Abstract
Ascorbate (AsA) plays a fundamental role in redox homeostasis in plants and animals, primarily by scavenging reactive oxygen species. Three genes, representing diverse steps putatively involved in plant AsA biosynthesis pathways, were cloned and independently expressed in Solanum lycopersicum (tomato) under the control of the CaMV 35S promoter. Yeast-derived GDP-mannose pyrophosphorylase (GMPase) and arabinono-1,4-lactone oxidase (ALO), as well as myo-inositol oxygenase 2 (MIOX2) from Arabidopsis thaliana, were targeted. Increases in GMPase activity were concomitant with increased AsA levels of up to 70% in leaves, 50% in green fruit, and 35% in red fruit. Expression of ALO significantly pulled biosynthetic flux towards AsA in leaves and green fruit by up to 54 and 25%, respectively. Changes in AsA content in plants transcribing the MIOX2 gene were inconsistent in different tissue. On the other hand, MIOX activity was strongly correlated with cell wall uronic acid levels, suggesting that MIOX may be a useful tool for the manipulation of cell wall composition. In conclusion, the Smirnoff–Wheeler pathway showed great promise as a target for biotechnological manipulation of ascorbate levels in tomato.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The l-enantiomer of ascorbate (AsA), or vitamin C, acts as a scavenger of the free radicals generated by photosynthesis, cellular respiration, and abiotic stresses such as ozone and UV radiation (Levine 1986; Conklin et al. 1996; Smirnoff and Pallanca 1996; Noctor and Foyer 1998; Smirnoff and Wheeler 2000). AsA has additionally been shown to play an important role as an enzyme cofactor while participating in defense, cellular elongation, division, and fruit ripening (Arrigoni and De Tullio 2000, 2002; Pastori et al. 2003; Green and Fry 2005). In animals, AsA is synthesized from d-glucose which is converted into l-gulono-1,4-lactone (l-GulL) via the intermediates d-glucuronic acid (GlucA) and l-gulonate (Fig. 1; Electronic Supplementary Material Fig. A). l-GulL is oxidized to AsA by l-gulono-1,4-lactone oxidase (Burns and Mosbach 1956). Humans cannot synthesize AsA due to a mutation in the l-gulono-1,4-lactone oxidase gene and have to acquire Vitamin C through the regular ingestion of fruit and vegetables (Nishikimi et al. 1994). Vitamin C micronutrient deficiency is associated with conditions such as scurvy and low immunity because of its integral role as enzyme cofactor and in the biosynthesis of carnitine and collagen (reviewed by Padayatty et al. 2003). The biofortification of crops has become a major focus in developing countries where poverty and micronutrient deficiencies are synonymous and are largely responsible for poor health and fatalities (reviewed by Müller and Krawinkel 2005).
A schematic representation of proposed ascorbic acidbiosynthesis pathways: the Smirnoff–Wheeler pathway (Wheeler et al. 1998) the pectin scavenging pathway (Agius et al. 2003) and the animal and animal-like AsA biosynthetic pathways (Wolucka and Van Montagu 2003; Lorence et al. 2004). GMPase GDP-mannose pyrophosphorylase; MIOX myo-inositol oxygenase; ALO arabinono-1,4-lactone oxidase; l -GulLDH l-gulono-1,4-lactone dehydrogenase; l -GalLDH l-galactono-1,4-lactone dehydrogenase
Several AsA biosynthetic pathways have been identified and characterized in plants (Fig. 1; Electronic Supplementary Material Fig. A). The “Smirnoff–Wheeler” pathway is considered the principal route for de novo synthesis of AsA and involves the conversion of d-mannose into AsA via a series of l-galactose containing intermediates (Barber 1979; Wheeler et al. 1998; Conklin et al. 1999, 2000, 2006; Bartoli et al. 2000; Wolucka and Van Montagu 2003; Smirnoff et al. 2004; Dowdle et al. 2007; Laing et al. 2007; Loannidi et al. 2009). Conklin et al. (1997) has demonstrated that ascorbate deficient Arabidopsis thaliana mutants display reduced GDP-mannose pyrophosphorylase (GMPase) activity, an enzyme that catalyzes one of the first steps of the “Smirnoff–Wheeler” pathway. Expression of an Acerola GMPase in tobacco resulted in up to 100% increased levels of AsA (Badejo et al. 2007). Loannidi et al. (2009) has shown that galactose-1-phosphate phosphatase expression is up regulated during fruit development, suggesting an important control point in ascorbate biosynthesis. The final biosynthetic step, oxidation of l-galactono-1,4-lactone (l-GalL) into AsA is catalyzed by galactono-1,4-lactone dehydrogenase (l-GalLDH), the only membrane-bound enzyme of this pathway (Hancock et al. 2003). A yeast homologue, arabinono-1,4-lactone oxidase (ALO), has been shown to promiscuously convert l-GalL, as well as l-guluno-1,4-lactone (l-GulL) into AsA (Huh et al. 1994; Lee et al. 1999; Hancock et al. 2000; Sauer et al. 2004; Hancock 2009). The “Smirnoff–Wheeler” pathway can, furthermore, be augmented through a “pectin scavenging” pathway whose products are directly utilized by l-GalLDH (Agius et al. 2003). Support for this alternative route to AsA stem from radiotracer, transcription, and expression studies of various pathway intermediates (Loewus 1999; Agius et al. 2003; Cruz-Rus et al. 2010).
Overexpression of a MIOX gene in Arabidopsis was shown to increase AsA levels two- to threefold (Lorence et al. 2004). A de novo “MIOX” or “animal-like” pathway, involving the ring cleavage of myo-inositol (MI) by myo-inositol oxygenase (MIOX) into d-glucuronic acid, was proposed (Fig. 1). Labeling experiments revealed that myo-inositol was incorporated not only into cell wall components but also into l-gulonate, which in turn may be converted into l-GulL (Lorence et al. 2004; Zhang et al. 2008). l-GulL was shown to serve a direct precursor of l-ascorbic acid in plant cells (Wolucka and Van Montagu 2003).
Our current study was initiated with the intent of increasing total AsA in tomato. Temporal analyses of changes in the levels of AsA, as well as precursors and breakdown products, have suggested that ascorbate metabolism is highly complex in tomato (Carrari and Fernie 2006; Wang et al. 2009; Garcia et al. 2009). Here we report on the heterologous expression of GMPase, ALO, and MIOX under the control of a constitutive promoter and the corresponding effect on AsA content within leaf and fruit tissue. GMPase has been shown to affect ascorbate biosynthesis in several Solanaceous species (Conklin et al. 1999; Keller et al. 1999; Badejo et al. 2007), ALO effectively metabolizes a range of substrates towards ascorbate production in situ (Huh et al. 1994), and MIOX is thought to play a central role in an “animal like” AsA biosynthetic pathway (Lorence et al. 2004).
Materials and methods
Constructs and transformations
GMPase (GenBank accession number NM_001180114) and ALO (accession number AY693120.1) were PCR amplified from Saccharomyces cerevisiae strain FY23 (S288C) (Winston et al. 1995) genomic DNA. The coding region of the Arabidopsis thaliana L. MIOX2 gene (accession number NM_127538) was amplified from A. thaliana Columbia-O cDNA [NASC (http://arabidopsis.info/)]. Appropriate PCR primer pairs are given in Table 1. Amplification, using pfu polymerase (Fermentas, Glen Burnie, MD, USA), introduced XhoI and HindIII restriction sites. PCR products were independently cloned into the pGEM®-T Easy vector (Promega, Madison, WI, USA) and sub-cloned into the pART7 vector (Gleave 1992) under control of the constitutive CaMV 35S promoter. Expression cartridges were transferred into the pART27 plant transformation vector as NotI fragments as described by Basson et al. (2010b). The constructs, i.e. pART27::GMPase, pART27::ALO, and pART27::MIOX2, were mobilized into Agrobacterium tumefaciens EHA 105 cells using the freeze–thaw method (Höfgen and Willmitzer 1988). The Solanum lycopersicum ‘Money maker’ cultivar was infiltrated as described by Obiadalla-Ali et al. (2004).
Plant material
Stem cuttings representing different transformation events were transferred onto MS agar (4.4 g/L Murashige and Skoog, 15 g/L sucrose and 3 g/L, agar, pH 7) and grown in tissue culture at 22°C under continuous light conditions. After 2 weeks, plants were transferred to the glass house and progressively hardened off in soil (Double Grow, Durbanville, South Africa) at 22°C in a 16/8 h day night cycle. Seeds were harvested from ripe fruit and germinated in the glasshouse. At 4 weeks, plantlets were moved to a greenhouse (summer between the months of November and March) and grown under controlled irrigation. Every 4 days, plants were supplied with 1 g/L calcium nitrate and 1.5 g/L carbon-free hydroponic nutrient supplement (Hygrotech Hygroponic Nutrients, Pretoria, South Africa Reg No. K5709). Leaf samples were collected at 8 weeks and whole fruit samples were harvested during green and red stages of maturity at 25 days and 60 ± 5 days, respectively, post anthesis (Basson et al. 2010a). The pericarp was not separated from the locular tissue as this would initiate a wound response thereby affecting ascorbate levels (Loannidi et al. 2009). Care was taken to harvest all samples at noon on days with non-overcast skies. In each case, five replicates were sampled for each line. Samples were immediately frozen, ground in liquid nitrogen, and stored at −80°C.
Selection of transformants by polymerase chain reaction
Plant material was ground in liquid nitrogen and genomic DNA extracted from 50 mg of tissue according to the method of McGarvey and Kaper (1991) and in the presence of 0.5 g/L spermidine. DNA concentration and quality were determined spectrophotometrically (Basson et al. 2010a, b). GMPase, ALO, and MIOX transgenic lines were screened using forward primer 10 and reverse primers 7, 8, and 9, respectively (Table 1). PCR screening reactions were performed with PromegaGoTaq® PCR (Promega, Madison, WI, USA). Amplicons were visualized in a 1% agarose gel containing ethidium bromide (4 μL/100 mL). WT plants and plasmids containing the cloned genes of interest were used as negative and positive controls, respectively.
RNA extraction and RT-PCR
RNA was extracted from frozen leaf and fruit material according to Burgos et al. (1995) with the following modifications. The extraction buffer contained 5% β-mercaptoethanol and RNA was precipitated with one-quarter volume 8 M lithium chloride. The dried RNA pellet was reconstituted in ~50 μL MQ water and RNA concentrations were normalized to 100 ng/μL. All samples were DNase-treated using DNase I (Fermentas). First strand cDNA synthesis was performed with 5 μg RNA using RevertAid H Minus Reverse Transcriptase (Fermentas). Gene-specific forward primers (Table 1, numbers 1, 3, and 5) and reverse primers (Table 1, numbers 2, 4, and 6) were used to amplify expressed sequences. TIP41, a reference gene for quantitative transcriptomics in Solanum lycopersicum (Expósito-Rodríguez et al. 2008) was used as a constitutively expressed gene control (Table 1, number 11 and 12). All RT-PCR reaction conditions were as follows: 3 min at 94°C; (25 cycles of: 30 s at 94°C, 30 s at 55°C, 30 s at 72°C); 7 min at 72°C.
Protein extraction
Total protein from GMPase expressing plants was extracted from frozen tissue in 10 volumes of ice cold buffer containing 50 mM Tris–HCl (pH 7.5), 0.05% Triton X-100, 5 mM EDTA, 5 mM DTT, 0.01% β-mercaptoethanol and 1 mM PMSF. Samples were centrifuged (18,000 g, 5 min, 4°C), one volume 50% PEG 6000 was added to the supernatant, and protein precipitated for 30 min on ice. Samples were centrifuged (14,000 g, 10 min, 4°C) and pellets resuspended in 100 mM Tris pH 7.5. MIOX protein was extracted in 10 volumes of ice-cold buffer containing 100 mM Tris–HCl pH 7.6, 2 mM l-cysteine, 1 mM ammonium ferrous sulfate hexahydrate, 1 mM EDTA, and 1% PVPP. Protein was precipitated as described above and resuspended in 100 mM KPO4 buffer (pH 7.2) containing 2 mM l-cysteine and 1 mM ammonium ferrous sulfate hexahydrate.
Activity assays
GMPase activity was measured using a stopped radioassay as described by Keller et al. (1999) with the following modifications. The assay was started by adding 400 μL crude protein extract to 400 μL assay mix (100 mM Tris pH 7.5, 4 mM MgCl2, 5 mM sodium pyrophosphate, 0.1 mM cold GDP-mannose, and 0.04 Cu 14C GDP-mannose) and stopped after 1 h with the addition of 2 mg activated charcoal. Scintillation fluid (5 mL) was added and 14C d-mannose-1-P determined using the Tri-Carb 2100 TR Liquid Scintillation Analyzer (Packard Instrument Company, Meriden, CT, USA).
MIOX activity was determined within the linear range of an endpoint assay (Reddy et al. 1981) modified as follows: Protein (500 μg per sample) was incubated for 30 min at 30°C in a buffer containing 100 mM KPO4 (pH 7.2), 2 mM l-cysteine, 1 mM ammonium ferrous sulfate hexahydrate, and 60 mM myo-inositol (Electronic Supplementary Material Fig. B). The reaction was stopped by boiling for 10 min and denatured protein removed by centrifugation (18,000 g, 10 min). Glucuronic acid was measured as described by Van den Hoogen et al. (1998).
Ascorbic acid measurement
Frozen plant tissue was ground in five volumes of 6% (w/v) meta-phosphoric acid and total AsA quantified with the aid of ascorbic acid oxidase (EC 1.10.3.3) and the reductant tris[2-carboxyethyl]phosphine hydrochloride (TCEP) as described by Basson et al. (2010a). Content was calculated against a standard curve of 0–80 μM ascorbic acid. Total AsA is given as the sum of oxidized AsA (l-ascorbic acid) and reduced AsA (DHA).
GC–MS for metabolite profiling
Extraction and derivatization of plant tissue was done according to the method of Roessner et al. (2000) with modifications. The polar fraction was extracted from 60 mg frozen leaf tissue homogenized in 1,400 μL 100% methanol and with 60 μL ribitol (0.2 mg/mL water) as internal standard. Samples were extracted at 70°C for 15 min, vortexed and centrifuged (18,000 g, 10 min). The supernatant was added to one volume chloroform and two volumes water, vortexed and centrifuged (5,500 g, 15 min), and the upper phase vacuum dried for derivatization. Dried samples were reconstituted in 40 μL methoxyamine hydrochloride (20 mg/mL in pyridine), derivatized for 2 h at 37°C, and incubated for a further 30 min (37°C) in the presence of 70 μL MSTFA and 40 μL internal retention time standard.
Analysis was performed using a 6890-N gas chromatograph and 5975 inert mass selective inhibitor mass spectrometer (Agilent Technologies; Santa Clara, CA, USA). 1-μL Volumes of were injected with a 7683B Series splitless injector (Agilent Technologies) and gas chromatography was performed on a 30-m Rtx®-5Sil MS Integra Guard column with 0.25 mm internal diameter and 0.25 μm film thickness (Restek, Bellefonte, PA, USA). Injection- and ion source temperatures were set at 230°C and 200°C, respectively, and the program was set to 5 min at 70°C, a first ramp of 1°C/min to 76°C, and a second ramp of 6°C/min to 350°C. Temperature was equilibrated to 70°C prior to injection of each sample and mass spectra recorded (2 scans per s in range of 50–600 m/z). Data were analyzed using the Automated Mass Spectral Deconvolution and Identification System (AMDIS, http://www.amdis.net/index.html, National Institute of Standards and Technology, Gaithersburg, MD, USA) (Stein 1999) and compared with a custom RI-annotated supervised plant metabolite mass spectral database (http://gmd.mpimp-golm.mpg.de/) (Schauer et al. 2005) and the NIST/EPA/NIH Mass Spectral Library (NIST 05) using the NIST Mass Spectral Search Program Version 2.0d.
Preparation of alcohol insoluble residues (AIR) and measurement of cell wall uronic acids
Ethanol was added to ground plant tissue (125 ± 10 mg) and incubated for 20 min at 70°C. Samples were centrifuged at 8,500 g for 10 min and supernatants discarded. Ethanol extraction was repeated four times. Samples were washed in acetone and vacuum dried. Cell wall uronic acids were measured using an adaptation of methods previously described (Blumenkrantz and Asboe-Hansen 1973; Van den Hoogen et al. 1998). Dried AIR samples (10 mg) were reconstituted in 200 μL 12 M sulfuric acid and incubated for 2 h at 4°C. The sulfuric acid was diluted to 2 M and cell wall polysaccharides hydrolyzed for 2 h at 80°C. Concentrated sulfuric acid containing 120 mM sodium tetraborate was added to 40-μL aliquots of AIR sample (200 μL per aliquot), incubated at room temperature for 30 min, and background OD measured at 540 nm. Uronic acids were measured as described by Van den Hoogen et al. (1998) against a galacturonic acid standard of 0–8 μg.
Results
Constructs, transformations, and selection
Regenerated plant transformants were screened by PCR for the presence of pART27::GMPase, pART27::ALO, and pART27::MIOX2 constructs, respectively. GMPase positive line G2 was not selected for further analyses due to the high probability that it exhibited somaclonal variation (Electronic Supplementary Material Fig. C), while ALO line A16 was rejected due to an uncharacteristically low fruit yield. Tomato seeds were collected and at least five biological replicates established per line.
GMPase activity
Lines positive for the presence of the yeast-derived GMPase gene were assayed for protein activity using a radiolabel incorporation assay. In comparison with untransformed controls, GMPase activity in leaves of transgenic lines increased between 26 and 31 times (Fig. 2). Similarly, in green fruit tissue activity increased 13–17 times. Despite the fact that the baseline activity in different wild-type tissues was very similar, transgenic leaf material displayed up to 100% more activity than transgenic green fruit.
GDP-mannose pyrophosphorylase (GMPase) activity measurements in plants expressing GMPase from Saccharomyces cerevisiae using [14C]GDP-mannose, cold GDP-mannose and PP i as substrates. Activity was measured as the amount of radio label incorporated into the product, mannose-1-phosphate. Values calculated as average ± standard deviation; n = 3; P < 0.05
ALO transcription
Arabinono-1,4-lactone oxidase (ALO) activity could not be reliably measured because the protein is embedded within the mitochondrial membrane. Membrane fractions contained varying amounts of active protein, complicating measurements, and standardization of enzymatic assays. Therefore, transcript levels of ALO were measured semi-quantitatively and compared with the expression level of the constitutively expressed TIP41 gene. RT-PCR confirmed the unique transcription of the heterologous gene in transgenic lines (Fig. 3).
MIOX activity
Transgenic lines displayed approximately three- to fourfold increased MIOX activity in leaves compared with wild-type controls (Fig. 4). In green fruit, activity in line M8 was not significantly higher than in wild-type plants, whereas lines M2 and M4 exhibited twofold increases (P < 0.1).
Myo-inositol oxygenase (MIOX) activity measurements in leaves of MIOX lines and wild-type controls. Myo-inositol was provided as substrate and MIOX activity measured relative to the amount of glucuronic acid produced. Optical density was determined at 540 nm before and after samples developed a pink color with addition of a 3-hydroxybiphenylphenol color reagent. Values calculated as average ± standard deviation; n = 3; P < 0.1 (green fruit); P < 0.05 (leaves)
Ascorbate
Total ascorbate, measured as the sum of l-AsA and DHA, was determined in leaves, green fruit and red fruit to study the effect of introduced transgenes on ascorbate biosynthesis or its steady-state levels. Due to the direct link between ascorbate levels and the wounding response, fruits were frozen and analyzed whole (Loannidi et al. 2009). During senescence, the locule becomes filled with water and soluble sugars. In red fruit, DHA concentrations per fresh weight were below the limits of detection, and ascorbate content was therefore represented by l-AsA alone. Increase in GMPase activity was concomitant with increased ascorbate levels in all tissues measured (Table 2). Ascorbate content in leaves was increased up to 66% compared with 50 and 35% in green and red fruit, respectively. Most transgenic ALO lines displayed increased ascorbate levels (P < 0.05) in leaf tissue, typically between 21 and 54% (Table 3). Levels in green fruit were increased up to 25% (P < 0.1), while red fruit contained levels invariant from the wild type. In leaf material, increased MIOX activity was associated with up to 30% reduction in ascorbate content (Table 4). Conversely, transgenic green fruit with increased MIOX activity displayed up to 35% increased ascorbate levels (P < 0.1).
Metabolite profiling
In order to determine whether precursor molecules within the various pathways of AsA biosynthesis were affected, GC–MS metabolite profiling was performed on leaf tissue. Comparison of the GC–MS chromatograms with plant metabolite and NIST mass spectral libraries revealed numerous metabolites consistently present in all samples and several significant deviations in the metabolite profiles of the transgenic plants (Table 5). GMPase transgenic lines showed an increase in galactono-1,4-lactone and galactonate, and a concomitant decrease in glucuronic acid. Major increases in citric acid cycle components, fumarate, and succinate were also observed. Principal component analysis (PCA) (Electronic Supplementary Material Fig. D) of the GC–MS data (Electronic Supplementary Material Table 1) revealed increases in threonate (P < 0.1). Galactonate, galactose, myo-inositol, and sucrose decreased significantly in most ALO lines. Decreases in myo-inositol content were most evident in MIOX lines, by between 72 and 90% (P < 0.05), with concomitant increases in gulonate.
Cell-wall analysis
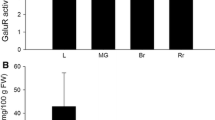
Cell wall uronic acids were determined in leaf and green fruit tissue of MIOX lines (Fig. 5). In leaf tissue, all three transgenic lines displayed small increases in cell wall uronic acids (P < 0.1). In green fruit, levels were increased by more than 100% in lines M2 and M4.
Uronic acid measurements in myo-inositol oxygenase (MIOX) lines representative of cell wall biosynthesis. Measurements were performed on leaf and green fruit material with wild-type controls and expressed as a weight percentage of total alcohol insoluble residues (AIR) extracted from the cell wall. Values calculated as average ± standard deviation; n = 3; P < 0.1 (leaves); P < 0.05 (green fruit)
Discussion
Three different genes, GMPase, MIOX, and ALO, were targeted for heterologous expression with the aim of (re)directing carbon flux toward AsA biosynthesis in plants. These genes were ectopically expressed in tomato in an attempt to overcome rate-limiting steps in production, or to increase the contribution of secondary pathways.
Expression of GDP-mannose pyrophosphorylase
A yeast-derived GMPase, catalyzing the conversion of d-mannose-1-P to GDP-d-mannose (Hashimoto et al. 1997) was expressed in an attempt to accelerate the flux of carbon through the Smirnoff–Wheeler AsA pathway (Fig. 1). Transgenic tomato lines exhibited up to 31 and 17-fold increased GMPase activity in leaves and green fruit, respectively. Total ascorbate levels increased up to 70%, most apparent in photosynthesizing tissues as reported earlier (Yabuta et al. 2008). Heterologous expression of a plant GMPase in tobacco leaves has previously resulted in about 100% increased AsA content (Badejo et al. 2007). In the current study, an increase in GMPase activity was accompanied by up to 375% more galactono-1,4-lactone, a downstream intermediate in the Smirnoff–Wheeler pathway, and a significant increase in galactonate, an intermediate in the cell wall scavenging pathway. DHA (the reduced form of ASA) was significantly increased in leaf tissue of all transgenic lines. Both the rate of AsA synthesis and recycling via DHA, and monodehydroascorbate reductase are critical in the maintenance of a high AsA redox state (Conklin and Barth 2004). Statistical principal component analysis (PCA) of metabolic profiles in leaves revealed an overall increase in threonate production in transgenic plants (Electronic Supplementary Material Fig. D). Pallanca and Smirnoff (2000) suggested that the rate at which AsA is recycled and catabolized can be inferred from the levels of DHA, glutathione or the breakdown products tartrate and threonate. Significant increases in the citric acid cycle components, fumarate and succinate, were measured in leaves. It has been shown that AsA biosynthetic rates are affected by the flow of electrons through the respiratory electron transport chain (Millar et al. 2003; Alhagdow et al. 2007). Increased flux through the Smirnoff–Wheeler pathway creates an increased demand for oxidized cytochrome c, which is diverted from ATP synthase. A resulting demand for citric acid cycle derived NADH could plausibly lead to increased turnover and intermediates such as succinate and fumarate. While GMPase may not exert majority metabolic control over this pathway, the study suggests that increased substrate supply from early steps of the l-galactose pathway positively affects vitamin C production, especially in photosynthesizing tissue.
Expression of arabinono-1,4-lactone oxidase
d-Arabinono-1,4-lactone oxidase (ALO), the yeast analog of galactono-1,4-lactone dehydrogenase (l-GalLDH), converts d-arabinono-1,4-lactone to erythroascorbate, while promiscuously converting l-galactono-1,4-lactone and l-guluno-1,4-lactone to AsA (Huh et al. 1994; Lee et al. 1999; Hancock et al. 2000; Sauer et al. 2004; Hancock 2009). ALO was expressed in order to assess if increased turnover of the terminal step in the ascorbate biosynthetic pathway would increase carbon flux towards AsA biosynthesis. l-GalLDH is sensitive to irradiance, ascorbate oxidase activity, cytochrome c activity, and respiration (Millar et al. 2003; Tamaoki et al. 2003; Nunes-Nesi et al. 2005; Bartoli et al. 2006, 2009; Bulley et al. 2009). By contrast, ALO has not shown sensitivity to light or reductant availability. ALO activity in tomato extracts could not be reliably quantified due to its presumed interaction with the inner mitochondrial membrane as demonstrated for its plant homologue l-GalLDH (Hancock et al. 2003). Transcription of the ALO transgene was, however, confirmed (Fig. 4) and has resulted in significantly higher AsA levels in leaves (up to 54%) and green fruit (up to 25%). DHA levels in transgenic green fruit also increased, suggesting an increase in AsA turnover. AsA feeding experiments have shown that AsA pool size is directly proportionate to turnover rate (Pallanca and Smirnoff 2000). Metabolite profiling of leaf tissue revealed up to 42% reduction in galactose (an intermediate in the Smirnoff–Wheeler pathway), up to 45% reduction of (galactonate an intermediate in the pectin degradation pathway) and up to 90% reduction of myo-inositol. GC–MS did not allow discrimination between d- and l-galactose. The yeast isoform (ALO) appears to pull carbon flux towards AsA biosynthesis. To our knowledge, this is the first report on the successful expression of ALO in planta.
Expression of myo-inositol oxygenase
Myo-inositol is converted into GlucA by the activity of MIOX. However, whether GlucA acts as a precursor to AsA in an “animal like” pathway in plants has not been established with certainty (Lorence et al. 2004; Zhang et al. 2008; Endres and Tenhaken 2009). The gene family for the MIOX enzyme from Arabidopsis was shown to be represented by four members (Kanter et al. 2005). The current study investigated expression of the MIOX2 isoform in tomato. Transcription of the transgene resulted in increased MIOX activity in leaf material without a concomitant increase in AsA content. In contrast, a significant decrease in AsA in leaf tissue, inversely proportionate to the level of MIOX activity, was apparent. Previously, expression of the MIOX4 gene in Arabidopsis was shown to increase AsA levels two- to threefold (Lorence et al. 2004; Zhang et al. 2008). In contrast, MIOX4 overexpressing Arabidopsis lines were recently shown to be largely invariant from the wild type (Endres and Tenhaken 2009).
Steady-state myo-inositol levels in lines with increased MIOX activity were decreased to as low as 10% of levels in wild type controls, while a tenfold increase in gulonate was observed (Table 5). Gulonate resides downstream of myo-inositol and is converted to l-gulono-1,4-lactone, the terminal substrate in the ‘animal-like’ AsA biosynthesis pathway (Fig. 1). While increased MIOX activity plays an ambiguous role in AsA biosynthesis, the enzyme clearly controls the metabolite level of myo-inositol and derivatives in plants as suggested previously (Endres and Tenhaken 2009). The authors have reported on increased incorporation of MIOX-derived sugars into cell wall polymers, while overexpressors exhibited a lower steady-state level of myo-inositol due to an enhanced turnover rate.
d-Glucuronic acid is a major precursor in cell wall biosynthesis (Kanter et al. 2005). Expressed as a percentage of the AIR of the cell wall, uronic acid content was significantly higher in the leaves of all MIOX lines. Increased uronic acid levels were also observed in green fruits with significantly higher MIOX activity, indicative of a shunt of glucuronic acid into the cell wall (Fig. 5). Green fruit with measurably higher MIOX activity levels and uronic acids also showed significant increases in AsA. Either carbon is being directed towards AsA biosynthesis through an ‘animal-like’ pathway, or increases in cell wall components provide more substrate for AsA biosynthesis via the pectin scavenging pathway. The strong correlation between MIOX activity and cell wall uronic acid levels suggests that MIOX may be a useful tool for the manipulation of cell wall composition. Downregulation of GDP-d-mannose 3,5-epimerase (GME) isoforms in tomato was recently shown to result in significant changes in cell wall composition (Gilbert et al. 2009). Garcia et al. (2009) showed direct correlations between intermediates of ascorbate and cell wall biosynthetic pathways. Such studies strengthen the concept of a cell wall-ascorbate nexus.
Abbreviations
- GMPase:
-
Guanidine-diphosphate mannose pyrophosphorylase
- ALO:
-
Arabinono-1,4-lactone oxidase
- MIOX:
-
Myo-inositol oxygenase
- MI:
-
Myo-inositol
- L-GulL:
-
l-Gulono-1,4-lactone
- GlucA:
-
d-Glucuronic acid
- DHA:
-
Dehydroascorbate
- L-Asc:
-
l-Ascorbate
- AsA:
-
Total ascorbate
- GalUR:
-
Galacturonic acid reductase
- L-GalLDH:
-
l-Galactono-1,4-lactone dehydrogenase
- GME:
-
GDP-d-mannose 3,5-epimerase
- O/N:
-
Over night
- GDP:
-
Guanidine-diphosphate
References
Agius F, Gonzalez-Lamothe R, Caballero JL, Munoz-Blanco J, Botella MA, Valpuesta V (2003) Engineering increased vitamin C levels in plants by overexpression of a d-galacturonic acid reductase. Nat Biotechnol 21:177–181
Alhagdow M, Mounet F, Gilbert L, Nunes-Nesi A, Garcia V, Just D, Petit J, Beauvoit B, Fernie AR, Rothan C, Baldet P (2007) Silencing of the mitochondrial ascorbate synthesizing enzyme l-galactono-1, 4-lactone dehydrogenase affects plant and fruit development in tomato. Plant Physiol 145:1408–1422
Arrigoni O, De Tullio MC (2000) The role of ascorbic acid in cell metabolism: between gene-directed functions and unpredictable chemical reactions. J Plant Physiol 157:481–488
Arrigoni O, De Tullio MC (2002) Ascorbic acid: much more than just an antioxidant. Biochim Biophys Acta 1569:1–9
Badejo AA, Jeong ST, Goto-Jamamoto N, Esaka M (2007) Cloning and expression of GDP-d-mannose pyrophosphorylase gene and ascorbic acid content of acerola (Malphighia glabra L.) fruit at ripening stages. Plant Physiol Biochem 45:665–672
Barber GA (1979) Observations on the mechanism of the reversible epimerization of GDP-mannose to GDP-l-galactose by an enzyme from Chlorella pyrenoidosa. J Biol Chem 254:7600–7603
Bartoli CG, Pastori GM, Foyer CH (2000) Ascorbate biosynthesis in mitochondria is linked to the electron transport chain between complexes III and IV. Plant Physiol 123:335–343
Bartoli CG, Yu J, Gómez F, Fernández L, McIntosh L, Foyer CH (2006) Inter-relationships between light and respiration in the control of ascorbic acid synthesis and accumulation in Arabidopsis thaliana leaves. J Exp Bot 57:1621–1631
Bartoli CG, Tambussi EA, Diego F, Foyer CH (2009) Control of ascorbic acid synthesis and accumulation and glutathione by the incident light red/far red ratio in Phaseolus vulgaris leaves. FEBS Lett 583:118–122
Basson CE, Groenewald JH, Kossmann J, Cronje C, Bauer R (2010a) Sugar and acid-related quality attributes and enzyme activities in strawberry fruits: invertase is the main sucrose hydrolyzing enzyme. Food Chem 121:1156–1162
Basson CE, Groenewald JH, Kossmann J, Cronjé C, Bauer R (2010b) Upregulation of pyrophosphate: fructose 6-phosphate 1-phosphotransferase (PFP) activity in strawberry. Transgenic Res 20:925–931
Blumenkrantz N, Asboe-Hansen G (1973) New method for quantitative determination of uronic acids. Anal Biochem 54:484–489
Bulley SM, Rassam M, Hoser D, Otto W, Schünemann N, Wright M, MacRae E, Gleave A, Laing W (2009) Gene expression studies in kiwifruit and gene over-expression in Arabidopsis indicates that GDP-l-galactose guanyltransferase is a major control point of vitamin C biosynthesis. J Exp Bot 60:765–778
Burgos RC, Chiang VL, Zhang XH, Campbell ER, Podila GK, Campbell WH (1995) RNA isolation from plant tissues recalcitrant to extraction in guanidine. Biotechniques 19:734–737
Burns JJ, Mosbach EH (1956) Further observations in the biosynthesis of l-ascorbic acid from d-glucose in the rat. J Biol Chem 221:107–111
Carrari F, Fernie AR (2006) Metabolic regulation underlying tomato fruit development. J Exp Bot 57:1883–1897
Conklin PL, Barth C (2004) Ascorbic acid, a familiar small molecule intertwined in the response of plants to ozone, pathogens, and the onset of senescence. Plant Cell Environ 27:959–970
Conklin PL, Williams EH, Last RL (1996) Environmental stress sensitivity of an ascorbic acid-deficient Arabidopsis mutant. Proc Natl Acad Sci USA 93:9970–9974
Conklin PL, Pallanca JE, Last RL, Smirnoff N (1997) l-Ascorbic acid metabolism in the ascorbate-deficient Arabidopsis mutant vtc1. Plant Physiol 115:1277–1285
Conklin PL, Norris SR, Wheeler GL, Williams EH, Smirnoff N, Last RL (1999) Genetic evidence for the role of GDP-mannose in plant ascorbic acid (vitamin C) biosynthesis. Proc Natl Acad Sci USA 96:4198–4203
Conklin PL, Saracco SA, Norris SR, Last RL (2000) Identification of ascorbic acid-deficient Arabidopsis thaliana mutants. Genetics 154:847–856
Conklin PL, Gatzek S, Wheeler GL, Dowdle J et al (2006) Arabidopsis thaliana VTC4 encodes l-galactose 1-P phosphatase, a plant ascorbic acid biosynthetic enzyme. J Biol Chem 281:15662–15670
Cruz-Rus E, Botella MA, Valpuesta V, Gomez-Jimenez MC (2010) Analysis of genes involved in l-ascorbic acid biosynthesis during growth and ripening of grape berries. J Plant Physiol 167:739–748
Dowdle J, Ishikawa T, Gatzek S, Rolinski S, Smirnoff N (2007) Two genes in Arabidopsis thaliana encoding GDP-l-galactose phosphorylase are required for ascorbate biosynthesis and seedling viability. Plant J 52:673–689
Endres S, Tenhaken R (2009) Myoinositol oxygenase controls the level of myoinositol in Arabidopsis, but does not increase ascorbic acid. Plant Physiol 149:1042–1049
Expósito-Rodríguez M, Borges AA, Borges-Pérez A, Pérez JA (2008) Selection of internal control genes for quantitative real-time RT-PCR studies during tomato development process. BMC Plant Biol 8:131
Garcia V, Stevens R, Gil L, Gibert L, Gest N, Petit J, Faurobert M et al (2009) An integrative genomics approach for deciphering the complex interactions between ascorbate metabolism and fruit growth and composition in tomato. C R Biol 332:1007–1021
Gilbert L, Alhagdow M, Nunes-Nesi A, Quemener B, Guillon F, Bouchet B, Faurobert M et al (2009) GDP-d-mannose 3,5-epimerase (GME) plays a key role at the intersection of ascorbate and non-cellulosic cell-wall biosynthesis in tomato. Plant J 60:499–508
Gleave AP (1992) A versatile binary vector system with a T-DNA organizational structure conducive to efficient integration of cloned DNA into the plant genome. Plant Mol Biol 20:1203–1207
Green MA, Fry SC (2005) Apoplastic degradation of ascorbate: novel enzymes and metabolites permeating the plant cell wall. Plant Biosyst 139:2–7
Hancock RD (2009) Recent patents on vitamin C: opportunities for crop improvement and single-step biological manufacture. Recent Pat Food Nutr Agric 1:39–49
Hancock RD, Galpin JR, Viola R (2000) Biosynthesis of l-ascorbic acid (vitamin C) by Saccharomyces cerevisiae. FEMS Microbiol Lett 186:245–250
Hancock RD, McRae D, Haupt S, Viola R (2003) Synthesis of l-ascorbic acid in the phloem. BMC Plant Bio 3:7
Hashimoto H, Sakakibara A, Yamasaki M, Yoda K (1997) Saccharomyces cerevisiae VIG9 encodes GDP-mannose pyrophosphorylase, which is essential for protein glycosylation. J Biol Chem 272:16308–16314
Höfgen R, Willmitzer L (1988) Storage of competent cells for Agrobacterium transformation. Nucleic Acids Res 16:9877
Huh W, Kim S, Yang K, Seok Y, Hah YC, Kang S (1994) Characterisation of D-arabinono-1, 4-lactone oxidase from Candida albicans ATCC 10231. Eur Biochem 225:1073–1079
Kanter U, Usadel B, Guerineau F, Li Y, Pauly M, Tenhanken R (2005) The inositol oxygenase gene family of Arabidopsis is involved in the biosynthesis of nucleotide sugar precursor for cell-wall matrix polysaccharides. Planta 221:243–254
Keller R, Springer F, Renz A, Kossmann J (1999) Antisense inhibition of the GDP-mannose pyrophosphorylase reduces the ascorbate content in transgenic plants leading to developmental changes during senescence. Plant J 19:131–141
Laing WA, Wright MA, Cooney J, Bulley SM (2007) The missing step of the l-galactose pathway of ascorbate biosynthesis in plants, an l-galactose guanylyltransferase, increases leaf ascorbate content. Proc Natl Acad Sci USA 104:9534–9539
Lee B, Huh W, Kim S, Lee J, Kang S (1999) Bacterial production of D-erythroascorbic acid and l-ascorbic acid through functional expression of Saccharomyces cerevisiae D-arabinono-1, 4-lactone oxidase in Escherichia coli. Appl Environ Microbiol 65:4685–4687
Levine M (1986) New concepts in the biology and biochemistry of ascorbic acid. N Eng J Med 314:892–902
Loannidi E, Kalamaki MS, Engineer C, Pateraki I, Alexandrou D, Mellidou I, Giovannonni J, Kanellis AK (2009) Expression profiling of ascorbic acid-related genes during tomato fruit development and ripening and in response to stress conditions. J Exp Bot 60:663–678
Loewus FA (1999) Biosynthesis and metabolism of ascorbic acid in plants and analogs of ascorbic acid in fungi. Phytochemistry 52:193–210
Lorence A, Chevone BI, Mendes P, Nessler CL (2004) Myo-inositol oxygenase offers a possible entry point into plant ascorbate biosynthesis. Plant Physiol 134:1200–1205
McGarvey P, Kaper JM (1991) A simple and rapid method for screening transgenic plants using the PCR. Biotechniques 11:428–432
Millar AH, Mittova V, Kiddle G, Haezlewood JL, Bartoli CG, Theodoulou FL, Foyer CH (2003) Control of ascorbate synthesis by respiration and its implications for stress response. Plant Physiol 133:443–447
Müller O, Krawinkel M (2005) Malnutrition and health in developing countries. Can Med Assoc J 173:279–286
Nishikimi M, Fukuyama R, Minoshima S, Shimizu N, Yagi K (1994) Cloning and chromosomal mapping of the human nonfunctional gene for l-gulono-gamma-lactone oxidase, the enzyme for l-ascorbic acid biosynthesis missing in man. J Biol Chem 269:13685–13688
Noctor G, Foyer CH (1998) Ascorbate and glutathione: keeping active oxygen under control. Annu Rev Plant Physiol Plant Mol Biol 49:249–279
Nunes-Nesi A, Lytovchenko A, Smith AM, Loueiro ME, Ratcliffe RG, Sweetlove LJ, Fernie AR (2005) Enhanced photosynthetic performance and growth as a consequence of decreasing mitochondrial malate dehydrogenase activity in transgenic tomato plants. Plant Physiol 137:611–622
Obiadalla-Ali H, Fernie AR, Lytovchenko A, Kossmann J, Lloyd JR (2004) Inhibition of chloroplastic fructose 1,6-bisphosphatase in tomato fruits leads to decreased fruit size, but only small changes in carbohydrate metabolism. Planta 219:533–540
Padayatty SJ, Katz A, Wang Y, Eck P, Kwon O, Lee J, Chen S, Corpe C, Dutta A, Dutta SK, Levine M (2003) Vitamin C as an antioxidant: evaluation of its role in disease prevention. J Am Coll Nutr 22:18–35
Pallanca JE, Smirnoff N (2000) The control of ascorbic acid synthesis and turnover in pea seedlings. J Exp Bot 51:669–674
Pastori GM, Kiddle G, Antoniw J, Bernard S, Veljovic-Jovanovic S, Verrier PJ, Noctor G, Foyer CH (2003) Leaf vitamin C contents modulate plant defense transcripts and regulate genes that control development through hormone signaling. Plant Cell 15:939–951
Reddy CC, Swan JS, Hamilton GA (1981) Myo-inositol oxygenase from hog kidney. Purification and characterization of the oxygenase and of an enzyme complex containing the oxygenase and d-glucuronate reductase. J Biol Chem 256:8510–8518
Roessner U, Wagner C, Kopka J, Trethewey RN, Willmitzer L (2000) Simultaneous analysis of metabolites in potato tuber by gas chromatography–mass spectrometry. Plant J 23:131–142
Sauer M, Branduardi P, Valli M, Porro D (2004) Production of l-ascorbic acid by metabolically engineered Saccharaomyces cerevisiae and Zygosaccharomyces bailii. Appl Environ Microbiol 70:6086–6091
Schauer N, Steinhauser D, Strelkov S, Schomburg D, Allison G et al (2005) GC-MS libraries for the rapid identification of metabolites in complex biological samples. FEBS Lett 579:1332–1337
Smirnoff N, Pallanca JE (1996) Ascorbate metabolism in relation to oxidative stress. Biochem Soc Trans 24:472–478
Smirnoff N, Wheeler GL (2000) Ascorbic acid in plants: biosynthesis and function. Crit Rev Biochem Mol Biol 35:291–314
Smirnoff N, Running JA, Gaztek S (2004) Ascorbate biosynthesis: a diversity of pathways. In: Asard H, May JM, Smirnoff N (eds) Vitamin C: functions and biochemistry in animals and plants. BIOS Scientific Publishers, London, pp 7–29
Stein SE (1999) An integrated method for spectrum extraction and compound identification from gas chromatography/mass spectrometry data. J Am Soc Mass Spectrom 10:770–781
Tamaoki M, Mukai F, Asai N, Nakajima N, Kubo A, Aono M, Saji H (2003) Light-controlled expression of a gene encoding l-galactono-1, 4-lactone dehydrogenase which affects ascorbate pool size in Arabidopsis thaliana. Plant Sci 164:1111–1117
Van den Hoogen BM, Van Weeren RP, Lopes-Cardozo M, Van Golde LMG, Barneveld A, Van de Lest CHA (1998) A microtiter plate assay for the determination of uronic acids. Anal Biochem 257:107–111
Wang H, Schauer N, Usadel B, Frasse P, Zouine M, Hernould M, Latche A, Pech JC, Fernie AR, Bouzyen M (2009) Regulatory features underlying pollination-dependent and independent tomato fruit set revealed by transcript and primary metabolite profiling. Plant Cell 21:1428–1452
Wheeler GL, Jones MA, Smirnoff N (1998) The biosynthetic pathway of vitamin C in higher plants. Nature 393:365–369
Winston F, Dollard C, Ricupero-Hovasse SL (1995) Construction of a set of convenient Saccharomyces cerevisiae strains that are isogenic to S288C. Yeast 11:53
Wolucka BA, Van Montagu M (2003) GDP-mannose 3′, 5′-epimerase forms GDP-l-gulose, a putative intermediate for the de novo biosynthesis of vitamin C in plants. J Biol Chem 278:47483–47490
Yabuta Y, Maruta T, Nakamura A, Mieda T, Yoshimura K, Ishikawa T, Shigeoka S (2008) Conversion of the l-galactono-1, 4-lactone to l-ascorbate is regulated by the photosynthetic electron transport chain in Arabidopsis. Biosci Biotechnol Biochem 72:2598–2607
Zhang W, Gruszewski HA, Chevone BI, Nessler CL (2008) An Arabidopsis purple acid phosphatase with phytase activity increases foliar ascorbate. Plant Physiol 146:431–440
Acknowledgments
Technical support from Ilse Balbo and scientific discussions with Prof. Adriano Nunes-Nesi (Max Planck Institute for Molecular Plant Physiology, Golm, Germany) are much appreciated. Dr Bénédicte A Lebouteiller (Institute for Plant Biotechnology; Stellenbosch University; South Africa) is thanked for her assistance as is funding from the National Research Foundation; South Africa.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Cronje, C., George, G.M., Fernie, A.R. et al. Manipulation of l-ascorbic acid biosynthesis pathways in Solanum lycopersicum: elevated GDP-mannose pyrophosphorylase activity enhances l-ascorbate levels in red fruit. Planta 235, 553–564 (2012). https://doi.org/10.1007/s00425-011-1525-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00425-011-1525-6