) suggest that most of this xylan is present in the walls of cells in the xylem. Small amounts of xylo-oligosaccharides containing approximately equal proportions of MeGlcA and GlcA were generated from the root hair xylan (Fig. 4b). Little if any labeling of root hairs was obtained with the mAbs that recognize xylan epitopes (Fig. 2). Nevertheless, our chemical data provide strong evidence that both root hair and root cell walls contain glucuronoxylan and suggest that the glucuronoxylan present in root hair walls has a lower degree of GlcA methylation than its counterpart in root cell walls.
A mannose-containing polysaccharide is present in soybean root hairs cell walls
The increased amount of Man was a notable feature of the 1 M KOH-soluble material from root hair walls (Table 1). Since mannan-like polysaccharides have been reported to be present in Arabidopsis root hairs (Yin et al. 2011), the 1 M KOH-soluble materials from stripped root and root hair cell walls were treated with an endo-1, 4-β-d-mannanase to determine if the Man originates from 1, 4-linked β-d-mannan. A series of oligosaccharides comprised of three to ten hexosyl residues were detected by MALDI-TOF–MS analysis of the endo-mannanase products from root hairs (Fig. 5). Oligosaccharides with a DP between 2 and 4 were enriched with Man, whereas Glc, galactose (Gal) and Man accounted for the bulk of the glycoses in the oligosaccharides with a DP between six and ten (Table 3). The origins of the arabinose (Ara) and Xyl are not known. These pentoses are unlikely to be associated with the mannan as no oligosaccharides composed of hexosyl and pentosyl residues were detected. No mannose-containing oligosaccharides were detected by MALDI-TOF MS when the 1 M KOH-soluble fraction from roots was treated with the endo-mannanase. Our data provide additional evidence that galactoglucomannan is a unique component of root hair cell walls.
MALDI-TOF mass spectra of the oligosaccharides isolated by SEC of the material generated by endo-mannanase treatment of the 1 M KOH-soluble material from root hair cell walls of soybean plants grown in the absence of B. japonicum. No discernible amounts of oligosaccharide were generated by endo-mannanase treatment of the 1 M KOH-soluble material from walls of roots stripped of their root hairs
Soybean root hair cell walls contain an acidic xyloglucan
XXXG-type xyloglucans have been identified in the cell walls of suspension-cultured soybean cells and in soybean meal (Hayashi et al. 1980; Huisman et al. 2000). These xyloglucans have subunits composed of three successive β-d-Glcp backbone residues bearing an α-d-Xylp residue (designated by the uppercase letter X) followed by a single unbranched backbone residue (designated by the uppercase letter G) (Tuomivaara et al. 2014). Many of the α-d-Xylp residues are themselves substituted at O-2 with a β-d-Galp residue to form the L side chains, as in XLXG, XXLG, and XLLG subunits. The Gal residue of the L side chain adjacent to the unbranched G is often substituted at O-2 with an α-l-Fucp residue to form an F side chain as in XXFG and XLFG subunits.
XEG treatment of the root hair and stripped root cell walls generated several xyloglucan oligosaccharides (XyGOs). The mono-O-acetylated XXFG subunit was the most abundant fragment observed irrespective of tissue (Fig. 6a, b). XXFG was also the most abundant subunit generated by treating the 4 M KOH-soluble materials with XEG (Fig. 6c, d). Small amounts of the GalA-containing subunit (YXXG) were detected in root hair xyloglucan but not in root xyloglucan. Such data are consistent with previously published data showing that GalA-containing subunits are present in Arabidopsis root hair xyloglucan (Peña et al. 2012).
MALDI-TOF mass spectra of the oligosaccharides generated by XEG treatment. of soybean root and root hair cell walls. a Cell walls of soybean roots stripped of their root hairs. b Cell walls of soybean root hairs. c The 4 M KOH soluble materials from cell walls of roots stripped of their root hairs. d The 4 M KOH soluble materials from cell walls of root hairs. The plants were grown in the absence of B. japonicum
Homologs of XUT1, an Arabidopsis gene that encodes a xyloglucan-specific galacturonosyl transferase, are highly expressed in soybean root hairs
Our chemical data established that the structures of the xyloglucan and glucuronoxylan present in soybean root hair and root cell walls are not identical and that root hair walls contain an acidic xyloglucan and a polysaccharide with a 1, 4-linked β-d-mannan backbone. To extend this study, we compared the tissue-specific expression of G. max homologs to Arabidopsis genes encoding glycosyltransferases known or believed to be involved in the synthesis of xyloglucan, glucuronoxylan, and glucomannan (Table 4).
The two soybean homologs (Glyma11g02640 and Glyma01g42830) of AtXUT1, which encodes the xyloglucan-specifc galacturonosyl transferase, are highly expressed in root hairs and either not expressed or expressed at much lower levels in other root cells. Such a result is consistent with our chemical analyses showing that the GalA-containing xyloglucan is present in soybean root hairs walls but not in the walls of roots that have been stripped of their root hairs. The G. max homologs of Arabidopsis genes (AtXXT1, AtXXT2, AtMUR3, AtXLT2, and AtFUT1) encoding the glycosyltransferases that form the side chains of xyloglucan (Zabotina 2012) are expressed in root hairs and roots, albeit at different levels.
The presence of glucuronoxylan in root hairs was somewhat unexpected as this polysaccharide is most often detected in the secondary cell walls of dicotyledons (McCartney et al. 2005). Nevertheless, small amounts of glucuronoxylan have been isolated from the walls of suspension-cultured plant cells (Darvill et al. 1980; Kato and Matsuda 1985). Xylans were also detected cytochemically in the primary cell walls of tobacco plants using xylan-specific mAbs and carbohydrate-binding modules (Hervé et al. 2010), suggesting that these polysaccharides are indeed a component of primary walls. Many of the G. max homologs of the genes (AtIRX9, AtIRX9-like, AtIRX10, AtIRX10-like, AtIRX14, AtGUX2, AtGXMT1, AtGXMT3) reported to be involved in glucuronoxylan biosynthesis in Arabidopsis (Scheller and Ulvskov 2010; Urbanowicz et al. 2012, 2014) are expressed in root hair cells, albeit at different levels. These genes are also expressed in root stripped of their hairs, a result consistent with our chemical analyses and immunolabeling data, which showed that the xylem walls of roots contain glucuronoxylan.
Several genes likely involved in cell wall glucomannan synthesis have been identified (Liepman et al. 2007; Yin et al. 2011). G. max homologs of some of these genes (AtTBL21, AtCSLA9, AtCSLC12, and AtCSLD2) are expressed in root and root hair cells, albeit at different levels. Soybean homologs of AtCSLA9 (Glyma03g34800), AtCSLC12 (Glyma04g05100), and AtCSLD2 (Glyma01g01780 and Glyma11g01230) are expressed more in hair cells than in stripped root cells (Table 4) suggesting they may be involved in root hair cell-specific mannan synthesis. Arabidopsis CSLD3 is expressed in root hair cells and also at much lower levels in epidermal cells that do not form root hairs (Favery et al. 2001). CSLD3 has been proposed to encode a root hair specific β-1, 4-glucan synthase rather than a mannan synthase (Park et al. 2011). We identified four soybean genes (Glyma01g44280, Glyma11g01230, Glyma01g01780, and Glyma09g34130), which encode proteins with between 75 and 86 % sequence identity to Arabidopsis CSLD3. These genes are expressed in root hairs and at somewhat lower levels in root cells. Thus, the role of CSLD3 proteins appear to be conserved in tip growing root hair cells of different plants.
Hydroxyproline-containing glycoproteins including the cell wall extensins and the cell surface arabinogalactan proteins (AGP) are believed to have important roles in the growth, development and function of roots and root hairs (Velasquez et al. 2011; Nguema-Ona et al. 2013). Thus, we compared the transcript abundances of selected G. max homologs of the Arabidopsis glycosyltransferases that have shown to be or are likely to be involved in the synthesis of extensins and AGPs (Supplementary Table S6). These glycosyltransferases include the arabinogalactan fucosyltransferases FUT4 and FUT6 (Wu et al. 2010), β-glucuronosyltransferases GlcAT14A-C (Knoch et al. 2013), and β-galactosyltransferase GALT31A (Geshi et al. 2013), the hydroxyproline-β-O-galactosyltransferase GALT2 (Basu et al. 2013), the hydroxyproline arabinosyltransferases HPAT1-3 (Ogawa-Ohnishi et al. 2013), the extensin arabinosyltransferases RRA3 and XEG113 (Gille et al. 2009; Velasquez et al. 2011), the arabinosyltransferases RRA1, RRA2, and RAY1 (Egelund et al. 2007; Gille et al. 2013), and the serine α-galactosyltransferases SGT1 (Saito et al. 2014). Numerous G. max homologs of the Arabidopsis enzymes were expressed, albeit at different levels, in both root hairs and roots stripped of root hairs (Supplementary Table 6). Such results are not entirely unexpected as the AGPs in particular exist in most if not all plant tissues and are structurally complex glycans whose synthesis requires a large number of glycosyltransferases (Knoch et al. 2014). Two homologs (Glyma13g05020; GlcAT14 family and Glyma05g06390; peptidyl serine α-galactosyltransferase family) showed >threefold increased transcript abundance in root hairs. Correlating changes in gene expression with the fine structures of extensins and AGPs in roots and root hairs will be challenging and will require the development of a new suite of analytical techniques that can probe the structural diversity of these glycoproteins and proteoglycans (Tan et al. 2012).
Discussion
Our chemical, glycome profiling and immunolabeling data together with analyses of selected gene expression provide evidence that root hair cell walls have structural features that distinguish them from the cell walls of roots stripped of their hairs. RG-I and glucuronoxylan are present in both walls but do not have identical structures. An acidic xyloglucan and a mannose-rich polysaccharide were detected only in root hair walls. Additional studies are required to determine if this mannan is O-acetylated.
The neutral xyloglucan in both root and root hair cell walls have comparable structures, since O-acetylated XXFG subunits account for the bulk of this xyloglucan. A predominance of fucosylated subunits may be a characteristic of root xyloglucan since XXFG and XLFG subunits also account for the bulk of Arabidopsis root xyloglucan (Peña et al. 2012). Both root and root hair walls contain RG-II that is predominantly cross-linked by a borate ester. Brewin (2004) has proposed that RG-II is important for infection thread growth during the nodulation process. This may reflect the reported sensitivity of infection thread growth to boron deprivation (Bolanos et al. 1996) and the importance of borate in RG-II cross-linking and in wall structure (O’Neill et al. 2004).
No significant differences were discernible in the glycosyl residue compositions of root and root hair cell walls from G. max grown in the presence or absence of B. japonicum. It is well documented that only a small fraction of the root hairs respond to B. japonicum inoculation (Calvert et al. 1984) or deform in response to lipo-oligosaccharide nodulation factors (Gage 2004). Furthermore, there are mechanisms in legumes to limit the number of nodules formed (Reid et al. 2011). Nevertheless, localized cell wall fragmentation has been observed in the initial stages of infection thread formation during the interaction between Rhizobium leguminosarum and pea (Van Spronsen et al. 1994). Highly localized legume-derived pectate lyase activity in the cell wall has also been reported to be required for infection thread initiation during Lotus japonicus nodulation (Xie et al. 2012). Thus, changes in wall composition that are a consequence of the root–bacterium interaction may be reduced to levels that are not readily detectable by the presence of non-responding tissue.
It is possible that cell wall composition does not change extensively in response to the bacterium. Rather, root hair curling and infection thread formation may reflect a re-direction of the cell wall synthesis machinery together with localized hydrolysis of some wall components. Such processes may lead to subtle changes in the types and distribution of polymers present in the wall. Such a conclusion is consistent with previous studies suggesting that changes do occur in the root hair cell wall, and most notably in the infection thread wall (Brewin 2004). For example, the levels of a number of cell wall glycoproteins are altered by inoculation with Rhizobium (Cassab and Varner 1988; Pichon et al. 1992) and some of these glycoproteins are abundant on the infection thread surface (Perotto et al. 1991; Sherrier et al. 1999). The abnormal distribution of glycoproteins/arabinogalactan proteins has led to the suggestion that targeted secretion of wall glycoproteins is correlated with the process of infection thread growth (Tsyganova et al. 2009).
Arabidopsis plants carrying mutations that affect neutral or acidic xyloglucan structure or prevent xyloglucan synthesis altogether have short or swollen root hairs (Cavalier et al. 2008; Zabotina et al. 2008; Peña et al. 2012). Rice plants carrying a point mutation in the homolog of Arabidopsis AtXXT1 also have root hairs that are shorter than wild type (Wang et al. 2014). Thus, xyloglucan is required for normal root hair growth and development in monocots and dicots. Interestingly, the roots and aerial portions of these xyloglucan-defective mutants appear near normal. Such data demonstrates that altering xyloglucan structure has a greater effect on cells that extend by tip growth (root hairs) than on cells that exhibit diffuse growth.
Studies with Arabidopsis have reported that a mannan-like polysaccharide is localized to the extending tip of the hair cell (Yin et al. 2011). These authors also reported that mutations that result in abnormal root hair growth are accompanied by the re-distribution of the mannan along the length of the root hair. Mannose-containing polysaccharides have also been detected in soybean root exudates (Timotiwu and Sakurai 2002). A glucomannan synthesized by Rhizobium leguminosarum was suggested to have a role in the attachment of this bacterium to pea root hairs (Williams et al. 2008). Thus, it is possible that an early step in the attachment process is mediated by interactions between glucomannan secreted by the root or present in the root hair cell wall and glucomannan on the surface of a rhizobium cell.
Peña and colleagues (Peña et al. 2012) proposed that anionic xyloglucan alone or in combination with other wall components may prevent the ordering of cellulose microfibrils at the root hair tip and thereby facilitate tip growth. Such growth is also facilitated by the deposition along the length of the root hair cell of a secondary wall that restricts lateral and longitudinal extension (Emons and Ketelaar 2009; Nielsen 2009; Akkerman et al. 2012). Altering the composition of these walls by, for example, depositing mannan may also alter the mechanical properties of the wall and thereby facilitate normal tip growth and development of the root hair cell.
Our study has revealed numerous differences in the polysaccharide components of root and root hair cell walls. Nevertheless, it still remains a challenge to establish the role of these walls during the nodulation process and to determine if they undergo changes in response to the bacterial symbiont. Such studies would benefit from the ability to increase the number of root hairs cells that respond to nodulation factors and to the availability of mAbs that recognize well-defined wall epitopes specific to root and root hair cell walls. The generation of legumes carrying mutations in genes involved in the synthesis of wall polysaccharides (Tadege et al. 2008) would also facilitate a reverse genetics approach to determine the role of the cell wall in the nodulation process.
Author contribution statement
RWC, GS, MAO, WSY, and MGH conceived and designed research. AM, MAO, ER, SP, UA, MJP1, ML, MdSH, LB, and RMB carried out experiments and analyzed the data. MAO, AM, MJP, WSY, GS, and RWC wrote the manuscript. All authors read and approved the manuscript.
Abbreviations
- AG:
-
Arabinogalactan protein
- CSLD:
-
Cellulose synthase-like d
- Dp:
-
Degree of polymerization
- MALDI-TOF MS:
-
Matrix assisted laser desorption time of flight mass spectrometry
- RG-I:
-
Rhamnogalacturonan I
- RG-II:
-
Rhamnogalacturonan II
- XEG:
-
Xyloglucan-specific endoglucanase
- XUT:
-
Xyloglucan-specific galacturonosyltransferase
- XXT:
-
Xyloglucan-specific xylosyltransferase
- XyGO:
-
Xyloglucan oligosaccharide
References
Aguiar G, Batista B, Rodrigues J, Silva L, Campiglia A, Barbosa R, Barbosa F Jr (2012) Determination of trace elements in bovine semen samples by inductively coupled plasma mass spectrometry and data mining techniques for identification of bovine class. J Dairy Sci 95:7066–7073
Akkerman M, Franseen-Verheijen MAW, Immerzeel P, Den Hollander L, Schel JHN, Emons AMC (2012) Texture of cellulose microfibrils of root hair cell walls of Arabidopsis thaliana, Medicago truncatula, and Vicia sativa. J Microscopy 247:60–67
Avci U, Pattathil S, Hahn MG (2012) Immunological approaches to plant cell wall and biomass characterization: Immunolocalization of glycan epitopes. In: Himmell ME (ed) Biomass conversion: methods and protocols. Humana Press, New York, pp 73–82
Barbosa R, Batista B, Varrique R, Coelho V, Campiglia A, Barbosa F Jr (2014) The use of advanced chemometric techniques and trace element levels for controlling the authenticity of organic coffee. Food Res Int 61:246–251
Basu D, Liang Y, Liu X, Himmeldirk K, Faik A, Kieliszewski M, Held M, Showalter A (2013) Functional identification of a hydroxyproline-O-galactosyltransferase specific for arabinogalactan protein biosynthesis in Arabidopsis. J Biol Chem 288:10132–10143
Bernal AJ, Yoo CM, Mutwil M, Jensen JK, Hou G, Blaukopf C, Sørensen I, Blancaflor EB, Scheller HV, Willats WGT (2008) Functional analysis of the cellulose synthase-like genes CSLD1, CSLD2, and CSLD4 in tip-growing Arabidopsis cells. Plant Physiol 148:1238–1253
Bibikova T, Gilroy S (2003) Root hair development. J Plant Growth Reg 21:383–415
Blumenkrantz N, Asboe-Hansen G (1973) New method for quantitative determination of uronic acids. Anal Biochem 54:484–489
Bolanos L, Brewin N, Bonilla I (1996) Effects of boron on Rhizobium-legume cell-surface interactions and nodule development. Plant Physiol 110:1249–1256
Brechenmacher L, Lee J, Sachdev S, Song Z, Nguyen THN, Joshi T, Oehrle N, Libault M, Mooney B, Xu D (2009) Establishment of a protein reference map for soybean root hair cells. Plant Physiol 149:670–682
Brewin N (2004) Plant cell wall remodelling in the rhizobium–legume symbiosis. Crit Rev Plant Sci 23:293–316
Broughton W, Dilworth M (1971) Control of leghaemoglobin synthesis in snake beans. Biochem J 125:1075–1080
Bussink H, Kester H, Visser J (1990) Molecular cloning, nucleotide sequence and expression of the gene encoding prepro-polygalacturonasell of Aspergillus niger. FEBS Lett 273:127–130
Calvert H, Pence M, Pierce M, Malik N, Bauer W (1984) Anatomical analysis of the development and distribution of rhizobium infections in soybean roots. Can J Bot 62:2375–2384
Cassab G, Varner J (1988) Cell wall proteins. Annu Rev Plant Physiol Plant Mol Biol 39:321–353
Cavalier DM, Lerouxel O, Neumetzler L, Yamauchi K, Reinecke A, Freshour G, Zabotina OA, Hahn MG, Burgert I, Pauly M (2008) Disrupting two Arabidopsis thaliana xylosyltransferase genes results in plants deficient in xyloglucan, a major primary cell wall component. Plant Cell 20:1519–1537
Clay R, Bergmann C, Fuller M (1997) Isolation and characterization of an endopolygalacturonase from Cochliobolus sativus and a cytological study of fungal penetration of barley. Phytopathology 87:1148–1159
Cosgrove DJ (2005) Growth of the plant cell wall. Nat Rev Mol Cell Biol 6:850–861
Cosgrove DJ, Jarvis MC (2012) Comparative structure and biomechanics of plant primary and secondary cell walls. Front Plant Sci 3:204
Darvill JE, McNeil M, Darvill AG, Albersheim P (1980) Structure of plant cell walls: XI. Glucuronoarabinoxylan, a second hemicellulose in the primary cell walls of suspension-cultured sycamore cells. Plant Physiol 66:1135–1141
DeMartini J, Pattathil S, Avci U, Szekalski K, Mazumder K, Hahn M, Wyman C (2011) Application of monoclonal antibodies to investigate plant cell wall deconstruction for biofuels production. Energy Environ Sci 4:4332–4339
Egelund J, Obel N, Ulvskov P, Geshi N, Pauly M, Bacic A, Petersen B (2007) Molecular characterization of two Arabidopsis thaliana glycosyltransferase mutants, rra1 and rra2, which have a reduced residual arabinose content in a polymer tightly associated with the cellulosic wall residue. Plant Mol Biol 64:439–451
Emons A, Ketelaar T (2009) Intracellular organization: A prerequisite for root hair elongation and cell wall deposition. In: Emons AMC, Ketelaar T (eds) Plant cell monographs. Root hairs. Springer, Berlin, pp 27–44
Favery B, Ryan E, Foreman J, Linstead P, Boudonck K, Steer M, Shaw P, Dolan L (2001) KOJAK encodes a cellulose synthase-like protein required for root hair cell morphogenesis in Arabidopsis. Genes Dev 15:79–89
Fransen C, Haseley S, Huisman M, Schols H, Voragen A, Kamerling J, Vliegenthart J (2000) Studies on the structure of a lithium-treated soybean pectin: characteristics of the fragments and determination of the carbohydrate substituents of galacturonic acid. Carbohydr Res 328:539–547
Freshour G, Clay RP, Fuller MS, Albersheim P, Darvill AG, Hahn MG (1996) Developmental and tissue-specific structural alterations of the cell-wall polysaccharides of Arabidopsis thaliana roots. Plant Physiol 110:1413–1429
Gage D (2004) Infection and invasion of roots by symbiotic, nitrogen-fixing rhizobia during nodulation of temperate legumes. Microbiol Mol Biol Rev 68:280–300
Galway ME, Eng RC, Schiefelbein JW, Wasteneys GO (2011) Root hair-specific disruption of cellulose and xyloglucan in AtCSLD3 mutants, and factors affecting the post-rupture resumption of mutant root hair growth. Planta 233:985–999
Geshi N, Johansen J, Dilokpimol A, Rolland A, Belcram K, Verger S, Kotake T, Tsumuraya Y, Kaneko S, Tryfona T (2013) A galactosyltransferase acting on arabinogalactan protein glycans is essential for embryo development in Arabidopsis. Plant J 76:128–137
Gille S, Hänsel U, Ziemann M, Pauly M (2009) Identification of plant cell wall mutants by means of a forward chemical genetic approach using hydrolases. Proc Nat Acad Sci USA 106:14699–14704
Gille S, Sharma V, Baidoo E, Keasling J, Scheller H, Pauly M (2013) Arabinosylation of a Yariv-precipitable cell wall polymer impacts plant growth as exemplified by the Arabidopsis glycosyltransferase mutant ray1. Mol Plant 6:1369–1372
Hayashi T, Kato Y, Matsuda K (1980) Xyloglucan from suspension-cultured soybean cells. Plant Cell Physiol 21:1405–1418
Haykin S (2008) Neural networks and learning machines. Prentice Hall, New Jersey
Hervé C, Rogowski A, Blake AW, Marcus SE, Gilbert HJ, Knox JP (2010) Carbohydrate-binding modules promote the enzymatic deconstruction of intact plant cell walls by targeting and proximity effects. Proc Nat Acad Sci USA 107:15293–15298
Hoffman M, Jia Z, Peña MJ, Cash M, Harper A, Blackburn AR II, Darvill A, York WS (2005) Structural analysis of xyloglucans in the primary cell walls of plants in the subclass Asteridae. Carbohydr Res 340:1826–1840
Huisman M, Weel K, Schols H, Voragen A (2000) Xyloglucan from soybean (Glycine max) meal is composed of XXXG-type building units. Carbohydr Polym 42:185–191
Huisman M, Fransen C, Kamerling J, Vliegenthart J, Schols HA, Voragen A (2001) The CDTA-soluble pectic substances from soybean meal are composed of rhamnogalacturonan and xylogalacturonan but not homogalacturonan. Biopolymers 58:279–294
Joshi T, Patil K, Fitzpatrick M, Franklin L, Yao Q, Cook J, Wang Z, Libault M, Brechenmacher L, Valliyodan B (2012) Soybean Knowledge Base (SoyKB): a web resource for soybean translational genomics. BMC Genom 13:S15
Jungk A (2001) Root hairs and the acquisition of plant nutrients from soil. J Plant Nutr Soil Sci 164:121–129
Kato Y, Matsuda K (1985) Acidic arabinoxylan as an extracellular polysaccharide of suspension-cultured soybean cells. Plant Cell Physiol 26:287–294
Knoch E, Dilokpimol A, Tryfona T, Poulsen C, Xiong G, Harholt J, Petersen B, Ulvskov P, Hadi M, Kotake T (2013) A β–glucuronosyltransferase from Arabidopsis thaliana involved in biosynthesis of type II arabinogalactan has a role in cell elongation during seedling growth. Plant J 76:1016–1029
Knoch E, Dilokpimol A, Geshi N (2014) Arabinogalactan proteins: focus on carbohydrate active enzymes. Frontiers Plant Sci 5:198
Libault M, Farmer A, Brechenmacher L, Drnevich J, Langley R, Bilgin D, Radwan O, Neece D, Clough S, May GD, Stacey G (2010) Complete transcriptome of the soybean root hair cell, a single-cell model, and its alteration in response to Bradyrhizobium japonicum infection. Plant Physiol 152:541–552
Liepman AH, Nairn CJ, Willats WGT, Sørensen I, Roberts AW, Keegstra K (2007) Functional genomic analysis supports conservation of function among cellulose synthase-like A gene family members and suggests diverse roles of mannans in plants. Plant Physiol 143:1881–1893
Matoh T, Ishigaki K, Ohno K, Azuma J (1993) Isolation and characterization of a boron-polysaccharide complex from radish roots. Plant Cell Physiol 34:639–642
McCartney L, Marcus SE, Knox JP (2005) Monoclonal antibodies to plant cell wall xylans and arabinoxylans. J Histochem Cytochem 53:543–546
Mort AJ, Grover PB (1988) Characterization of root hair cell walls as potential barriers to the infection of plants by rhizobia: the carbohydrate component. Plant Physiol 86:638–642
Nakamura A, Furuta H, Maeda H, Takao T, Nagamatsu Y (2002) Structural studies by stepwise enzymatic degradation of the main backbone of soybean soluble polysaccharides consisting of galacturonan and rhamnogalacturonan. Biosci Biotechnol Biochem 66:1301–1313
Nevins D, English PD, Albersheim P (1967) The specific nature of plant cell wall polysaccharides. Plant Physiol 42:900–906
Nguema-Ona E, Vicré-Gibouin M, Cannesan M-A, Driouich A (2013) Arabinogalactan proteins in root–microbe interactions. Trends Plant Sci 18:440–449
Nielsen E (2009) Plant cell wall biogenesis during tip growth in root hair cells. In: Emons AMC, Ketelaar T (eds) Plant cell monographs. Root hairs. Springer, Berlin, pp 85–102
Ogawa-Ohnishi M, Matsushita W, Matsubayashi Y (2013) Identification of three hydroxyproline O-arabinosyltransferases in Arabidopsis thaliana. Nature Chem Biol 9:726–730
Oldroyd G, Downie J (2008) Coordinating nodule morphogenesis with rhizobial infection in legumes. Annu Rev Plant Biol 59:519–546
O’Neill MA, Ishii T, Albersheim P, Darvill AG (2004) Rhamnogalacturonan II: structure and function of a borate cross-linked cell wall pectic polysaccharide. Annu Rev Plant Biol 55:109–139
Park S, Szumlanski AL, Gu F, Guo F, Nielsen E (2011) A role for CSLD3 during cell-wall synthesis in apical plasma membranes of tip-growing root-hair cells. Nat Cell Biol 13:973–980
Pattathil S, Avci U, Baldwin D, Swennes A, McGill J, Popper Z, Bootten T, Albert A, Davis R, Chennareddy C, Dong R, O’Shea B, Rossi R, Leoff C, Freshour G, Narra R, O’Neil M, York W, Hahn M (2010) A comprehensive toolkit of plant cell wall glycan-directed monoclonal antibodies. Plant Physiol 153:514–525
Pattathil S, Avci U, Miller J, Hahn M (2012) Immunological approaches to plant cell wall and biomass characterization: glycome profiling. In: ME H (ed) Biomass conversion: Methods and protocols. Humana Press, New York, pp 61–72
Pauly M, Andersen LN, Kauppinen S, Kofod LV, York WS, Albersheim P, Darvill A (1999) A xyloglucan-specific endo-β-1, 4-glucanase from Aspergillus aculeatus: expression cloning in yeast, purification and characterization of the recombinant enzyme. Glycobiology 9:93–100
Peña MJ, Kong Y, York WS, O’Neill MA (2012) A galacturonic acid–containing xyloglucan is involved in Arabidopsis root hair tip growth. Plant Cell 24:4511–4524
Perotto S, VandenBosch K, Butcher G, Brewin N (1991) Molecular composition and development of the plant with the peribacteroid membrane of pea root nodules. Development 112:763–773
Pichon M, Journet E-P, Dedieu A, de Billy F, Truchet G, Barker D (1992) Rhizobium meliloti elicits transient expression of the early nodulin gene ENOD12 in the differentiating root epidermis of transgenic alfalfa. Plant Cell 4:1199–1211
Raven P, Evert R, Eichhorn S (2005) Biology of plants. WH Freeman, New York
Reid D, Ferguson B, Gresshoff P (2011) Inoculation-and nitrate-induced CLE peptides of soybean control NARK-dependent nodule formation. Mol Plant Microbe 24:606–618
Saito F, Suyama A, Oka T, Yoko-o T, Matsuoka K, Jigami Y, Shimma Y (2014) Identification of novel peptidyl serine α-galactosyltransferase gene family in plants. J Biol Chem 289:20405–20420
Scheller HV, Ulvskov P (2010) Hemicelluloses. Annu Rev Plant Biol 61:263–289
Schubert K (1986) Products of biological nitrogen fixation in higher plants: synthesis, transport, and metabolism. Annu Rev Plant Physiol 37:539–574
Sherrier D, Prime T, Dupree P (1999) Glycosylphosphatidylinositol-anchored cell-surface proteins from Arabidopsis. Electrophoresis 20:2027–2035
So J-S, Hodgson L, Haugland R, Leavitt M, Banfalvi Z, Nieuwkoop A, Stacey G (1987) Transposon-induced symbiotic mutants of Bradyrhizobium japonicum: isolation of two gene regions essential for nodulation. Mol Gen Genet 207:15–23
Tadege M, Wen J, He J, Tu H, Kwak Y, Eschstruth A, Cayrel A, Endre G, Zhao P, Chabaud M, Ratet P, Mysore K (2008) Large-scale insertional mutagenesis using the Tnt1 retrotransposon in the model legume Medicago truncatula. Plant J 54:335–347
Tan L, Showalter A, Egelund J, Hernandez-Sanchez A, Doblin M, Bacic A (2012) Arabinogalactan-proteins and the research challenges for these enigmatic plant cell surface proteoglycans. Frontiers Plant Sci 3:140
Timotiwu P, Sakurai N (2002) Identification of mono-, oligo-, and polysaccharides secreted from soybean roots. J Plant Res 115:77–85
Tsyganova A, Tsyganov V, Findlay K, Borisov AY, Tikhonovich I, Brewin N (2009) Distribution of legume arabinogalactan protein-extensin (AGPE) glycoproteins in symbiotically defective pea mutants with abnormal infection threads. Cell Tissue Biol 3:93–102
Tuomivaara ST, Yaoi K, O’Neill MA, York WS (2014) Generation and structural validation of a library of diverse xyloglucan-derived oligosaccharides, including an update on xyloglucan nomenclature. Carbohydr Res 402:55–66
Urbanowicz BR, Peña MJ, Ratnaparkhe S, Avci U, Backe J, Steet HF, Foston M, Li H, O’Neill MA, Ragauskas AJ, Gilbert H, York W (2012) 4-O-methylation of glucuronic acid in Arabidopsis glucuronoxylan is catalyzed by a domain of unknown function family 579 protein. Proc Nat Acad Sci USA 109:14253–14258
Urbanowicz BR, Peña MJ, Moniz HA, Moremen KW, York WS (2014) Two Arabidopsis proteins synthesize acetylated xylan in vitro. Plant J 80:197–206
Van Spronsen P, Bakhuizen R, Van Brussel A, Kijne J (1994) Cell wall degradation during infection thread formation by the root nodule bacterium Rhizobium leguminosarum is a two-step process. Eur J Cell Biol 64:88–94
Velasquez SM, Ricardi MM, Dorosz JG, Fernandez PV, Nadra AD, Pol-Fachin L, Egelund J, Gille S, Harholt J, Ciancia M (2011) O-glycosylated cell wall proteins are essential in root hair growth. Science 332:1401–1405
Wan J, Torres M, Ganapathy A, Thelen J, DaGue B, Mooney B, Xu D, Stacey G (2005) Proteomic analysis of soybean root hairs after infection by Bradyrhizobium japonicum. Mol Plant Microbe In 18:458–467
Wang C, Li S, Ng S, Zhang B, Zhou Y, Whelan J, Wu P, Shou H (2014) Mutation in xyloglucan 6-xylosytransferase results in abnormal root hair development in Oryza sativa. J Exp Bot 65:4149–4157
Warren M, Kester H, Benen J, Colangelo J, Visser J, Bergmann C, Orlando R (2002) Studies on the glycosylation of wild-type and mutant forms of Aspergillus niger pectin methylesterase. Carbohydr Res 337:803–812
Williams A, Wilkinson A, Krehenbrink M, Russo D, Zorreguieta A, Downie JA (2008) Glucomannan-mediated attachment of Rhizobium leguminosarum to pea root hairs is required for competitive nodule infection. J Bacteriol 190:4706–4715
Wu Y, Williams M, Bernard S, Driouich A, Showalter AM, Faik A (2010) Functional identification of two nonredundant Arabidopsis α (1, 2) fucosyltransferases specific to arabinogalactan proteins. J Biol Chem 285:13638–13647
Xie F, Murray JD, Kim J, Heckmann AB, Edwards A, Oldroyd G, Downie JA (2012) Legume pectate lyase required for root infection by rhizobia. Proc Nat Acad Sci USA 109:633–638
Yamaguchi F, Ota Y, Hatanaka C (1996) Extraction and purification of pectic polysaccharides from soybean okara and enzymatic analysis of their structures. Carbohydr Polymers 30:265–273
Yin L, Verhertbruggen Y, Oikawa A, Manisseri C, Knierim B, Prak L, Jensen JK, Knox JP, Auer M, Willats WGT, Scheller H (2011) The cooperative activities of CSLD2, CSLD3, and CSLD5 are required for normal Arabidopsis development. Mol Plant 4:1024–1037
York WS, Darvill AG, McNeil M, Stevenson TT, Albersheim P (1986) Isolation and characterization of plant cell walls and cell wall components. Method Enzymol 118:3–40
Zabotina OA (2012) Xyloglucan and its biosynthesis. Front Plant Sci 3:1–5
Zabotina OA, Van De Ven WTG, Freshour G, Drakakaki G, Cavalier D, Mouille G, Hahn MG, Keegstra K, Raikhel NV (2008) Arabidopsis XXT5 gene encodes a putative α-1, 6-xylosyltransferase that is involved in xyloglucan biosynthesis. Plant J 56:101–115
Zabotina OA, Avci U, Cavalier D, Pattathil S, Chou Y-H, Eberhard S, Danhof L, Keegstra K, Hahn MG (2012) Mutations in multiple XXT genes of Arabidopsis reveal the complexity of xyloglucan biosynthesis. Plant Physiol 159:1367–1384
Acknowledgments
The authors acknowledge the Division of Chemical Sciences, Geosciences, and Biosciences, Office of Basic Energy Sciences of the U.S. Department of Energy through Grants DE-FG02-93ER20097 (to R.W.C.), DE-FG02-12ER16324 (to W.S.Y, M.A.O., M.J.P.), and DE-FG02-12ER16326 (to M.A.O.) for funding the structural studies of soybean root and root hair cell walls. The National Science Foundation is acknowledged through Plant Genome Grant DBI-041683 and IOB-0923992 (to M.G.H., W.S.Y., M.A.O.) for funding the development of cell wall antibodies and Plant Genome Grant DBI-0421620 (to G.S.) for funding studies of soybean functional genomics.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Muszyński, A., O’Neill, M.A., Ramasamy, E. et al. Xyloglucan, galactomannan, glucuronoxylan, and rhamnogalacturonan I do not have identical structures in soybean root and root hair cell walls. Planta 242, 1123–1138 (2015). https://doi.org/10.1007/s00425-015-2344-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00425-015-2344-y