Abstract
Physcomitrella patens is a model bryophyte representing an early land plant in the green plant lineage. This organism possesses many advantages as a model organism. Its genome has been sequenced, its predominant life cycle stage is the haploid gametophyte, it is readily transformable and it can integrate transformed DNA into its genome by homologous recombination. One limitation for the use of P. patens in photosynthesis research is its reported inability to grow photoheterotrophically, in the presence of sucrose and the Photosystem II inhibitor 3-(3,4-dichlorophenyl)-1,1-dimethylurea, which prevents linear photosynthetic electron transport. In this communication we describe the facile isolation of a P. patens strain which can grow photoheterotrophically. Additionally, we have examined a number of photosynthetic parameters for this strain grown under photoautotrophic, mixotrophic (in the presence of sucrose) and photoheterotrophic conditions, as well as the 3-(3,4-dichlorophenyl)-1,1-dimethylurea-inhibited state. The ability to grow P. patens photoheterotrophically should significantly facilitate its use in photosynthetic studies.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The moss Physcomitrella patens is an important developing model organism. As a bryophyte, it represents a non-vascular land plant, the earliest representatives of which were present at least 350–400 million years ago (Hueber 1961). The predominant life cycle stage of these organisms is the haploid gametophyte. The P. patens genome has been sequenced (Rensing et al. 2008), the organism is transformable and can integrate exogenous DNA into its genome via homologous recombination (Schaefer and Zrÿd 1997). The ability to specifically target genes for knockout and replacement has proved a very useful characteristic of this organism. Recently, P. patens has been used in an increasing number of studies examining plant evolution, physiology and metabolism (Cove 2005; Cove et al. 2006).
One limitation on the use of P. patens in the field of photosynthesis is its reported inability to grow heterotrophically (Thornton et al. 2005), i.e. in the absence of linear chain photosynthetic electron transport when supplied with a carbon source. These authors observed no growth of P. patens when gametophytic explants were transferred to media containing 3-(3,4-dichlorophenyl)-1,1-dimethylurea (DCMU) and 0.5 % glucose. They also tested a number of other carbon sources, none of which could apparently support heterotrophic growth. The two organisms which have proved, arguably, among the most useful for photosynthesis research, are the cyanobacterium Synechocystis sp. PCC 6803 (henceforth Synechocystis 6803) and Chlamydomonas reinhardtii (henceforth Chlamydomonas); both can utilize exogenous carbon sources to support growth in the absence of linear chain electron transport. Synechocystis can be cultured photoheterotrophically in the presence of the Photosystem II (PS II) herbicide DCMU when supplied with glucose (Williams 1988). This has allowed the isolation of numerous PS II mutants (Williams 1988; Vermaas et al. 1987; Bricker et al. 1998; Pakrasi et al. 1989). This organism can also grow very slowly via light-activated heterotrophic growth on glucose in darkness if supplied with a brief pulse of blue light on a daily basis (Anderson and McIntosh 1991). Chlamydomonas can grow heterotrophically in the dark when supplied with acetate (Rochaix 1987). Mutants completely lacking PS II have also been isolated and characterized in this organism (de Vitry et al. 1989). Consequently, the ability to grow heterotrophically allows the genetic manipulation of genes involved in photosynthesis and the recovery of otherwise lethal mutations in the photosynthetic apparatus.
Interestingly, a number of reports have indicated that P. patens can utilize sucrose to supplement photosynthetic growth. For instance, gametophytic tissue grown in the presence of sucrose grows more rapidly than in its absence (Frank et al. 2005). Additionally, if sucrose is provided under dark growth conditions, the growth of caulonemal tissue, which contains few chloroplasts, is enhanced, while the growth of chloronemal tissue, which contains abundant chloroplasts, is suppressed (Cove et al. 1978). Under low light intensities or very short day lengths the presence of sucrose enhances the growth rate of chloronemal tissue (Cove et al. 1978). Finally, putative sucrose uptake transporters are present in the P. patens genome. Two type IIA and three type III sucrose transporters appear to be present, although their subcellular localization and physiological roles have yet to be determined. It has been suggested that sucrose uptake transporters may be important for scavenging sucrose from the environment in non-vascular plants (Reinders et al. 2012). All of these studies seem to indicate that some capacity for the utilization of exogenously supplied sucrose is present in wild-type P. patens.
Materials and methods
Plant materials and growth conditions
Physcomitrella patens, strain Gradsen was maintained on agar plates containing BCD medium (Cove et al. 2009) + 1 % sucrose prior to the initiation of these experiments. For the examination of growth under different culture conditions, 1.5–2 mm gametophytic explants were transferred to agar plates containing BCD medium (photoautotrophic growth), BCD medium + 1 % sucrose (mixotrophic growth), BCD medium + 1 % sucrose + 10 μM DCMU (photoheterotrophic growth) or BCD medium + 10 μM DCMU (PS II-inhibited). The growth temperature was 22 °C with continuous illumination (60 μmol photons m−2 s−1). Except where indicated, plants were grown for 5–12 weeks prior to characterization.
Fluorescence and spectroscopic measurements
For all of the fluorescence and spectroscopic measurements, gametophytes were dark-incubated for 5 min before initiation of the experiments. OJIP fluorescence induction and non-photochemical quenching (NPQ) measurements were performed using a Photon Systems Instruments FluorCam 800MF. Both measuring and saturating flashes are provided by computer-controlled photodiode arrays. Data analysis was performed using proprietary Photon Systems Instruments software. The steady-state P700 and cytochrome f measurements were performed using a Joliot-Type Spectrophotometer (JTS-10, Bio-Logic Scientific Instruments) operating in the absorbance mode using the ‘pulse of dark’ method. For the P700 measurements, samples were illuminated with a broadband actinic orange light source with a peak of 630 nm for 5 s illumination and the absorbance changes at 705 nm monitored P700 oxidation. At the end of the 5 s actinic illumination period, P700 + was reduced in the dark. The broadband actinic light source excites both PS II and PS I. For the cytochrome f measurements the same actinic illumination protocol was used and absorbance data were collected at 546, 554, 563 and 573 nm. Data were analyzed using proprietary software provided by Bio-Logic Scientific Instruments and Origin version 6.1 (OriginLab, Corp.)
Electrophoresis and protein detection
Thylakoids from gametophytes were isolated by grinding in a glass homogenizer using a buffer containing 20 mM Tricine–NaOH, pH 8.4, 0.45 M sorbitol, 10 mM EDTA, 0.1 % BSA and 1 % polyvinylpyrrolidone. The homogenate was filtered through two layers of Miracloth (Calbiochemical Co.) and the thylakoids pelleted at 2,500×g for 5 min. The thylakoid pellet was resuspended in a small volume of 0.3 M sorbitol, 20 mM Tricine–NaOH, pH 7.6 and 5 mM MgCl2 and frozen at −80 °C until use. The chl concentration was determined by the method of Arnon (1949). Lithium dodecyl sulfate polyacrylamide gel electrophoresis (LiDS-PAGE) was performed under conditions described by Delepelaire and Chua (Delepelaire and Chua 1979) using gradient 12.5–20 % polyacrylamide gels. The resolved polypeptides were electroblotted onto PVDF membranes (Immobilon-P, Millipore Corp.). After blocking for 2 h with 5 % nonfat dry milk in TS buffer (150 mM NaCl, 10 mM Tris–HCl, pH 7.4), the blots were washed extensively with TS buffer and then incubated with diluted primary antibody in TS buffer + 1 % bovine serum albumin overnight. This was followed by washing in TS buffer and incubation with either anti-rabbit or anti-mouse IgG-peroxidase conjugate (Sigma) diluted in TS buffer + 1 % bovine serum albumin. After washing in TS buffer, the labeled protein was detected using chemiluminescence (Super Signal West Pico, Pierce Chemical Co.) and semi-quantified as described previously (Yi et al. 2009).
Results and discussion
Initially, we were able to fully replicate the results of Thornton et al. (2005). In the presence of 10 μM DCMU, no growth of gametophytic tissue was observed on plates supplemented with glucose or a variety of other carbon sources (fructose, sucrose, acetate or pyruvate, all at three different concentrations, data not shown). These cultures were all started from parent cultures maintained autotrophically on standard BCD media in the absence of any additional carbon source.
We hypothesized that the use of sucrose by gametophytic tissue might require acclimatization to the exogenously supplied sugar. Consequently, P. patens cultures were maintained for 1 month on BCD medium + 1 % sucrose. Explants of gametophytic tissue (≈2 mm) grown in this manner were then plated onto various media. The results, after 8 weeks of incubation, are shown in Fig. 1. Luxuriant growth was observed on BCD medium, alone (photoautotrophic growth, Fig. 1a) and on BCD medium supplemented with 1 % sucrose (mixotrophic growth, Fig. 1b). Interestingly, significant gametophytic growth was also observed for BCD medium containing 1 % sucrose + 10 μM DCMU (photoheterotrophic growth, Fig. 1c). No growth was observed on BCD medium containing 10 μM DCMU in the absence of sucrose (DCMU-inhibited state, Fig. 1d). It should be noted that microscopic observation of the explant borders of the photoheterotrophically grown tissue indicated that new chloronemal filaments were being formed as early as 1 week after transplantation (data not shown). Clearly the extent of the apparent photoheterotrophic growth of P. patens (Fig. 1c) is lower than observed for either photoautotrophically (Fig. 1a) or mixotrophically (Fig. 1b) grown cultures. This was not unexpected. Both, the photoheterotrophic growth of Synechocystis 6803 on glucose and the heterotrophic growth of Chlamydomonas on acetate also are much slower than observed for photoautotrophically or mixotrophically grown cultures.
Illustrated is the growth of 1.5–2 mm gametophytic explants after 8 weeks; all panels are at the same magnification. a Photoautotrophic growth, b mixotrophic growth, c photoheterotrophic growth and d PS II-inhibited gametophytes. At 8 weeks, the DCMU-inhibited gametophytes have fully bleached and are apparently dead. Clearly, photoheterotrophic growth is slower than photoautotrophic or mixotrophic growth conditions. This was fully expected and is similar to that observed in Synechocystis 6803 and Chlamydomonas grown under analogous growth conditions
Figure 2 shows the general morphology of the gametophytic tissue under these various growth conditions. The gametophylls of photoautotrophically (Fig. 2a), mixotrophically (Fig. 2b) and photoheterotrophically (Fig. 2c) grown tissue all appear very similar. These are typically bright green and have the same general shape. However, those from mixotrophically grown tissue are somewhat larger than those grown autotrophically; the gametophylls from photoheterotrophically grown tissue are quite small. The DCMU-inhibited tissue (Fig. 2d) is bleached and, as noted above, no growth was observed.
Details of the appearance of gametophytes cultured under the various growth conditions; all panels are at the same magnification. a Photoautotrophic growth, b mixotrophic growth, c photoheterotrophic growth and d PS II-inhibited gametophytes. The gametophylls of the photoheterotrophically grown gametophytes are smaller than those grown under photoautotrophic or mixotrophic conditions
These results indicated that P. patens was capable of growth on media containing sucrose in the presence of DCMU. This apparent photoheterotrophic growth could, however, be due to a spontaneous mutation which yielded DCMU-resistant mutant gametophytes. Such mutants have been isolated in the D1 protein of PS II, which bears the Q B binding site and are known in both Synechocystis 6803 (Bouyoub et al. 1993; Dalla-Chiesa et al. 1997) and Chlamydomonas (Galloway and Mets 1982; Erickson et al. 1984). It is also formally possible that in the presence of sucrose, DCMU uptake is inhibited. In either of these instances it would be expected that the tissue exhibiting apparent photoheterotrophic growth would possess relatively normal PS II electron transport characteristics, with electrons being transported to Q B, the plastoquinone pool and beyond even in the presence of DCMU. Table 1 shows the analysis of an OJIP fluorescence induction experiment (Strasser et al. 2000) performed on P. patens grown for 12 weeks under the different test conditions. Please note that after 12 weeks, gametophytes transferred to the DCMU-inhibited medium were bleached and dead (Fig. 1d). For the fluorescence induction experiment, gametophytes were characterized after 2 weeks of incubation on this medium. At this time point, these gametophytes had not bleached and were still quite green.
Only modest differences are evident between autotrophically and mixotrophically grown gametophytes. While the quantum yield for energy trapping by PS II (F v/F M) is slightly higher in the mixotrophically grown gametophytes, the absorption of photons on a reaction center basis (ABS/RC), their trapping by PS II (TR/RC), electron transport beyond Q −A (ET/RC) and energy dissipation (DI/RC) are all somewhat higher in the gametophytes grown autotrophically. No differences were observed for the quantum yield of electron transport (φ Eo) or the efficiency of a trapped photon leading to productive electron transport past Q −A (Ψ0). Overall, the differences between the fluorescence parameters observed under these two different growth conditions are quite small. The gametophytes grown under photoheterotrophic conditions and the DCMU-inhibited gametophytes, however, exhibited highly altered fluorescence kinetics. The ET0/RC and Ψ0 is ≈0 under these conditions, indicating that in the presence of DCMU, no electron transport can occur past Q −A . The ABS/RC and DI/RC were very high. The small apparent number of fully functional reaction centers, indicated by a very low F v/F M, leads to these high values. Clearly, the gametophytes grown under photoheterotrophic conditions and the DCMU-inhibited gametophytes exhibit very similar fluorescence characteristics. These fluorescence parameters indicate that the photoheterotrophically grown and the DCMU-inhibited gametophytes have no capacity for electron transport past Q −A , and electron transport appears fully inhibited by DCMU. Consequently, true photoheterotrophic growth has been observed in Physcomitrella.
The observation of a low F v/F M in gametophytes grown in the presence of DCMU is quite interesting. This could be due to a loss of variable fluorescence brought about by either an increase in F 0, a decrease in F M, or a combination of these two conditions. Figure 3 illustrates the unprocessed fluorescence data obtained from the different growth states. Both the autotrophically and mixotrophically grown gametophytes exhibited relatively high F M and relatively low F 0 values. The photoheterotrophically grown and DCMU-inhibited gametophytes exhibited a similarly high F M as observed under the other growth conditions; however, these also exhibited a high F 0. These results indicate that while a similar number of reaction centers were present under all growth conditions, the number of reaction centers which could carry out successful charge separation was much smaller in the gametophytes grown photoheterotrophically and those which were DCMU-inhibited.
Raw fluorescence data for the gametophytes grown under the different growth conditions. Autotrophically and mixotrophically grown gametophytes exhibited a low initial level of fluorescence (F 0) and a high level of maximal fluorescence (F M). While the photoheterotrophically grown and DCMU-inhibited gametophytes also exhibited a relatively high F M, these had a high F 0. This leads to a low F V/F M value in these gametophytes. Plotted are means ± 1 SD, n = 9; light gray, F 0; dark gray, F M. For reference, the F v/F M values are shown below
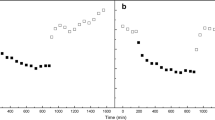
We also examined the function of PS I and the cytochrome b 6/f complex under the different growth conditions. Figure 4 illustrates the steady-state oxidation (Fig. 4a) and reduction (Fig. 4b) kinetics of P700. The steady-state oxidation kinetics are very similar for the gametophytes grown under all conditions. These results indicate that PS I charge separation was not perturbed under mixotrophic or photoheterotrophic growth conditions. Autotrophically and mixotrophically grown gametophytes exhibited similar rates of P700 + reduction, while this was markedly slowed under photoheterotrophic growth conditions. This was expected since in the presence of DCMU both the plastoquinone and the plastocyanin pools are in a predominately oxidized state; consequently only a small amount of reduced plastocyanin is available to donate electrons to PS I. Similar results were observed for the cytochrome b 6/f complex as monitored by steady-state cytochrome f oxidation (Fig. 4c) and reduction (Fig. 4d). The rate of cytochrome f oxidation was similar under all growth conditions while the reduction of oxidized cytochrome f was seriously retarded during photoheterotrophic growth, again due to the oxidized plastoquinone pool present under photoheterotrophic growth conditions. The rates of oxidation and reduction for both P700 and cytochrome f are summarized in Table 2.
Steady-state P700 and cytochrome f oxidation and reduction kinetics. For the P700 measurements (a, b), orange actinic light (560 μmol photons m−2 s−1) was provided and the relative absorption was measured at 705 nm. For the cytochrome f measurements (c, d), orange actinic light (560 μmol photons m−2 s−1) was provided and relative absorption was measured at 546, 554, 563 and 573 nm. These data were then deconvoluted to yield the cytochrome f absorption. Data were normalized to the value at time 0 representing the onset of actinic illumination (a, c) and at 5 s representing the cessation of actinic illumination (b, d)
It is possible that the presence of sucrose during mixotrophic growth could lead to an alteration in the rate or extent of NPQ development. This could theoretically occur if there were either a direct or indirect redox coupling between the respiratory and photosynthetic electron transport chains. Figure 5 illustrates the result of an NPQ experiment performed at a moderate (365 μmol photons m2 s) actinic light intensity. Both the development of NPQ upon onset of the actinic illumination and its dissipation upon cessation of illumination were nearly identical in both the autotrophically and mixotrophically grown gametophytes. It should be noted that no NPQ development was observed in the photoheterotrophically grown gametophytes (data not shown). These results indicate that no effect on NPQ was evident under mixotrophic growth conditions.
NPQ development and relaxation of autotrophically and mixotrophically grown gametophytes. Measurements were taken at an actinic light intensity of 365 μmol photons m−2 s−1. Arrows indicate actinic light on and off. Photoheterotrophically grown and DCMU-inhibited gametophytes exhibited no NPQ. Plotted are means ± 1 SD; n = 9
Finally, a number of thylakoid proteins associated with the different photosynthetic electron transport complexes were also examined under the various growth conditions (Fig. 6). In most instances, the examined proteins accumulated to somewhat lower levels (50–80 %) under mixotrophic and photoheterotrophic conditions when compared with autotrophically grown gametophytes. One exception was the AtpB protein, which accumulated to high levels during photoheterotrophic growth. This observation may indicate that the chloroplast ATP synthase accumulates under this growth condition. This, however, must be tested. While it is clear that genetic disruption of individual subunits of the ATP synthase leads to a coordinated down-regulation of the other subunits (Dal Bosco et al. 2004; Drapier et al. 1992; Gatenby et al. 1989), the converse may not be generally true. Indeed, overexpression of the γ-subunit does not lead to increased abundances of other ATP synthase subunits (Dal Bosco et al. 2004).
Thylakoid proteins from autotrophically, mixotrophically and photoheterotrophically grown gametophytes were separated by LiDS-PAGE and identified by immunoblotting with specific antibodies. Left panel dilutions of the proteins from autotrophically grown gametophytes; right panel proteins from mixotrophically and photoheterotrophically grown gametophytes. The 100 % lanes contain 3 μg chlorophyll
Conclusions
We have demonstrated the ability to grow P. patens gametophytes photoheterotrophically in the presence of sucrose as a carbon source and the PS II inhibitor DCMU. Our studies indicate that successful photoheterotrophic growth requires prior acclimatization of P. patens to growth on sucrose-containing medium. Earlier, it had been shown that the moss Ceratodon purpureus can grow photoheterotrophically using glucose as a carbon source (Thornton et al. 2005). The use of C. purpureus to examine photosynthetic processes, however, is problematic. The draft genomic sequence is currently in the analysis stage and has not yet been released. Additionally, the molecular tools for manipulating genes in C. purpureus are not as well developed as those for P. patens. More critically, the function and/or stability of PS II appears to be different in C. purpureus than in P. patens and all other oxygenic photosynthetic organisms which have been studied. All wild-type organisms previously examined in flash oxygen yield experiments including higher plants (Joliot et al. 1969; Kok et al. 1970), cyanobacteria (Burnap et al. 1992), green algae (Jursinic 1979) and the moss P. patens (Thornton et al. 2005) exhibit a maximum oxygen yield on the third saturating flash after an extended dark incubation. This indicates that the oxygen-evolving complex is predominantly in the S1 oxidation state (Kok et al. 1970). C. purpureus, however, exhibits a maximum yield on the fifth flash (Thornton et al. 2005). Taken at face value this indicates that the oxygen-evolving complex is in the S-1 state, usually associated with damaged oxygen-evolving complexes. Also, examination of the flash oxygen yield pattern for C. purpureus (Thornton et al. 2005) indicates that its oxygen yield oscillations are much more highly dampened than observed for other oxygenic organisms. This result indicates misses, double hits or deactivations (or a combination of these) are more prevalent in C. purpureus than in other oxygenic organisms. The basis for these differences is unknown although one can speculate that PS II from C. purpureus is more labile during thylakoid membrane isolation.
Our results indicate that during photoautotrophic and mixotrophic growth the photosynthetic characteristics of P. patens are very similar. The fluorescence characteristics (primarily probing PS II), as well as oxidation and reduction rates of both cytochrome f and P700, and the rate of development and dissipation of NPQ all indicate that only small differences exist between these two growth states. The observed differences in the protein composition of the thylakoid membrane are also quite modest. The differences observed for gametophytes grown under photoheterotrophic conditions are all consistent with loss of PS II function in the presence of DCMU. This is highlighted by the similarities between photoheterotrophically grown gametophytes and those which are DCMU-inhibited. While, in general, the alteration of the protein complement in thylakoids from photoheterotrophically grown gametophytes is very similar to those grown mixotrophically, the possible upregulation of the ATP synthase will require further study. Finally, the demonstrated ability to grow P. patens photoheterotrophically should augment and extend the utility of this model organism for use in photosynthesis research.
References
Anderson SL, McIntosh L (1991) Light-activated heterotrophic growth of the cyanobacterium Synechocystis sp. strain PCC 6803: a blue-light-requiring process. J Bacteriol 173:2761–2767
Arnon DI (1949) Copper enzymes in isolated chloroplasts. Polyphenol oxidase in Beta vulgaris. Plant Physiol 24:1–15
Bouyoub A, Vernotte C, Astier C (1993) Functional analysis of the two homologous psbA gene copies in Synechocystis PCC 6714 and PCC 6803. Plant Mol Biol 21:249–258
Bricker TM, Putnam-Evans C, Wu J (1998) Mutagenesis in the study of the structure and function of Photosystem II. Meth Enzymol 297:320–337
Burnap RL, Shen J-R, Jursinic PA, Inoue Y, Sherman LA (1992) Oxygen yield and thermoluminescence characteristics of a cyanobacterium lacking the manganese-stabilizing protein of Photosystem II. Biochemistry 31:7404–7410
Cove DJ (2005) The moss Physcomitrella patens. Annu Rev Genet 39:339–358
Cove DJ, Schild A, Ashton NW, Hartmann E (1978) Genetic and physiological studies of the effect of light on the development of the moss, Physcomitrella patens. Photochem Photobiol 27:249–254
Cove DJ, Bezanilla M, Harries P, Quatrano RS (2006) Mosses as model systems for the study of metabolism and development. Annu Rev Plant Biol 57:497–520
Cove DJ, Perroud P-F, Charron AJ, McDanie SFl, Khandelwal A, Quatrano RS (2009) Culturing the Moss Physcomitrella patens. Cold Spring Harb Protoc. doi:10.1101/pdb.prot5136
Dal Bosco C, Lezhneva L, Biehl A, Leister D, Strotmann H, Wanner G, Meurer J (2004) Inactivation of the chloroplast ATP synthase gamma subunit results in high non-photochemical fluorescence quenching and altered nuclear gene expression in Arabidopsis thaliana. J Biol Chem 279:1060–1069
Dalla-Chiesa M, Friso G, Deak Z, Vass I, Barber J, Nixon PJ (1997) Reduced turnover of the D1 polypeptide and photoactivation of electron transfer in novel herbicide resistant mutants of Synechocystis sp. PCC 6803. Eur J Biochem 248(3):731–740
de Vitry C, Olive J, Drapier D, Recouvreur M, Wollman FA (1989) Post-translational events leading to the assembly of Photosystem II protein complex a study using photosynthesis mutants from Chlamydomonas reinhardtii. J Cell Biol 109:991–1006
Delepelaire P, Chua N (1979) Lithium dodecyl sulfate/polyacrylamide gel electrophoresis of thylakoid membranes at 4 °C: characterizations of two additional chlorophyll a-protein complexes. Proc Natl Acad Sci (USA) 76:111–115
Drapier D, Girard-Bascou J, Wollman F-A (1992) Evidence for nuclear control of the expression of the atpA and atpB chloroplast genes in Chlamydomonas. Plant Cell 4:283–295
Erickson JM, Rahire M, Bennoun P, Delepelaire P, Diner B, Rochaix JD (1984) Herbicide resistance in Chlamydomonas reinhardtii results from a mutation in the chloroplast gene for the 32 kilodalton protein of Photosystem II. Proc Natl Acad Sci USA 81(12):3617–3621
Frank W, Dekker EL, Reski R (2005) Molecular tools to study Physcomitrella patens. Plant Biol 7:220–227
Galloway RE, Mets L (1982) Non-mendelian inheritance of 3-(3,4-dichlorophenyl)-1,1-dimethylurea resistant thylakoid membrane properties in Chlamydomonas reinhardii. Plant Physiol 70:1673–1677
Gatenby AA, Rothstein SJ, Nomura M (1989) Translational coupling of the maize chloroplast atpB and atpE genes. Proc Natl Acad Sci (USA) 86:4066–4070
Hueber FM (1961) Hepaticites devonicus : a new fossil liverwort from the Devonian of New York. Ann Miss Bot Gard 48:125–132
Joliot P, Barbieri G, Chabaud R (1969) Un nouveau modele des centres photochimique du systeme II. Photochem Photobiol 10:309–329
Jursinic PA (1979) Flash-yield pattern for photosynthetic oxygen evolution in Chlorella and chloroplasts as a function of excitation intensity. Arch Biochem Biophys 196:484–492
Kok B, Forbush B, McGloin M (1970) Cooperation of charges in photosynthetic oxygen evolution: I. A linear four step mechanism. Photochem Photobiol 11:457–475
Pakrasi HB, Diner BA, Williams JGK, Arntzen CJ (1989) Deletion mutagenesis of the cytochrome b 559 protein inactivates the reaction center of Photosystem II. Plant Cell 1(6):591–598
Reinders A, Sivitz AB, Ward JM (2012) Evolution of plant sucrose uptake transporters. Front Plant Sci 3:22–31
Rensing SA, Lang D, Zimmer AD, Terry A, Salamov A, Shapiro H, Nishiyama T, Perroud P-F, Lindquist EA, Kamisugi Y, Tanahashi T, Sakakibara K, Fujita T, Oishi K, Shin-I T, Kuroki Y, Toyoda A, Suzuki Y, S-i Hashimoto, Yamaguchi K, Sugano S, Kohara Y, Fujiyama A, Anterola A, Aoki S, Ashton N, Barbazuk WB, Barker E, Bennetzen JL, Blankenship R, Cho SH, Dutcher SK, Estelle M, Fawcett JA, Gundlach H, Hanada K, Heyl A, Hicks KA, Hughes J, Lohr M, Mayer K, Melkozernov A, Murata T, Nelson DR, Pils B, Prigge M, Reiss B, Renner T, Rombauts S, Rushton PJ, Sanderfoot A, Schween G, Shiu S-H, Stueber K, Theodoulou FL, Tu H, Van de Peer Y, Verrier PJ, Waters E, Wood A, Yang L, Cove D, Cuming AC, Hasebe M, Lucas S, Mishler BD, Reski R, Grigoriev IV, Quatrano RS, Boore JL (2008) The Physcomitrella genome reveals evolutionary insights into the conquest of land by plants. Science 319:64–69
Rochaix J-D (1987) Molecular genetics of chloroplasts and mitochondria in the unicellular green alga Chlamydomonas. FEMS Microbiol Rev 46:13–34
Schaefer DG, Zrÿd J-P (1997) Efficient gene targeting in the moss Physcomitrella patens. Plant J 11:1195–1206
Strasser RJ, Srivastava A, Tsimilli-Michael M (2000) The fluorescence transient as a tool to characterize and screen photosynthetic samples. In: Yunus M, Pathre U, Mohanty P (eds) Probing photosynthesis: mechanism, regulation and adaptation. Taylor and Francis, London, pp 443–480
Thornton LE, Keren N, Ohad I, Pakrasi HB (2005) Physcomitrella patens and Ceratodon purpureus, mosses as model organisms in photosynthesis studies. Photosynth Res 83:87–96
Vermaas WFJ, Williams JGK, Arntzen CJ (1987) Sequencing and modification of psbB, the gene encoding the CP 47 protein of Photosystem II in the cyanobacterium Synechocystis 6803. Plant Mol Biol 8:317–326
Williams JGK (1988) Construction of specific mutations in Photosystem II photosynthetic reaction center by genetic engineering methods in Synechocystis 6803. Methods Enzymol 167:766–778
Yi X, Hargett SR, Frankel LK, Bricker TM (2009) The PsbP protein, but not the PsbQ protein, is required for normal thylakoid membrane architecture in Arabidopsis thaliana. FEBS Lett 583:2142–2147
Acknowledgments
This work was supported by the Division of Chemical Sciences, Geosciences, and Biosciences, Office of Basic Energy Sciences of the U.S. Department of Energy through Grant DE-FG02-98ER20310 to T.M.B and L.K.F and Grant DE-FG02-03ER1540 to S.M.T.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bricker, T.M., Bell, A.J., Tran, L. et al. Photoheterotrophic growth of Physcomitrella patens . Planta 239, 605–613 (2014). https://doi.org/10.1007/s00425-013-2000-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00425-013-2000-3