Abstract
Investigating how plants cope with different abiotic stresses—mainly drought and extreme temperatures—is pivotal for both understanding the underlying signaling pathways and improving genetically engineered crops. Plant cells are known to react defensively to mild and severe dehydration by initiating several signal transduction pathways that result in the accumulation of different proteins, sugar molecules and lipophilic anti-oxidants. Among the proteins that build up under these adverse conditions are members of the ancestral ASR (ABA-stress-ripening) family, which is conserved in the plant kingdom but lacks orthologs in Arabidopsis. This review provides a comprehensive summary of the state of the art regarding ASRs, going back to the original description and cloning of the tomato ASR cDNA. That seminal discovery sparked worldwide interest amongst research groups spanning multiple fields: biochemistry, cell biology, evolution, physiology and epigenetics. As these proteins function as both chaperones and transcription factors; this review also covers the progress made on relevant molecular features that account for these dual roles—including the recent identification of their target genes—which may inspire future basic research. In addition, we address reports of drought-tolerant ASR-transgenic plants of different species, highlighting the influential work of authors taking more biotechnological approaches.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Original cloning in tomato and recognition of the existence of a gene family
Back in 1993, a fruit cDNA library derived from tomato (Solanum lycopersicum) ripe fruit was screened by differential hybridisation with transcripts from tomato leaves under normal or water-stress conditions. As a result, a cDNA clone corresponding to an mRNA that is abundant in both stressed leaves and ripe fruits was isolated (Iusem et al. 1993). In parallel, the abscisic acid (ABA)-dependence of this transcript was demonstrated in leaves using a tomato mutant deficient for ABA synthesis (unpublished data). Soon later, a genomic clone with significant sequence identity to the original cDNA was isolated and named ABA-stress and ripening (Asr2) (Rossi and Iusem 1994), while the gene originally discovered in 1993 was referred as Asr1. This finding sheds light on the recognition of a new gene family rather than different alleles of the same locus.
Soon thereafter, Amitai et al. identified a third member of this family, Asr3 (sequence deposited in GenBank, unpublished data) and Rossi et al. (1996) reported that the three known tomato sequences originate from different but tightly linked loci located on chromosome 4. Ten years later, our lab found a fourth member (Asr4) in tomato (Frankel et al. 2006). In addition, wild Solanum species related to commercial tomato and chronically exposed to arid environments, namely, Solanum chilense and Solanum peruvianum, display a fifth paralogous gene, termed Asr5 (Fischer et al. 2011).
Further evidence on the organization of Asr genes in families in different species
Over the past 20 years, Asr genes have been reported in many plant species other than tomato, ranging from ancient gymnosperms to monocots and dicots (Table 1). With regard to monocots, the landscape of the Asr gene family in rice (OsAsr) is rather different and is composed of six members (Philippe et al. 2010). In contrast to tomato Asr genes, the rice genes are located on different chromosomes, except for OsAsr3 and OsAsr4, which are tightly linked on chromosome 1 (Philippe et al. 2010). Strikingly, OsAsr6 is the most divergent of the rice paralogs but is in turn very similar to the orthologous tomato Asr4 gene, particularly in its size, which encodes for a larger protein (Philippe et al. 2010).
As far as maize (Zea mays) is concerned, its Asr gene (ZmAsr) family consists of nine paralogous genes, thus being larger than in tomato (four members, Frankel et al. 2006), banana (four members, Henry et al. 2011) and rice (six members, Philippe et al. 2010).
Other monocot species, such as those of the genus Musa (plantain and banana), possess a four-member Asr family, much like tomato. These Asr genes are similarly clustered on the same chromosome (Henry et al. 2011).
Regarding dicots, an Asr ortholog was also reported in wild potato (Solanum chacoense) and named DS2 (Silhavy et al. 1995). Single Asr genes have also been found in other Solanaceous plants, for example, in Nicotiana species (Yang et al. 2012). Halophyte plants such as Salicornia brachiata, which grow luxuriantly on salty shores, also contain Asr genes, which are induced by high salinity in such extreme environments (Jha et al. 2009).
Gene structure and evolution
Tomato Asr genes are short with a very simple structure: two exons separated by an intron (Gonzalez et al. 2011). In particular, the Asr2 genomic sequence is 73 % homologous to Asr1 (Rossi and Iusem 1994), with an AT-rich regulatory region that has been studied in depth (Rossi et al. 1998; Rossi and Iusem 1995). For example, the Asr2 promoter has a putative ABA-response motif, and intriguingly, its 3′ untranslated region (3′ UTR) shares 92 % homology with an intron from the polygalacturonase gene, whose expression increases during fruit ripening (Rossi and Iusem 1995).
The general simple structure of tomato Asr genes with only two exons separated by an intron is also shared by other species, for example, those of the genus Musa (plantain and banana) (Henry et al. 2011) and the halophyte plant S. brachiata (Jha et al. 2009).
When members of the commercial tomato Asr gene family were compared with their orthologous genes from different Solanum wild species, Asr1 showed a very low level of non-synonymous substitutions throughout its entire sequence, indicative of a strong purifying selection (Fischer et al. 2011; Frankel et al. 2006). In contrast, Asr2 showed a higher ratio of non-synonymous to synonymous substitutions when compared with species inhabiting arid regions, implying the action of positive, adaptive selection on this gene during evolution (Frankel et al. 2003). Similarly, the Asr2 gene showed intra-specific variations in wild tomato populations that were subject to different rainfall regimes (Giombini et al. 2009). However, Fisher et al. found no evidence for adaptive evolution in the Asr2 coding sequence, in contrast to Asr4 (Fischer et al. 2011). Nevertheless, the same author recently found that the Asr2 regulatory region was indeed subject to positive selection in Solanum chilense, a species adapted to very dry environments (Fischer et al. 2013).
Interestingly, orthologous genes were much more alike in pair-wise comparisons (Asr1 vs. ci21a; Asr2 vs. ci21b; and Asr4 vs. DS2) than were paralogous genes (Asr1 vs. Asr2; ci21a vs. ci21b; etc.) (Frankel et al. 2006). One conclusion from that analysis was that the Asr family is ancient (300 million years ago), and its origin derives from gene duplications occurring before the divergence of the tomato and potato branches. Strikingly, Asr3 does not have a potato ortholog. A similar evolutionary history is inferred from the rice and maize Asr genes, as they cluster together and exhibit more similarity between orthologs than between paralogs (Frankel et al. 2006).
Unlike tomato, rice Asr genes are located on different chromosomes, though OsAsr1 and OsAsr2 are tightly linked in chromosome 1, most likely as a consequence of a recent duplication event. OsAsr6 is also located on chromosome 1 but is distant from the first two paralogs. Finally, OsAsr3, OsAsr4 and OsAsr5 are located on different chromosomes. This divergence between locations could be the result of duplication events taking place on chromosome segments or even involving whole genomes (Philippe et al. 2010).
Another evolutionary lesson arose from data regarding the common bean (Phaseolus vulgaris), which has two Asr genes, each of which have a different evolutionary history. The first, Asr1, underwent little nucleotide variation between wild and cultivated bean, indicative of a strong purifying selection, whereas Asr2 seems to have been the target of adaptive selection (Cortes et al. 2012).
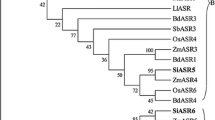
To present an overall picture of the relatedness of ASR proteins in terms of sequence, a phylogenetic tree is displayed in Fig. 1 (Frankel et al. 2006). Another evolutionary study on this protein family has been performed in depth by Henry et al. (2011), focusing on Musa species (plantain and banana). This analysis revealed that, unlike tomato ASRs, those from Musa cluster together rather than with ASRs from other species, suggesting that they were the product of recent duplication events.
Neighbor-joining tree of ASR proteins from different plant species (taken from Frankel et al. 2006). The numbers in nodes are bootstrap values (only values >70 % are shown). Bar length indicates p-distance. The tree is rooted with the Ginkgo-Pine group (gymnosperms). Permission to reproduce this figure was obtained under License Agreement Number 3313060861664 between N. D. I. and Elsevier publishing company
Epigenetics
Tomato is the only species in which epigenetic studies on Asr genes have been conducted. Asr1 and Asr2 revealed epigenetic marks on both DNA cytosines and histones (Gonzalez et al. 2011, 2013). For example, in leaves, Asr1 exhibits H3K27me3 marks along with cytosine methylation in all methylation contexts (CG, CNG and CNN), with the latter context (asymmetric methylation) being atypical within the body of a non-repetitive gene such as Asr1 (Gonzalez et al. 2011). Interestingly, CNN methylation and H3K27me3 decrease after water deprivation, concomitant with an increase in Asr1 mRNA levels (Gonzalez et al. 2011). In roots, CNN methylation in the regulatory region decreases under water stress, along with a drop in H3K9me2 within the regulatory and coding regions of Asr2 (Gonzalez et al. 2013).
Expression analyses
In tomato, Asr1 transcript was found in stressed leaves and fruit pericarp (Iusem et al. 1993). The ASR1 protein levels also increase upon to the osmotic stress induced by polyethyleneglycol (PEG) (Amitai-Zeigerson et al. 1995). Both Asr1 and Asr2 expression levels increased in dehydrated leaves (whole organ), but only Asr2 levels rose in roots (Maskin et al. 2001). A deeper examination of the cell types involved revealed Asr1 mRNA in leaf vascular tissue irrespective of stress, whereas Asr2 mRNA was observed in phloem companion cells, expanding to mesophyll cells (Maskin et al. 2008). In addition, Asr1 expression levels were high and constant throughout fruit ripening (Maskin et al. 2001). The ASR1 protein also accumulates in tomato seeds, an organ subjected to physiological desiccation during final maturation drying (Maskin et al. 2007).
Consistently, ASR proteins are also crucial in pollen, especially during the drying stage, where protection against drying conditions is vital for subsequent grain maturation (Wang et al. 2013). In contrast to tomato, the orthologous potato (Solanum tuberosum) water stress-inducible Asr gene (named DS2) is insensitive to ABA (Dóczi et al. 2002).
Of the six rice Asr genes, at least OsAsr5 is induced by gibberellins (GA), as well as by ABA. This could imply that Asr5 is not only involved in stress tolerance but also in plant growth, particularly in leaf expansion (Takasaki et al. 2008). OsAsr5 is also implicated in aluminum (Al) tolerance (Arenhart et al. 2013b). In the presence of Al ions, all six OsAsr genes increase their expression level, particularly OsAsr5, whose induction turned out to be the highest in Al-resistant cultivars (Arenhart et al. 2013b). Furthermore, the encoded OsASR5 protein is present in the chloroplast probably as an inactive transcription factor that could be released to the nucleus in response to Al to regulate genes related to photosynthesis (Arenhart et al. 2012). On the other hand, OsAsr1 is induced by ABA, a situation that in turn leads to higher tolerance to water and osmotic stress (Joo et al. 2013a). Similar to tomato, rice Asr genes exhibit non-overlapping expression patterns in different tissues and have different responses to ABA and water stress, as demonstrated by qRT-PCR and GUS staining (Perez-Diaz et al. 2014). For example, OsAsr1 is expressed mostly in leaves, whereas OsAsr3 is preferentially expressed in roots. Together, these two proteins are the most abundant ASR proteins in rice, being present in a variety of tissues. In addition, they are induced by abiotic stress but stimulated differentially by plant hormones (ABA or GA) and by different sugars, such as sucrose and glucose (Joo et al. 2013b).
In wheat, another important cereal crop, a proteomic study revealed that an ASR protein is up-regulated during water stress (Bazargani et al. 2011), whereas in the large maize Asr family, ZmAsr1 has the highest expression levels (Virlouvet et al. 2011).
Studies conducted with other species showed that, for example, in pinus (Pinus taeda), the only orthologous gene analyzed (named lp3) is expressed mostly in roots under water-deficit conditions (Padmanabhan et al. 1997), while in lily (Lilium longiflorum), the orthologous protein is present at least in developing pollen, induced by ABA and osmotic stress, i.e., PEG (Huang et al. 2000).
When tobacco plants were engineered with the pinus Asr (named lp3) coding sequence driven by its own promoter, the exogenous lp3 regulatory region was widely recognized by the heterologous transcription machinery in root and shoot meristematic regions, most cell types in leaves except the petiole, trichomes, root hairs, stems, pistils of developing floral buds, developing ovary and embryos, and developing seeds. Moreover, such a promoter proved to display elements that respond to hormones including methyl jasmonate and ABA in the recipient species. As with many LEA genes, lp3 expression decreased after seed germination, most likely as a consequence of methylation (Wang et al. 2002).
Subcellular localisation
Tomato ASR1 was first reported solely as a nuclear protein (Iusem et al. 1993) but was later detected in both the cytosol and the nucleus (Kalifa et al. 2004a). Consistent with this compartmentalized distribution, a nuclear localisation signal (NLS) was putatively identified (Kalifa et al. 2004a) but turned out to be non-functional upon experimental testing (Ricardi et al. 2012). In tobacco plants, ASR was also found in the nucleus (Yang et al. 2012).
Interestingly, during the early stages of pollen maturation, the native ASR protein from lily translocates from the cytosol into the nucleus (Yang et al. 2008). However, in this case, the existence of a functional NLS was concluded based on the fact that when this signal was artificially removed, the protein was found only in the cytosol (Wang et al. 2005).
The use of heterologous in vivo systems such as Saccharomyces cerevisiae and Nicotiana benthamiana showed that tomato ASR localizes mostly in the cytosol and usually forms granules that are readily visible using confocal microscopy (Bermudez-Moretti et al. 2006; Ricardi et al. 2012; Urtasun et al. 2010). A different pattern of subcellular distribution was observed in another heterologous system consisting of tobacco expressing the pinus Asr (named lp3), which revealed that the exogenous LP3 protein localizes exclusively in the nucleus, where it might protect against dehydration (Wang et al. 2002).
Protein structure
Tomato is the species in which ASR proteins have been analyzed most deeply at the structural level. In particular, ASR1 from this species turned out to be a small, highly charged 13-kDa polypeptide rich in Gly (7 %), Ala, (13 %), Glu (15 %), His (15 %), and Lys (17 %) with an isoelectric point of 7.9 (Iusem et al. 1993). It exhibits two distinct domains: a DNA-binding domain located at the carboxy-terminal end, and a zinc-binding domain capable of binding two Zn atoms at the amino-terminal end (Rom et al. 2006).
Recombinant ASR1, purified via Ni2+-affinity chromatography, is an intrinsically unfolded monomer in vitro, as shown by several biophysical methods. However, in the presence of Zn2+ ions, it undergoes a conformational transition from unfolded to folded as it gains more α-helix and β-strand domains, which implies a more highly ordered polypeptide structure (Goldgur et al. 2007). In addition, the expected solubility of ASR1 at high temperatures in the presence of Zn2+ was confirmed by microcalorimetry, where the peak of heat absorbance was detected between 70 and 80 °C (Goldgur et al. 2007), in line with the observed solubility at 90 °C of the ASR ortholog from lily (Wang et al. 1996).
Recently, soybean ASR (GmASR) has also been shown by in vitro intrinsic fluorescence spectroscopic assays (circular dicroism) to bind metal ions like Fe3+, Ni2+ and Cu2+ (Li et al. 2013).
To the best of our knowledge, the only in vivo structural study on an ASR protein is the one reporting ASR1 to self-assemble as homodimers and even displaying more complex quaternary structures (Ricardi et al. 2012).
Classification of ASR proteins
ASR proteins have been independently proposed by Caramelo and Iusem (2009), Battaglia et al. (2008) and Hunault and Jaspard (2010) to constitute a novel group of the LEA (Late Embryogenesis Abundant) superfamily based on: (1) their accumulation in seeds (see “Expression analysis” section), (2) some of their basic structural features mentioned in the previous section and (3) their expression under drought conditions. In this respect, Battaglia and colleagues’ group 7 corresponds to LEAPdb class 12 [PF 10714] in the classification of Hunault and Jaspard (http://forge.info.univ-angers.fr/~gh/Leadb/leaclasses.png). However, the same latter authors do not include ASR proteins in the LEA family in their recent bioinformatic study (Jaspard et al. 2012). Considering that definition of LEA proteins is not always clear-cut, it might be more practical to comply with the Sanger Institute’s Pfam database, which classifies ASRs under the ABA/WDS (ABA/water-deficit stress) category (PF02496). Besides, as ASR proteins have no sequence similarity to the accepted groups of LEA proteins and as their function as transcription factors (see next section) also distinguishes them from the known LEA proteins, we support the view that ASRs are classified as an important group of ABA/WDS proteins with distinct and unique functions and roles.
Molecular function and possible physiological roles
The elusive function of ASR proteins stimulated interesting research through both in vivo and in vitro experiments. For example, ASR1 was reported to bind in vitro to chromatin in a zinc-dependent fashion (Kalifa et al. 2004a). The physical association between DNA and both ASR1 monomers and dimers was directly observed in vitro by Atomic Force Microscopy at the single molecule level (Maskin et al. 2007) and most likely involved the binding to the short consensus DNA sequence previously determined by SELEX (Kalifa et al. 2004a).
It is worth mentioning that the grape (Vitis vinifera) ASR ortholog named VvMSA was claimed to bind to the enhancer of a sugar transporter gene (VvHT1), although the reported regulation was not clear (Cakir et al. 2003). Therefore, a transcription factor function was later proposed (Cakir et al. 2003), as was an ASR protein-mediated cross-talk between ABA and sugars (Carrari et al. 2004).
As far as their role as transcription factors is concerned, it was not until very recently that in vivo (preserving the native chromatin structure) genome-wide Chromatin Immunoprecipitation (ChIP) data were reported in rice (Arenhart et al. 2013a) and tomato (Ricardi et al. 2014). In rice, some of the putative targets of ASR5 in roots are related to Al tolerance like the ABC transporter STAR1 (Arenhart et al. 2013a). On the other hand, the targets of tomato leaf ASR1 turned out to be gene encoding proteins involved in water transport like aquaporins and cell wall remodeling proteins, namely cellulose synthase and glucanases (Ricardi et al. 2014). However, ASR1 does not seem to interact with sugar transporter genes (Ricardi et al. 2014), as reported in grape (Cakir et al. 2003).
In addition to direct targets, some ASR proteins seem to regulate different target genes, albeit indirectly, as revealed by double hybrid assays in yeast. From example, the grape ortholog interacts in vivo with a drought responsive element (DRE)-binding transcription factor to form a heterodimer (Saumonneau et al. 2008). Similarly, tobacco ASR in vivo binds to a transcription factor involved in leaf senescence and floral development (Yang et al. 2012).
Interestingly, ASRs (supposedly the cytosolic pool) might also act as chaperone-like proteins. For example, tomato ASR1 protected reporter enzymes against freezing or heat denaturation in vitro (Konrad and Bar-Zvi 2008). Similar results were found with ASRs from plantain (Dai et al. 2011) and lily (Hsu et al. 2011).
Also worthy of note is the fact that ASR from soybean exhibited a novel biochemical function: an antioxidant activity in vitro, but no model of underlying mechanism was formulated (Li et al. 2013). On the other hand, maize Asr genes seem to be involved in regulating the biosynthesis of branched-chain amino acids such as Val, Leu and Ile (Virlouvet et al. 2011).
At the physiological level, the function of ASRs as sugar transporter regulators was observed in transgenic potato and tobacco plants in which endogenous expression of Asr was artificially silenced. In these cases, glucose transport was altered as a consequence of the changes in the expression pattern of sugar transporter genes, which, in turn, were indirectly caused by the loss of ASR (Dominguez et al. 2013; Frankel et al. 2007).
Physiological effects of Asr genes in Arabidopsis and yeast
Classical model organisms lacking endogenous Asr genes but carrying foreign ones have also been tested at the physiological level. For example, tomato Asr1 was used to transform yeast (S. cerevisiae) strains that lack the MAP kinase hog1 and are, therefore, deficient in the high-osmolarity glycerol pathway. This strain is unable to grow under high salt conditions, but its resistance increased notably when carrying Asr1 through an unknown mechanism (Bermudez-Moretti et al. 2006).
On the other hand, transgenic Arabidopsis plants carrying and overexpressing the Asr gene from lily exhibit reduced sensitivity to ABA, diminished levels of dormancy and increased resistance to salt, osmotic, drought and cold stresses (Yang et al. 2005). Another report on the same species claims that, when transformed with tomato Asr1, individuals exhibited a phenotype similar to abi4 (ABA-insensitive 4) mutants, namely, no sensitivity to ABA-triggered inhibition of seed germination. This behavior resulted from competition between the endogenous transcription factor ABI4 and the exogenous ASR1 for binding to a cis-regulatory DNA sequence (Shkolnik and Bar-Zvi 2008). Arabidopsis plants were also engineered with the plantain (Musa paradisiaca) Asr transgene, which conferred resistance to osmotic stress (Dai et al. 2011).
Phenotypes of transgenic crop plants eventual biotechnological applications
There have been several studies on the heterologous expression of Asr genes in crop plants. The first was carried out by Wang et al. (2002), who produced tobacco expressing the pinus Asr coding sequence driven by its own promoter. However, no phenotypes were reported therein except for expression data described in the corresponding section of this review.
In another study also with tobacco, plants carrying the Asr1 cDNA from tomato driven by the constitutive 35S promoter showed improved tolerance to osmotic stress, likely due to decreased uptake of Na+ ions from the environment (Kalifa et al. 2004b). Leaves from these plants turned out to lose less water than wild type leaves, suggesting regulation at the stomatal level. Similar results were obtained in transgenic tobacco plants bearing the Asr cDNA from the halophyte S. brachiata (Jha et al. 2012). Analogously, when wheat Asr was overexpressed in tobacco, plant tissues showed higher water content and higher catalase and superoxide dismutase [enzymes involved in reactive oxygen species (ROS) detoxification] activities, resulting in a promising high tolerance to water stress (Hu et al. 2013).
In maize, plants constitutively overexpressing an additional copy of their own Asr1 gene exhibited a higher foliar senescence ratio than wild type plants under drought conditions (Jeanneau et al. 2002). The resulting ASR1 accumulation also caused an increase in biomass and yield (Virlouvet et al. 2011), which could be attractive for biotechnological purposes. Also appealing is the case of transgenic rice plants overexpressing an extra copy of their endogenous Asr1, in which a higher tolerance to cold stress was observed in terms of photosynthetic efficiency (Kim et al. 2009).
Potato plants have been the target of genetic transformation as well and those overexpressing tomato Asr1 showed an increase in the concentration of sugars in tubers, allowing for bigger tubercles but reducing their number. Conversely, the silencing of endogenous ci21a in potato plants led to smaller tubers with lower glucose concentrations (Frankel et al. 2007).
Concluding remarks and perspectives
The widespread ASR proteins, which have been studied by many groups worldwide, are only a component of the drought stress response, albeit an important one, making them attractive for interdisciplinary research. As a matter of fact, over the last 20 years, the field has drawn the attention of physiologists, biochemists, biophysicists, evolutionary biologists and bioinformaticians as well as experts on genomics and plant biotechnology.
Making a critical appraisal of the progress made up to date on the subject, in vivo structural approaches are still scarce (Ricardi et al. 2012) and should be considered for future studies to deepen our understanding of the chaperone-like functions of ASR proteins by revealing details of the changes in their 3-D structure resulting from stress (Caramelo and Iusem 2009). Concerning the role of ASRs as transcription factors, the recent and long-awaited breakthrough on the discovery of their target genes (Ricardi et al. 2014; Arenhart et al. 2013b), combined with further research, surely will gain insight into both the early environmental stress-sensing molecular events triggered by ABA and the late physiological adjustments that finally confer drought tolerance.
From an applied perspective, despite a natural skepticism towards engineering drought tolerance using a single transgene (Caramelo and Iusem 2009), we were able to compile several promising examples of ASR proteins that improve drought resistance in the laboratory. Nevertheless, it is imperative to perform field tests to ascertain the agronomic usefulness of the reported transgenic plants.
Finally, we express our apologies to those whose work could not be cited due to space limitations.
References
Amitai-Zeigerson H, Scolnik PA, Bar-Zvi D (1995) Tomato Asr1 mRNA and protein are transiently expressed following salt stress, osmotic stress and treatment with abscisic acid. Plant Sci 110:205–213
Arenhart RA, Margis R, Margis-Pinheiro M (2012) The rice ASR5 protein: a putative role in the response to aluminium photosynthesis disturbance. Plant Signal Behav 7:1263–1266
Arenhart RA, Bai Y, Valter de Oliveira LF, Bucker Neto L, Schunemann M, Maraschin FD, Mariath J, Silverio A, Sachetto-Martins G, Margis R, Wang ZY, Margis-Pinheiro M (2013a) New Insights into Aluminum Tolerance in Rice: The ASR5 Protein Binds the STAR1 Promoter and Other Aluminum-Responsive Genes. Mol Plant [Epub ahead of print]
Arenhart RA, Lima JC, Pedron M, Carvalho FE, Silveira JA, Rosa SB, Caverzan A, Andrade CM, Schunemann M, Margis R, Margis-Pinheiro M (2013b) Involvement of ASR genes in aluminium tolerance mechanisms in rice. Plant Cell Environ 36:52–67
Battaglia M, Olvera-Carrillo Y, Garciarrubio A, Campos F, Covarrubias AA (2008) The enigmatic LEA proteins and other hydrophilins. Plant Physiol 148:6–24
Bazargani MM, Sarhadi E, Bushehri AA, Matros A, Mock HP, Naghavi MR, Hajihoseini V, Mardi M, Hajirezaei MR, Moradi F, Ehdaie B, Salekdeh GH (2011) A proteomics view on the role of drought-induced senescence and oxidative stress defense in enhanced stem reserves remobilization in wheat. J Proteomics 74:1959–1973
Bermudez-Moretti M, Maskin L, Gudesblat G, García SC, Iusem ND (2006) ASR1, a stress-induced tomato protein, protects yeast from osmotic stress. Physiol Plant 127:111–118
Cakir B, Agasse A, Gaillard C, Saumonneau A, Delrot S, Atanassova R (2003) A grape ASR protein involved in sugar and abscisic acid signaling. Plant Cell 15:2165–2180
Caramelo JJ, Iusem ND (2009) When cells lose water: lessons from biophysics and molecular biology. Prog Biophys Mol Biol 99:1–6
Carrari F, Fernie AR, Iusem ND (2004) Heard it through the grapevine? ABA and sugar cross-talk: the ASR story. Trends Plant Sci 9:57–59
Cortes AJ, Chavarro MC, Madrinan S, This D, Blair MW (2012) Molecular ecology and selection in the drought-related Asr gene polymorphisms in wild and cultivated common bean (Phaseolus vulgaris L.). BMC Genet 13:58
Dai JR, Liu B, Feng DR, Liu HY, He YM, Qi KB, Wang HB, Wang JF (2011) MpAsr encodes an intrinsically unstructured protein and enhances osmotic tolerance in transgenic Arabidopsis. Plant Cell Rep 30:1219–1230
Dóczi R, Csanaki C, Bánfalvi Z (2002) Expression and promoter activity of the desiccation-specific Solanum tuberosum gene, StDS2. Plant Cell Environ 25:1197–1203
Dominguez PG, Frankel N, Mazuch J, Balbo I, Iusem N, Fernie AR, Carrari F (2013) ASR1 mediates glucose-hormone cross talk by affecting sugar trafficking in tobacco plants. Plant Physiol 161:1486–1500
Fischer I, Camus-Kulandaivelu L, Allal F, Stephan W (2011) Adaptation to drought in two wild tomato species: the evolution of the Asr gene family. New Phytol 190:1032–1044
Fischer I, Steige KA, Stephan W, Mboup M (2013) Sequence evolution and expression regulation of stress-responsive genes in natural populations of wild tomato. PLoS ONE 8:e78182
Frankel N, Hasson E, Iusem ND, Rossi MS (2003) Adaptive evolution of the water stress-induced gene Asr2 in Lycopersicon species dwelling in arid habitats. Mol Biol Evol 20:1955–1962
Frankel N, Carrari F, Hasson E, Iusem ND (2006) Evolutionary history of the Asr gene family. Gene 378:74–83
Frankel N, Nunes-Nesi A, Balbo I, Mazuch J, Centeno D, Iusem ND, Fernie AR, Carrari F (2007) ci21A/Asr1 expression influences glucose accumulation in potato tubers. Plant Mol Biol 63:719–730
Giombini MI, Frankel N, Iusem ND, Hasson E (2009) Nucleotide polymorphism in the drought responsive gene Asr2 in wild populations of tomato. Genetica 136:13–25
Goldgur Y, Rom S, Ghirlando R, Shkolnik D, Shadrin N, Konrad Z, Bar-Zvi D (2007) Desiccation and zinc binding induce transition of tomato abscisic acid stress ripening 1, a water stress- and salt stress-regulated plant-specific protein, from unfolded to folded state. Plant Physiol 143:617–628
Gonzalez RM, Ricardi MM, Iusem ND (2011) Atypical epigenetic mark in an atypical location: cytosine methylation at asymmetric (CNN) sites within the body of a non-repetitive tomato gene. BMC Plant Biol 11:94
Gonzalez RM, Ricardi MM, Iusem ND (2013) Epigenetic marks in an adaptive water stress-responsive gene in tomato roots under normal and drought conditions. Epigenetics 8(8):864–872
Henry IM, Carpentier SC, Pampurova S, Van Hoylandt A, Panis B, Swennen R, Remy S (2011) Structure and regulation of the Asr gene family in banana. Planta 234:785–798
Hsu YF, Yu SC, Yang CY, Wang CS (2011) Lily ASR protein-conferred cold and freezing resistance in Arabidopsis. Plant Physiol Biochem 49:937–945
Hu W, Huang C, Deng X, Zhou S, Chen L, Li Y, Wang C, Ma Z, Yuan Q, Wang Y, Cai R, Liang X, Yang G, He G (2013) TaASR1, a transcription factor gene in wheat, confers drought stress tolerance in transgenic tobacco. Plant Cell Environ 36:1449–1464
Huang JC, Lin SM, Wang CS (2000) A pollen-specific and desiccation-associated transcript in Lilium longiflorum during development and stress. Plant Cell Physiol 41:477–485
Hunault G, Jaspard E (2010) LEAPdb: a database for the late embryogenesis abundant proteins. BMC Genom 11:221
Iusem ND, Bartholomew DM, Hitz WD, Scolnik PA (1993) Tomato (Lycopersicon esculentum) transcript induced by water deficit and ripening. Plant Physiol 102:1353–1354
Jaspard E, Macherel D, Hunault G (2012) Computational and statistical analyses of amino acid usage and physico-chemical properties of the twelve late embryogenesis abundant protein classes. PLoS ONE 7(5):e36968
Jeanneau M, Gerentes D, Foueillassar X, Zivy M, Vidal J, Toppan A, Perez P (2002) Improvement of drought tolerance in maize: towards the functional validation of the Zm-Asr1 gene and increase of water use efficiency by over-expressing C4-PEPC. Biochimie 84:1127–1135
Jha B, Agarwal PK, Reddy PS, Lal S, Sopory SK, Reddy MK (2009) Identification of salt-induced genes from Salicornia brachiata, an extreme halophyte through expressed sequence tags analysis. Genes Genet Syst 84:111–120
Jha B, Lal S, Tiwari V, Yadav SK, Agarwal PK (2012) The SbASR-1 gene cloned from an extreme halophyte Salicornia brachiata enhances salt tolerance in transgenic tobacco. Mar Biotechnol (NY) 14:782–792
Joo J, Lee Y, Choi D, Cheong J-J, Kim Y-K, Song S (2013a) Rice ASR1 has function in abiotic stress tolerance during early growth stages of rice. J Korean Soc Appl Biol Chem 56:349–352
Joo J, Lee Y, Kim Y-K, Nahm B, Song S (2013b) Abiotic stress responsive rice ASR1 and ASR3 exhibit different tissue-dependent sugar and hormone-sensitivities. Mol Cells 35:421–435
Kalifa Y, Gilad A, Konrad Z, Zaccai M, Scolnik PA, Bar-Zvi D (2004a) The water- and salt-stress-regulated Asr1 (abscisic acid stress ripening) gene encodes a zinc-dependent DNA-binding protein. Biochem J 381:373–378
Kalifa Y, Perlson E, Gilad A, Konrad Z, Scolnik PA, Bar-Zvi D (2004b) Over-expression of the water and salt stress-regulated Asr1 gene confers an increased salt tolerance. Plant Cell Environ 27:1459–1468
Kim SJ, Lee SC, Hong SK, An K, An G, Kim SR (2009) Ectopic expression of a cold-responsive OsAsr1 cDNA gives enhanced cold tolerance in transgenic rice plants. Mol Cells 27:449–458
Konrad Z, Bar-Zvi D (2008) Synergism between the chaperone-like activity of the stress regulated ASR1 protein and the osmolyte glycine-betaine. Planta 227:1213–1219
Li RH, Liu GB, Wang H, Zheng YZ (2013) Effects of Fe3+ and Zn2+ on the structural and thermodynamic properties of a soybean ASR protein. Biosci Biotechnol Biochem 77:475–481
Maskin L, Gudesblat GE, Moreno JE, Carrari FO, Ns Frankel, An Sambade, Rossi M, Iusem ND (2001) Differential expression of the members of the Asr gene family in tomato (Lycopersicon esculentum). Plant Sci 161:739–746
Maskin L, Frankel N, Gudesblat G, Demergasso MJ, Pietrasanta LI, Iusem ND (2007) Dimerization and DNA-binding of ASR1, a small hydrophilic protein abundant in plant tissues suffering from water loss. Biochem Biophys Res Commun 352:831–835
Maskin L, Maldonado S, Iusem ND (2008) Tomato leaf spatial expression of stress-induced Asr genes. Mol Biol Rep 35:501–505
Padmanabhan V, Dias DM, Newton RJ (1997) Expression analysis of a gene family in loblolly pine (Pinus taeda L.) induced by water deficit stress. Plant Mol Biol 35:801–807
Perez-Diaz J, Wu TM, Perez-Diaz R, Ruiz-Lara S, Hong CY, Casaretto JA (2014) Organ- and stress-specific expression of the ASR genes in rice. Plant Cell Rep 33(1):61–73
Philippe R, Courtois B, McNally KL, Mournet P, El-Malki R, Le Paslier MC, Fabre D, Billot C, Brunel D, Glaszmann JC, This D (2010) Structure, allelic diversity and selection of Asr genes, candidate for drought tolerance, in Oryza sativa L. and wild relatives. Theor Appl Genet 121:769–787
Ricardi MM, Guaimas FF, Gonzalez RM, Burrieza HP, Lopez-Fernandez MP, Jares-Erijman EA, Estevez JM, Iusem ND (2012) Nuclear import and dimerization of tomato ASR1, a water stress-inducible protein exclusive to plants. PLoS ONE 7:e41008
Ricardi MM, González RM, Zhong S, Domínguez PG, Duffy T, Turjanski PG, Salgado Salter JD, Alleva K, Carrari F, Giovannoni JJ, Estévez JM, Iusem ND (2014) Genome-wide data (ChIP-seq) enabled identification of cell wall-related and aquaporin genes as targets of tomato ASR1, a drought stress-responsive transcription factor. BMC Plant Biol 14(1):29
Rom S, Gilad A, Kalifa Y, Konrad Z, Karpasas MM, Goldgur Y, Bar-Zvi D (2006) Mapping the DNA- and zinc-binding domains of ASR1 (abscisic acid stress ripening), an abiotic-stress regulated plant specific protein. Biochimie 88:621–628
Rossi M, Iusem ND (1994) Tomato (Lycopersicon esculentum) genomic clone homologous to a gene encoding an abscisic acid-induced protein. Plant Physiol 104:1073–1074
Rossi M, Iusem ND (1995) Sequence of Asr2, a member of a gene family from Lycopersicon esculentum encoding chromosomal proteins: homology to an intron of the polygalacturonase gene. DNA Seq 5:225–227
Rossi M, Lijavetzky D, Bernacchi D, Hopp HE, Iusem N (1996) Asr genes belong to a gene family comprising at least three closely linked loci on chromosome 4 in tomato. Mol Gen Genet 252:489–492
Rossi M, Carrari F, Cabrera-Ponce JL, Vazquez-Rovere C, Herrera-Estrella L, Gudesblat G, Iusem ND (1998) Analysis of an abscisic acid (ABA)-responsive gene promoter belonging to the Asr gene family from tomato in homologous and heterologous systems. Mol Gen Genet 258:1–8
Saumonneau A, Agasse A, Bidoyen MT, Lallemand M, Cantereau A, Medici A, Laloi M, Atanassova R (2008) Interaction of grape ASR proteins with a DREB transcription factor in the nucleus. FEBS Lett 582:3281–3287
Shkolnik D, Bar-Zvi D (2008) Tomato ASR1 abrogates the response to abscisic acid and glucose in Arabidopsis by competing with ABI4 for DNA binding. Plant Biotechnol J 6:368–378
Silhavy D, Hutvagner G, Barta E, Banfalvi Z (1995) Isolation and characterization of a water-stress-inducible cDNA clone from Solanum chacoense. Plant Mol Biol 27:587–595
Takasaki H, Mahmood T, Matsuoka M, Matsumoto H, Komatsu S (2008) Identification and characterization of a gibberellin-regulated protein, which is ASR5, in the basal region of rice leaf sheaths. Mol Genet Genomics 279:359–370
Urtasun N, Correa Garcia S, Iusem ND, Bermudez Moretti M (2010) Predominantly cytoplasmic localization in yeast of ASR1, a non-receptor transcription factor from plants. Open Biochem J 4:68–71
Virlouvet L, Jacquemot MP, Gerentes D, Corti H, Bouton S, Gilard F, Valot B, Trouverie J, Tcherkez G, Falque M, Damerval C, Rogowsky P, Perez P, Noctor G, Zivy M, Coursol S (2011) The ZmASR1 protein influences branched-chain amino acid biosynthesis and maintains kernel yield in maize under water-limited conditions. Plant Physiol 157:917–936
Wang C-S, Wu T-D, Chung C-KW, Lord EM (1996) Two classes of pollen-specific, heat-stable proteins in Lilium longiflorum. Physiol Plant 97:643–650
Wang J-T, Gould J, Padmanabhan V, Newton R (2002) Analysis and localization of the water-deficit stress-induced gene (lp3). J Plant Growth Regul 21:469–478
Wang H-J, Hsu C-M, Jauh GY, Wang C-S (2005) A lily pollen ASR protein localizes to both cytoplasm and nuclei requiring a nuclear localization signal. Physiol Plant 123:314–320
Wang CS, Hsu SW, Hsu YF (2013) New insights into desiccation-associated gene regulation by Lilium longiflorum ASR during pollen maturation and in transgenic Arabidopsis. Int Rev Cell Mol Biol 301:37–94
Yang CY, Chen YC, Jauh GY, Wang CS (2005) A Lily ASR protein involves abscisic acid signaling and confers drought and salt resistance in Arabidopsis. Plant Physiol 139:836–846
Yang CY, Wu CH, Jauh GY, Huang JC, Lin CC, Wang CS (2008) The LLA23 protein translocates into nuclei shortly before desiccation in developing pollen grains and regulates gene expression in Arabidopsis. Protoplasma 233:241–254
Yang SH, Kim SH, Berberich T, Kusano T (2012) Identification and properties of a small protein that interacts with a tobacco bZIP-type transcription factor TBZF. Plant Biotechnol 29:395–399
Acknowledgments
The authors are indebted to the following Argentinian institutions: CONICET (Consejo Nacional de Investigaciones Científicas y Tecnológicas) for salaries, fellowships and grants, ANPCyT (Agencia Nacional de Promoción de Ciencia y Tecnología) for grants and UBA (the University of Buenos Aires) for salaries and grants. The authors are also thankful to the Editor and the anonymous reviewers for constructive comments and suggestions.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
González, R.M., Iusem, N.D. Twenty years of research on Asr (ABA-stress-ripening) genes and proteins. Planta 239, 941–949 (2014). https://doi.org/10.1007/s00425-014-2039-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00425-014-2039-9