Abstract
The fruit of Actinidia are unusual in that they contain very high concentrations of vitamin C—over 80 mg/100 g fresh weight (FW) in commercial cultivars and over 800 mg/100 g FW in some wild species. In this review, we describe the genes for various proposed pathways for ascorbate production, via l-galactose, via glucuronate from myo-inositol and via galacturonate from pectin. We then focus on the l-galactose pathway genes and enzymes identified in kiwifruit. We also discuss the presence of genes that recycle ascorbate and the production of oxalate, another metabolite with a high concentration in kiwifruit. Lastly, we discuss two levels of regulation of ascorbate biosynthesis in kiwifruit, at the transcriptional level through the gene that encodes the enzyme GDP-galactose phosphorylase (GGP) and at the translational level through feedback control of GGP translation involving a upstream open reading frame on the 5′ untranslated region of GGP.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Fruit Development
- Ascorbate Concentration
- Phosphomannose Isomerase
- Ascorbate Biosynthesis
- Actinidia Species
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
13.1 Introduction
Vitamin C or ascorbate is not synthesised by humans and is consequently needed in the diet with the best dietary sources of ascorbate being green vegetables and fruits. Of the commercially significant and traded dessert fruit, kiwifruit has the highest concentration of vitamin C. While other fruit have higher ascorbate (e.g. acerola), they are either not significantly traded or mainly processed (frozen or juiced, e.g. blackcurrants and dried, e.g. acerola). Actinidia chinensis var. deliciosa ‘Hayward’ kiwifruit has 85 mg ascorbate/100 g fresh weight (FW); Actinidia chinensis var. chinensis ‘Hort16A’ marketed as Zespri® Gold Kiwifruit has 105–110 mg ascorbate/100 g FW while Actinidia eriantha can have over 800 mg/100 g FW. On a fresh weight basis, green ‘Hayward’ and gold ‘Hort16A’ kiwifruit contain 50 % more vitamin C than an orange, five or six times as much as a banana or ten times as much as an apple (Huang and Ferguson 2007). High vitamin C concentration is one of the reasons that kiwifruit are rated as very healthy (Ferguson and Ferguson 2003).
There is considerable variation between Actinidia species in ascorbate concentration, from low values in fruit of Actinidia henryi (4.4 mg/100 g FW) and Actinidia rudis (5) to very high in Actinidia latifolia (671–2140) and A. eriantha (500–1379 mg/100 g FW) (Huang et al. 2004). Fruit of Actinidia kolomikta also contain high amounts of ascorbate (650–850) (Chesoniene et al. 2004). When the total amount of vitamin C concentration per fruit is calculated, the best sources are the fruit of A. eriantha, A. chinensis var. chinensis and A. chinensis var. deliciosa, all of which have larger fruit. There is also large variation in the vitamin C concentration within a species (Huang and Ferguson 2007). Accessions of A. chinensis var. chinensis range from 50 to 420 mg ascorbate/100 g FW (Huang et al. 2004) while 143 fruiting plants from a single cross of A. chinensis var. chinensis ranged from 49 to 209 mg/100 g FW [A.R. Ferguson, unpublished, (Bulley et al. 2009)]. Values for ascorbate concentrations in fruit of accessions of A. chinensis var. deliciosa range from 30 to ca. 400 mg/100 g FW (Ferguson 1991). While the levels of the acids citrate, quinate and malate are usually much higher than ascorbate (Marsh et al. 2009) in cultivars of A. chinensis var. chinensis, A. chinensis var. deliciosa and Actinidia arguta, this is not the case in ultra-high ascorbate Actinidia genotypes.
In addition, in some Actinidia species, ascorbate content has been shown to have a high heritability (Cheng et al. 2004) which is linked to increased soluble sugar content. In the A. chinensis var. chinensis cross mentioned above, a significant QTL for ascorbate was detected in the 143 progeny (M.A. McNeilage et al. personal communication) on the genetic map (Fraser et al. 2009). In segregating back-cross populations (A. eriantha × A. chinensis var. chinensis or A. chinensis var. deliciosa back-crossed to either A. chinensis var. deliciosa or A. chinensis var. chinensis), a simple genetic model with two main loci will fit the measured ascorbate data (W.A. Laing and A.G. Seal unpublished).
13.2 Biochemical Pathways of Ascorbate Biosynthesis
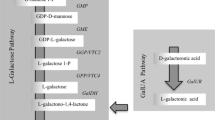
One main pathway of ascorbate biosynthesis has been fully documented in various species of higher plants, named the l-Galactose pathway (Wheeler et al. 1998; Linster and Clarke 2008; Wheeler et al. 2015), starting from glucose, and all the genes in the pathway have been identified (Fig. 13.1) and shown to have activity as proteins (including kiwifruit, W.A. Laing et al. unpublished). The genes for this pathway have been identified, cloned and validated from various Actinidia species (Laing et al. 2004a, 2007; Richardson et al. 2004; Linster et al. 2008; Bulley et al. 2009; Torabinejad et al. 2009; Li et al. 2011; Bulley et al. 2012; Li et al. 2013a, b, c, 2014). Alternative pathways through galacturonate and glucuronate (Li et al. 2010b) have been proposed but not all the genes and enzymes have been identified. The glucuronate pathway could either derive from glucose via UDP-glucose or from conversion of myo-inositol conversion to glucuronate by myo-inositol oxygenase. The most likely precursor to the galacturonate pathway would be either pectin or UDP-glucuronate epimerisation to UDP-galacturonate. However, polygalacturonase gene expression (Wang et al. 2000) and kiwifruit cell wall breakdown occurs later in fruit development and ripening when ascorbate concentrations are stable (Bulley et al. 2009).
Reactions, enzymes and context of ascorbic acid biosynthesis and regeneration in plants. (A) l-Galactose pathway, reactions 2–9. (B) myo-Inositol/glucuronate pathway, reactions 7, 18–26. (C) Galacturonate pathway, reactions 14–17. (D) l-Gulose pathway, possible reactions 5, 6, 7, 8 and 10. Reactions with question marks after the number are hypothetical and the exact enzyme is yet to be identified. Underlined chemical names are those that appear in more than one position in the diagram. Gene expression of transcripts of numbered enzymes in bold type was analysed. 1, glucose-6-phosphate isomerase; 2, mannose-6-phosphate isomerase; 3, phosphomannomutase; 4, GDP-mannose pyrophosphorylase; 5, GDP-mannose-3′,5′-epimerase; 6, GDP-l-galactose transferase; 7, l-galactose-1-phosphate phosphatase; 8, l-galactose dehydrogenase; 9, l-galactono-1,4-lactone dehydrogenase; 10, l-gulono-1,4-lactone oxidase; 11, GDP-d-mannose-4,6-dehydratase; 12, GDP-l-fucose synthase; 13, UDP-galacturonate epimerase; 14, polygalacturonate 4-α-galacturonosyltransferase; 15, galacturonate-1-phosphate uridylyltransferase and galacturonate-1-phosphate phosphatase (hypothetical); 16, d-galacturonic acid reductase; 17, aldonolactonase; 18, l-myo-inositol 1-phosphate synthase; 19, myo-inositol oxygenase; 20, d-glucurono-1-phosphate phosphatase; 21, glucuronate reductase; 22, gulonolactonase; 23, phosphoglucomutase; 24, UDP-glucose-pyrophosphorylase; 25, UDP-glucose dehydrogenase; 26, glucuronate-1-phosphate uridylyltransferase; 27, monodehydroascorbate reductase; 28, dehydroascorbate reductase; vtc, vitamin C content (Bulley et al. 2009. With the permission of Oxford University Press)
13.2.1 l-Galactose Pathway
13.2.1.1 Conversion of Glucose-6-P to Gannose-1-P
The genes encoding the enzymes for these conversions have all been identified in kiwifruit and do not appear to be limiting ascorbate formation, although overexpression of phosphomannomutase (PMM) has been shown to increase ascorbate in leaves of other species (Qian et al. 2007; Badejo et al. 2009). It is possible that, under some circumstances, carbon supply to ascorbate biosynthesis might limit ascorbate synthesis. Two phosphomannose isomerase (PMI) genes exist in Arabidopsis with PMI1 being shown to be essential for ascorbate synthesis and the only one to be expressed constitutively, with PMI2 not being expressed in light (Maruta et al. 2008). Both PMI isoforms were feedback inhibited by ascorbate showing a higher level general cut-off mechanism to be in place before metabolism enters ascorbate biosynthesis proper (Maruta et al. 2008).
Phosphoglucoisomerase (PGI) , PMI and PMM are all represented in the kiwifruit genome (Huang et al. 2013) and found in the kiwifruit EST collection (Crowhurst et al. 2008) (Table 13.1).
13.2.1.2 Conversion of Mannose-1-P to Galactose-1-P
GDP-mannose pyrophosphorylase (GMP) converts mannose-1-P to GDP-mannose . GDP-mannose provides carbon both for ascorbate synthesis and for cell wall components and protein modification (Zablackis et al. 1996; Keller et al. 1999; Handford et al. 2003). In some circumstances, it is probable that the supply of carbon skeletons may limit ascorbate biosynthesis which may be why transformation with the GMP gene has variable effects of ascorbate concentration (Badejo et al. 2007; Wang et al. 2011; Cronje et al. 2012; Imai et al. 2012; Zhou et al. 2012; Zhang et al. 2015a). It is possible that upstream PMI expression levels may also be limiting and this suggests that coexpression of PMI might overcome such limitations.
GDP-mannose epimerase (GME) is another key gene in that it results in the formation of GDP-l-galactose which is almost all used for ascorbate production, with little l-galactose being found elsewhere in the metabolome (Gilbert et al. 2009). However, by itself GME has little effect of ascorbate concentrations in plant tissues (see below).
GDP-galactose phosphorylase (GGP) which converts GDP-galactose to galactose-1-phosphate is the key enzyme in the ascorbate pathway and has been shown to control ascorbate in a wide range of species (Laing et al. 2007; Bulley et al. 2009, 2012; Zhang et al. 2011, 2015a; Zhou et al. 2012; Li et al. 2013a, b, c; Huang et al. 2014; Ma et al. 2014). The evidence is either from the studying patterns of gene expression of these genes during fruit development in kiwifruit or from the overexpression of kiwifruit genes in other species.
Transformation with GGP results in very significant increases in tissue ascorbate concentrations (Laing et al. 2007; Bulley et al. 2012; Zhou et al. 2012; Zhang et al. 2015a). While transformation with GME alone has small effects on ascorbate in a range of species (Zhang et al. 2011, 2015b; Huang et al. 2014; Ma et al. 2014), coexpression of GME and GGP results in a strong synergistic increase on ascorbate concentration (Bulley et al. 2009; Laing et al. 2015). GME gene transcription also tracks ascorbate production along with GGP transcription (Bulley et al. 2009; Li et al. 2013c).
13.2.1.3 Conversion of Galactose-1-P to Ascorbate
Galactose-1-P phosphatase (GPP) (Laing et al. 2004b), galactose dehydrogenase (GalDH) (Laing et al. 2004a; Li et al. 2010b) and galactono lactone dehydrogenase (GalLDH) (W.A. Laing unpublished) have all been identified in kiwifruit, but again do not appear to regulate ascorbate formation, except when activity is reduced (Tabata et al. 2001; Gatzek et al. 2002; Conklin et al. 2006; Alhagdow et al. 2007; Imai et al. 2009; Torabinejad et al. 2009; Zhou et al. 2012; Zhang et al. 2015b). However, it has been suggested GPP may be involved in light and abiotic stress responses (Li et al. 2013b). In tomato, GPP has been suggested to play an important role in regulating ascorbate accumulation during fruit development (Ioannidi et al. 2009), and while its expression was the highest of the l-galactose pathway genes, GME and more particularly GGP expression also correlated well with ascorbate accumulation. A later study, also in tomato, found that ‘translocation from source leaves and biosynthesis via the d-mannose/l-galactose pathway are dominant sources in immature fruits, while the alternative d-galacturonate pathway contributes to AsA [ascorbate] accumulation in ripened Micro-Tom fruits’ (Badejo et al. 2012). It therefore appears that a possible explanation for the very high expression of GPP in ripening tomato fruit may be it is there to convert the l-galactose-1P postulated to be derived from the d-galacturonate pathway via the conversion of UDP-d-galacturonate to l-galactose-1P (Fig. 13.1).
13.2.2 l-Glucuronate Pathway and Myo-Inositol
A. chinensis var. deliciosa and A. arguta contain high concentrations of myo-inositol in the leaves and fruit (Bieleski et al. 1997; Klages et al. 1998) which potentially could serve as a substrate of ascorbate production by conversion of myo-inositol to glucuronate by myo-inositol oxygenase. Myo-inositol-1-P is synthesised from glucose-6-P by myo-inositol synthase and then, myo-inositol-1-P is dephosphorylated by the enzyme myo-inositol phosphatase (Gillaspy et al. 1995). This latter enzyme is the same as that encoded by GPP, l-galactose-1-P phosphatase (Laing et al. 2004b) in both kiwifruit and Arabidopsis (Torabinejad et al. 2009). Good homologues of the oxygenase and the synthase have also been identified in a kiwifruit EST collection (Crowhurst et al. 2008). The glucuronate is then converted to l-gulonate by an unknown enzyme, then to the gulonate 1,4 lactone and thence to ascorbate, again by enzymes encoded by unknown genes. However, possible candidates for these genes are found in the kiwifruit EST database (Crowhurst et al. 2008) although they are not validated, even in other species. The other genes that encode enzymes that convert glucose to glucuronate (UDP-glucose dehydrogenase , glucuronate-1-P uridyltransferase and glucurono-1-P phosphatase ) are validated in other species and good homologues are present in the kiwifruit genome and EST databases.
13.2.3 l-Galacturonate Pathway
This pathway is proposed to result in ascorbate production from the breakdown of cell wall pectin into galacturonate (Di Matteo et al. 2010) or from the conversion of UDP-glucuronate into UDP-galacturonate by UDP-glucuronate epimerase (Gu and Bar-Peled 2004; Usadel et al. 2004). The UDP-galacturonate is then hydrolysed to galacturonate (this requires a uridylyltransferase and a phosphatase, no enzymes characterised) and then reduced to galactonate by galacturonate reductase (Agius et al. 2003). A homologue to the validated strawberry galacturonate reductase is found in kiwifruit ESTs (Crowhurst et al. 2008) and in the kiwifruit genome (Huang et al. 2013) with 65–67 % identity and 83 % similarity, respectively. The kiwifruit gene has been cloned and expressed in Escherichia coli, but it showed no reductase activity (Li et al. 2010b, 2011). While the strawberry gene has been shown to increase ascorbate in tomato (Amaya et al. 2014), potato (Hemavathi et al. 2009) and Arabidopsis (Agius et al. 2003), this gene is not thought to control ascorbate in kiwifruit (Li et al. 2010b, 2011).
13.3 Ascorbate Recycling Enzymes
A reactive oxygen species or an oxidising agent such as H2O2 oxidises ascorbate to monodehydroascorbate (MDHA), which is either directly reduced back to ascorbate by monodehydroascorbate reductase (MDHAR) or non-enzymatically disproportionated into ascorbate and dehydroascorbate (DHA) . DHA is then reduced back to ascorbate through reducing equivalents provided by glutathione either chemically or by dehydroascorbate reductase (DHAR) . Oxidised glutathione is finally reduced by glutathione reductase . This redox hub maintains the ascorbate in a mostly reduced state (Foyer and Noctor 2011). There are five MDHAR genes and three DHAR genes in Arabidopsis.
Different studies have overexpressed DHAR (Chen et al. 2003; Kwon et al. 2003; Eltayeb et al. 2006; Goo et al. 2008; Naqvi et al. 2009; Yin et al. 2010; Haroldsen et al. 2011; Qin et al. 2011; Huang et al. 2014) and MDHAR (Kavitha et al. 2010; Li et al. 2010a; Yin et al. 2010; Haroldsen et al. 2011; Gest et al. 2012) in a range of plants and, except for maize kernels (only very low ascorbate is present in seeds), the concentration of ascorbate was mostly little affected. None of these studies was performed in kiwifruit. However, many papers reported an increase in the ratio of reduced to oxidised ascorbate and an improvement in stress resistance.
Various versions of the two ascorbate recycling genes are found in the kiwifruit genome (Table 13.1). However, there appears to be no published work on MDHAR and DHAR genes in kiwifruit. We have constitutively overexpressed a kiwifruit DHAR in Arabidopsis which resulted in changes to the ratio of reduced/oxidised ascorbate and also appeared to increase salinity tolerance (S.M. Bulley, unpublished).
13.4 Oxalate in Kiwifruit
Kiwifruit fruit also contain high amounts of oxalate, much in crystalline (raphide) form (Rassam and Laing 2005; Rassam et al. 2007). In other species, it has been proposed that ascorbate breaks down into oxalate (Keates et al. 2000; Kostman et al. 2001) although little is known about genes involved in the pathway (Green and Fry 2005). The relationship between the amount of oxalate and ascorbate in a plant tissue is not straightforward, suggesting that the breakdown of ascorbate is a regulated process (Rassam and Laing 2005; Rassam et al. 2007).
13.5 Regulation of Ascorbate Biosynthesis
13.5.1 Regulation Through Gene Expression of Pathway Genes
There have been several studies of expression of vitamin C-related genes in kiwifruit (Bulley et al. 2009; Li et al. 2010b, 2013a, c, 2014) and these have shown changes during fruit development and between kiwifruit species and accessions. For example, Bulley et al. 2009 showed large differences during fruit development with a peak in ascorbate accumulation around four to seven weeks after anthesis and gene expression maxima around the same time. However, the biggest differences between taxa (A. chinensis var. chinensis, A. chinensis var. deliciosa and A. eriantha) were for GGP and GME, and to some extent for GMP, with the high ascorbate A. eriantha having much higher expression levels of these genes. Through transient and stable transformation of Arabidopsis thaliana and Nicotiana benthamiana with kiwifruit genes, it was concluded that GGP and GME synergistically controlled ascorbate biosynthesis. Other results (Li et al. 2014) also supported the difference in GGP expression between A. eriantha (high ascorbate) and Actinidia rufa (low ascorbate). On the other hand, Li et al. 2010b showed a similar pattern of ascorbate accumulation and gene expression during fruit development, for the one cultivar A. chinensis var. deliciosa ‘Qinmei’. Based on correlations between gene expression and ascorbate concentrations, they concluded GPP controlled ascorbate biosynthesis. Various light and stress conditions have also been shown to affect gene expression of GGP, GME (Li et al. 2013c) and GPP (Li et al. 2013b) in kiwifruit, but these studies do not determine where the control of ascorbate biosynthesis lies in fruit. Correlations between gene expression and a metabolite support but do not prove that a gene product regulates the metabolite, and it is likely some degree of coordination between genes in a pathway would occur. Some recent sequencing of an A. chinensis var. chinensis kiwifruit during fruit development is shown in Fig. 13.2. These data (L. Luo et al. unpublished) are consistent with other published studies mentioned above.
Various studies have established GGP as a key regulator of ascorbate biosynthesis in many plants, and most work has been done on this gene (Laing et al. 2007; Bulley et al. 2009; Li et al. 2013a, b, 2014). This is reasonable given that the GGP enzyme catalyses the first committed step in ascorbate biosynthesis, although others have suggested GMP as the key control gene (Wang et al. 2013a). Gene expression of GGP and GME in a range of species is strongly regulated by light (Dowdle et al. 2007; Yabuta et al. 2007; Gao et al. 2011; Massot et al. 2012) and abiotic stress (Li et al. 2013a), both factors regulate ascorbate concentrations. In addition, as discussed above, expression of these two genes relates to the ascorbate concentrations in different kiwifruit species with different ascorbate concentrations (Bulley et al. 2009; Li et al. 2014). More significantly, transient transformation of Nicotiana benthamiana with various kiwifruit genes from the ascorbate biosynthetic pathway shows that GGP strongly affects ascorbate, and GME has little effect while a combination of GME and GGP synergistically stimulates ascorbate concentration (Bulley et al. 2009, 2012; Laing et al. 2015). Other genes in the pathway except GMP have little effect on ascorbate concentration ((Zhou et al. 2012), W.A. Laing unpublished). In addition, genetic mapping in apple has established that the QTLs for ascorbate colocate with GGP orthlogs (Mellidou et al. 2012) and that transformation of strawberry, potato and tomato with kiwifruit or Arabidopsis GGP results in significantly increased ascorbate (Bulley et al. 2012). GGP gene expression also shows a strong diurnal trend, peaking during the morning and falling during the day in Arabidopsis (Dowdle et al. 2007), suggesting that ascorbate biosynthesis potential is primed for the period of the day when peak ascorbate is required, at midday maximum light intensity. We have also observed similar diurnal trends in gene expression in the leaves of Arabidopsis (W.A. Laing unpublished).
13.5.2 Regulation Through Translation of GGP
It appears well established that transcriptional regulation of the key genes GGP and GME controls ascorbate concentrations. However, translational regulation of ascorbate concentrations also plays a significant part. Recently, it was shown that the 5′ untranslated region (UTR) of GGP from a wide range of species including kiwifruit contains a highly conserved upstream open reading frame (uORF) (Laing et al. 2015). This uORF is unusual in that it starts with a non-canonical start codon, ACG. The model proposed was that under high ascorbate concentrations, the uORF is translated and acts as an inhibitor of GGP translation. Under low ascorbate, the uORF is skipped and GGP is translated. This model provides a direct link between the ascorbate concentration and the production of GGP, the key regulatory enzyme in ascorbate biosynthesis, and allows rapid and feedback responsive control of ascorbate biosynthesis under demand conditions (e.g. high light and low temperatures).
13.5.3 Other Ascorbate Affecting Genes
Four genes that regulate ascorbate in Arabidopsis have been identified and characterised. These are a protein kinase/phosphatase (VTC3) (Conklin et al. 2013), a transcription factor (AtERF98) (Zhang et al. 2012), photomorphogenic factor COP9 signalosome subunit 5B (CSN5B) (Wang et al. 2013b) and an F box protein (AMR1) (Zhang et al. 2009). Actinidia homologues of the first three genes have been found but not of the F box protein. Little is known how VTC3 regulates ascorbate except that it is constitutively expressed and does not vary much under a wide range of conditions, suggesting that regulation may be at a post-transcriptional level (Conklin et al. 2013). It is possible that VTC3 may be a factor in the uORF regulation described above (Laing et al. 2015). AMR1 appears to be a negative regulator of ascorbate and a negative regulator of the l-galactose pathway of ascorbate biosynthesis genes (Zhang et al. 2009). Inspection of this paper shows that GGP and GME are the genes most affected by AMR1. An obvious hypothesis is that AMR1 targets for degradation a transcription factor that increases transcription of the ascorbate genes. The AtERF98 transcription factor increases the transcription of many of the genes in the l-galactose pathway, GMP especially and GGP (Zhang et al. 2012) and binds with the promoter of GMP. However, the authors did not explore how AtERF98 interacted with other promoters or whether it was a target for AMR1. Lastly, CSN5B was shown to interact with the N terminus of GMP and target it for degradation (Wang et al. 2013b). CSN5B appears to promote degradation of GMP protein in the dark through ubiquitination and the proteasome. However, the authors did not test whether CSN5B interacted with other genes in the l-galactose pathway. Interestingly, knockout of another COP9 signalosome subunit, CSN8, increased ascorbate even further than a knockout of CSN5B (Wang et al. 2013b).
In addition, an ascorbate transporter located in the chloroplast has also been identified (Miyaji et al. 2015) and an Actinidia homologue is present. The transporter would serve to transport ascorbate into the chloroplast from its point of synthesis in the mitochondria.
13.6 Conclusions
The ascorbate biosynthetic pathway in kiwifruit is similar to that found in other species. The most likely pathway to provide the great bulk of ascorbate in kiwifruit is the l-Galactose pathway and regulation of ascorbate concentration is through the GGP gene and enzyme, with possibly some higher level feedback by ascorbate against PMI1. Ascorbate content plant tissues appear to be mainly the result of variation in transcription of GGP (and GME in synergy with GGP) as well as at the translational level through feedback regulation by ascorbate of the translation of GGP. Regulation through GGP makes sense as it the first committed step in the biosynthesis of ascorbate.
References
Agius F, González-Lamothe R, Caballero JL, Muñoz-Blanco J, Botella MA, Valpuesta V (2003) Engineering increased vitamin C levels in plants by overexpression of a d-galacturonic acid reductase. Nat Biotechnol 21:177–181
Alhagdow M, Mounet F, Gilbert L, Nunes-Nesi A, Garcia V, Just D et al (2007) Silencing of the mitochondrial ascorbate synthesizing enzyme l-galactono-1,4-lactone dehydrogenase affects plant and fruit development in tomato. Plant Physiol 145:1408–1422
Amaya I, Osorio S, Martinez-Ferri E, Lima-Silva V, Doblas VG, Fernández-Muñoz R et al (2014) Increased antioxidant capacity in tomato by ectopic expression of the strawberry d-galacturonate reductase gene. Biotechnol J 10:490–500
Badejo AA, Tanaka N, Esaka M (2007) Analysis of GDP-d-mannose pyrophosphorylase gene promoter from acerola (Malpighia glabra) and increase in ascorbate content of transgenic tobacco expressing acerola gene. Plant Cell Physiol 49:126–132
Badejo AA, Eltelib HA, Fukunaga K, Fujikawa Y, Esaka M (2009) Increase in ascorbate content of transgenic tobacco plants overexpressing the acerola (Malpighia glabra) phosphomannomutase gene. Plant Cell Physiol 50:423–428
Badejo AA, Wada K, Gao YS, Maruta T, Sawa Y, Shigeoka S, Ishikawa T (2012) Translocation and the alternative d-galacturonate pathway contribute to increasing the ascorbate level in ripening tomato fruits together with the d-mannose/l-galactose pathway. J Exp Bot 63:229–239
Bieleski RL, Clark CJ, Klages KU (1997) Identification of myo-inositol as a major carbohydrate in kiwifruit, Actinidia deliciosa. Phytochemistry 46:51–55
Bulley SM, Rassam M, Hoser D, Otto W, Schünemann N, Wright M et al (2009) Gene expression studies in kiwifruit and gene over-expression in Arabidopsis indicates that GDP-l-galactose guanyltransferase is a major control point of vitamin C biosynthesis. J Exp Bot 60(3):765–778
Bulley S, Wright M, Rommens C, Yan H, Rassam M, Lin-Wang K et al (2012) Enhancing ascorbate in fruits and tubers through over-expression of the l-galactose pathway gene GDP-l-galactose phosphorylase. Plant Biotechnol J 10:390–397
Chen Z, Young TE, Ling J, Chang S-C, Gallie DR (2003) Increasing vitamin C content of plants through enhanced ascorbate recycling. Proc Nat Acad Sci USA 100:3525–3530
Cheng CH, Seal AG, Boldingh HL, Marsh KB, MacRae EA, Murphy SJ et al (2004) Inheritance of taste characters and fruit size and number in a diploid Actinidia chinensis (kiwifruit) population. Euphytica 138:185–195
Chesoniene L, Daubaras R, Viskelis P (2004) Biochemical composition of berries of some Kolomikta kiwi (Actinidia kolomikta) cultivars and detection of harvest maturity. Acta Hort 663:305–308
Conklin PL, Gatzek S, Wheeler GL, Dowdle J, Raymond MJ, Rolinski S et al (2006) Arabidopsis thaliana VTC4 encodes l-galactose-1-P phosphatase, a plant ascorbic acid biosynthetic enzyme. J Biol Chem 281:15662–15670
Conklin PL, DePaolo D, Wintle B, Schatz C, Buckenmeyer G (2013) Identification of Arabidopsis VTC3 as a putative and unique dual function protein kinase: protein phosphatase involved in the regulation of the ascorbic acid pool in plants. J Exp Bot 64:2793–2804
Cronje C, George GM, Fernie AR, Bekker J, Kossmann J, Bauer R (2012) Manipulation of l-ascorbic acid biosynthesis pathways in Solanum lycopersicum: elevated GDP-mannose pyrophosphorylase activity enhances l-ascorbate levels in red fruit. Planta 235:553–564
Crowhurst RN, Gleave AP, MacRae EA, Ampomah-Dwamena C, Atkinson RG, Beuning LL et al (2008) Analysis of expressed sequence tags from Actinidia: applications of a cross species EST database for gene discovery in the areas of flavor, health, color and ripening. BMC Genom 9:351
Di Matteo A, Sacco A, Anacleria M, Pezzotti M, Delledonne M, Ferrarini A et al (2010) The ascorbic acid content of tomato fruits is associated with the expression of genes involved in pectin degradation. BMC Plant Biol 10:163
Dowdle J, Ishikawa T, Gatzek S, Rolinski S, Smirnoff N (2007) Two genes in Arabidopsis thaliana encoding GDP-l-galactose phosphorylase are required for ascorbate biosynthesis and seedling viability. Plant J 52:673–689
Eltayeb AE, Kawano N, Badawi GH, Kaminaka H, Sanekata T, Morishima I et al (2006) Enhanced tolerance to ozone and drought stresses in transgenic tobacco overexpressing dehydroascorbate reductase in cytosol. Physiol Plant 127:57–65
Ferguson AR (1991) Kiwifruit (Actinidia). Acta Hort 290:603–656
Ferguson AR, Ferguson LR (2003) Are kiwifruit really good for you? Acta Hort 610:131–138
Foyer CH, Noctor G (2011) Ascorbate and glutathione: the heart of the redox hub. Plant Physiol 155:2–18
Fraser LG, Tsang GK, Datson PM, De Silva HN, Harvey CF, Gill GP et al (2009) A gene-rich linkage map in the dioecious species Actinidia chinensis (kiwifruit) reveals putative X/Y sex-determining chromosomes. BMC Genom 10:102
Gao Y, Badejo AA, Shibata H, Sawa Y, Maruta T, Shigeoka S et al (2011) Expression analysis of the VTC2 and VTC5 genes encoding GDP-l-galactose phosphorylase, an enzyme involved in ascorbate biosynthesis, in Arabidopsis thaliana. Biosci Biotechnol Biochem 75:1783–1788
Gatzek S, Wheeler GL, Smirnoff N (2002) Antisense suppression of l-galactose dehydrogenase in Arabidopsis thaliana provides evidence for its role in ascorbate synthesis and reveals light modulated l-galactose synthesis. Plant J 30:541–553
Gest N, Garchery C, Gautier H, Jiménez A, Stevens R (2012) Light-dependent regulation of ascorbate in tomato by a monodehydroascorbate reductase localized in peroxisomes and the cytosol. Plant Biotechnol J 11:344–354
Gilbert L, Alhagdow M, Nunes-Nesi A, Quemener B, Guillon F, Bouchet B et al (2009) GDP-d-mannose 3,5-epimerase (GME) plays a key role at the intersection of ascorbate and non-cellulosic cell-wall biosynthesis in tomato. Plant J 60:499–508
Gillaspy GE, Keddie JS, Oda K, Gruissem W (1995) Plant inositol monophosphatase is a lithium-sensitive enzyme encoded by a multigene family. Plant Cell 7:2175–2185
Goo Y-M, Chun H, Kim T-W, Lee C-H, Ahn M-J, Bae S-C et al (2008) Expressional characterization of dehydroascorbate reductase cDNA in transgenic potato plants. J Plant Biol 51:35–41
Green MA, Fry SC (2005) Vitamin C degradation in plant cells via enzymatic hydrolysis of 4-O-oxalyl-l-threonate. Nature 433:83–87
Gu X, Bar-Peled M (2004) The biosynthesis of UDP-galacturonic acid in plants. Functional cloning and characterization of Arabidopsis UDP-d-glucuronic acid 4-epimerase. Plant Physiol 136:4256–4264
Handford MG, Baldwin TC, Goubet F, Prime TA, Miles J, Yu X et al (2003) Localisation and characterisation of cell wall mannan polysaccharides in Arabidopsis thaliana. Planta 218:27–36
Haroldsen VM, Chi-Ham CL, Kulkarni S, Lorence A, Bennett AB (2011) Constitutively expressed DHAR and MDHAR influence fruit, but not foliar ascorbate levels in tomato. Plant Physiol Biochem 49:1244–1249
Hemavathi, Upadhyaya CP, Young KE, Akula N, soon Kim H et al (2009) Over-expression of strawberry d-galacturonic acid reductase in potato leads to accumulation of vitamin C with enhanced abiotic stress tolerance. Plant Sci 177:659–667
Huang H-W, Ferguson AR (2007) [incorrectly published as Ferguson AR, Huang H-W] Genetic resources of kiwifruit: Domestication and breeding. Hort Rev 33: 1-121
Huang H-W, Wang Y, Zhang Z-H, Jiang Z-W, Wang S-M (2004) Actinidia germplasm resources and kiwifruit industry In China. HortScience 39:1165–1172
Huang S, Ding J, Deng D, Tang W, Sun H, Liu D et al (2013) Draft genome of the kiwifruit Actinidia chinensis. Nat Commun 4:2640
Huang M, Xu Q, Deng X-X (2014) l-Ascorbic acid metabolism during fruit development in an ascorbate-rich fruit crop chestnut rose (Rosa roxburghii Tratt). J Plant Physiol 171:1205–1216
Imai T, Niwa M, Ban Y, Hirai M, Ôba K, Moriguchi T (2009) Importance of the l-galactonolactone pool for enhancing the ascorbate content revealed by l-galactonolactone dehydrogenase overexpressing tobacco plants. Plant Cell Tiss Org Cult 96:105–112
Imai T, Ban Y, Yamamoto T, Moriguchi T (2012) Ectopic overexpression of peach GDP- d -mannose pyrophosphorylase and GDP- d -mannose-3′,5′-epimerase in transgenic tobacco. Plant Cell Tiss Organ Cult 111:1–13
Ioannidi E, Kalamaki MS, Engineer C, Pateraki I, Alexandrou D, Mellidou I et al (2009) Expression profiling of ascorbic acid-related genes during tomato fruit development and ripening and in response to stress conditions. J Exp Bot 60:663–678
Kavitha K, George S, Venkataraman G, Parida A (2010) A salt-inducible chloroplastic monodehydroascorbate reductase from halophyte Avicennia marina confers salt stress tolerance on transgenic plants. Biochimie 92:1321–1329
Keates SE, Tarlyn NM, Loewus FA, Franceschi VR (2000) l-Ascorbic acid and l-galactose are sources for oxalic acid and calcium oxalate in Pistia stratiotes. Phytochemistry 53:433–440
Keller R, Springer F, Renz FS, Kossmann J (1999) Antisense inhibition of the GDP-mannose pyrophosphorylase reduces the ascorbate content in transgenic plants leading to developmental changes during senescence. Plant J 19:131–141
Klages K, Donnison H, Boldingh H, MacRae E (1998) myo-Inositol is the major sugar in Actinidia arguta during early fruit development. Aust J Plant Physiol 25:61–67
Kostman TA, Tarlyn NM, Loewus FA, Franceschi VR (2001) Biosynthesis of l-ascorbic acid and conversion of carbons 1 and 2 of l-ascorbic acid to oxalic acid occurs within individual calcium oxalate crystal idioblasts. Plant Physiol 125:634–640
Kwon S-Y, Choi S-M, Ahn Y-O, Lee H-S, Lee H-B, Park Y-M et al (2003) Enhanced stress-tolerance of transgenic tobacco plants expressing a human dehydroascorbate reductase gene. J Plant Physiol 160:347–353
Laing WA, Frearson N, Bulley S, MacRae, E (2004a) Kiwifruit l-galactose dehydrogenase; molecular, biochemical and physiological aspects of the enzyme. Funct Plant Biol 31:1015–1025
Laing WA, Bulley S, Wright M, Cooney J, Jensen D, Barraclough D, MacRae E (2004) A highly specific l-galactose-1-phosphate phosphatase on the path to ascorbate biosynthesis. Proc Natl Acad Sci USA 101:16976–16981
Laing WA, Wright MA, Cooney J, Bulley SM (2007) The missing step of the l-galactose pathway of ascorbate biosynthesis in plants, an l-galactose guanyltransferase, increases leaf ascorbate content. Proc Natl Acad Sci USA 104:9534–9539
Laing WA, Martínez-Sánchez M, Wright MA, Bulley SM, Brewster D, Dare AP et al (2015) An upstream open reading frame is essential for feedback regulation of ascorbate biosynthesis in Arabidopsis. Plant Cell 27:772–786
Li F, Wu Q-Y, Sun Y-L, Wang L-Y, Yang X-H, Meng Q-W (2010a) Overexpression of chloroplastic monodehydroascorbate reductase enhanced tolerance to temperature and methyl viologen-mediated oxidative stresses. Physiol Plant 139:421–434
Li M-J, Ma F-W, Liang D, Li J, Wang Y-L (2010b) Ascorbate biosynthesis during early fruit development is the main reason for its accumulation in kiwi. PLoS ONE 5:e14281
Li M-J, Liu J, Liang D, Guo C-M, Ma F-W (2011) The relationship between GalUR expression and ascorbate accumulation in kiwifruit. Acta Hort Sin 38:1641–1649
Li J, Liang D, Li M, Ma F (2013a) Light and abiotic stresses regulate the expression of GDP-l-galactose phosphorylase and levels of ascorbic acid in two kiwifruit genotypes via light-responsive and stress-inducible cis-elements in their promoters. Planta 238:535–547
Li J, Li M, Liang D, Cui M, Ma F (2013b) Expression patterns and promoter characteristics of the gene encoding Actinidia deliciosa l-galactose-1-phosphate phosphatase involved in the response to light and abiotic stresses. Mol Biol Rep 40:1473–1485
Li J, Cui M, Li M, Wang X, Liang D, Ma F (2013c) Expression pattern and promoter analysis of the gene encoding GDP-d-mannose 3′,5′-epimerase under abiotic stresses and applications of hormones by kiwifruit. Scientia Hort 150:187–194
Li J, Li M, Liang D, Ma F, Lei Y (2014) Comparison of expression pattern, genomic structure, and promoter analysis of the gene encoding GDP-l-galactose phosphorylase from two Actinidia species. Scientia Hort 169:206–213
Linster CL, Clarke SG (2008) l-Ascorbate biosynthesis in higher plants: the role of VTC2. Trends Plant Sci 13:567–573
Linster CL, Adler LN, Webb K, Christensen KC, Brenner C, Clarke SG (2008) A second GDP-l-galactose phosphorylase in Arabidopsis en route to vitamin C: Covalent intermediate and substrate requirements for the conserved reaction. J Biol Chem 283:18483–18492
Ma L, Wang Y, Liu W, Liu Z (2014) Overexpression of an alfalfa GDP-mannose 3, 5-epimerase gene enhances acid, drought and salt tolerance in transgenic Arabidopsis by increasing ascorbate accumulation. Biotechnol Lett 36:2331–2341
Marsh KB, Boldingh HL, Shilton RS, Laing WA (2009) Changes in quinic acid metabolism during fruit development in three kiwifruit species. Funct Plant Biol 36:463–470
Maruta T, Yonemitsu M, Yabuta Y, Tamoi M, Ishikawa T, Shigeoka S (2008) Arabidopsis phosphomannose isomerase 1, but not phosphomannose isomerase 2, is essential for ascorbic acid biosynthesis. J Biol Chem 283:28842–28851
Massot C, Stevens R, Génard M, Longuenesse J-J, Gautier H (2012) Light affects ascorbate content and ascorbate-related gene expression in tomato leaves more than in fruits. Planta 235:153–163
Mellidou I, Chagné D, Laing WA, Keulemans J, Davey MW (2012) Allelic variation in paralogs of GDP-l-galactose phosphorylase is a major determinant of vitamin C concentrations in apple fruit. Plant Physiol 160:1613–1629
Miyaji T, Kuromori T, Takeuchi Y, Yamaji N, Yokosho K, Shimazawa A et al (2015) AtPHT4;4 is a chloroplast-localized ascorbate transporter in Arabidopsis. Nat Commun 6:5928
Naqvi S, Zhu C, Farre G, Ramessar K, Bassie L, Breitenbach J et al (2009) Transgenic multivitamin corn through biofortification of endosperm with three vitamins representing three distinct metabolic pathways. Proc Natl Acad Sci USA 106:7762–7767
Qian W, Yu C, Qin H, Liu X, Zhang A, Johansen IE, Wang D (2007) Molecular and functional analysis of phosphomannomutase (PMM) from higher plants and genetic evidence for the involvement of PMM in ascorbic acid biosynthesis in Arabidopsis and Nicotiana benthamiana. Plant J 49:399–413
Qin A, Shi Q, Yu X (2011) Ascorbic acid contents in transgenic potato plants overexpressing two dehydroascorbate reductase genes. Mol Biol Rep 38:1557–1566
Rassam M, Laing W (2005) Variation in ascorbic acid and oxalate levels in the fruit of Actinidia chinensis tissues and genotypes. J Agric Food Chem 53:2322–2326
Rassam M, Bulley SM, Laing WA (2007) Oxalate and ascorbate in Actinidia fruit and leaves. Acta Hort 753:479–484
Richardson AC, Marsh KB, Boldingh HL, Pickering AH, Bulley SM, Frearson NJ et al (2004) High growing temperatures reduce fruit carbohydrate and vitamin C in kiwifruit. Plant Cell Environ 27:423–435
Tabata K, Oba K, Suzuki K, Esaka M (2001) Generation and properties of ascorbic acid-deficient transgenic tobacco cells expressing antisense RNA for l-galactono-1,4- actone dehydrogenase. Plant J 27:139–148
Torabinejad J, Donahue JL, Gunesekera BN, Allen-Daniels MJ, Gillaspy GE (2009) VTC4 is a bifunctional enzyme that affects myoinositol and ascorbate biosynthesis in plants. Plant Physiol 150:951–961
Usadel B, Schlüter U, Mølhøj M, Gipmans M, Verma R, Kossmann J, Reiter W-D et al (2004) Identification and characterization of a UDP-glucuronate 4-epimerase in Arabidopsis. FEBS Lett 569:327–331
Wang Z-Y, MacRae EA, Wright MA, Bolitho KM, Ross GS, Atkinson RG (2000) Polygalacturonase gene expression in kiwifruit: relationship to fruit softening and ethylene production. Plant Mol Biol 42:317–328
Wang H-S, Yu C, Zhu Z-J, Yu X-C (2011) Overexpression in tobacco of a tomato GMPase gene improves tolerance to both low and high temperature stress by enhancing antioxidation capacity. Plant Cell Rep 30:1029–1040
Wang J, Zhang Z, Huang R (2013a) Regulation of ascorbic acid synthesis in plants. Plant Signaling Behavior 8:e24536
Wang J, Yu Y, Zhang Z, Quan R, Zhang H, Ma L et al (2013b) Arabidopsis CSN5B interacts with VTC1 and modulates ascorbic acid synthesis. Plant Cell Online 25:625–636
Wheeler GL, Jones MA, Smirnoff N (1998) The biosynthetic pathway of vitamin C in higher plants. Nature 393:365–369
Wheeler G, Ishikawa T, Pornsaksit V, Smirnoff N (2015) Evolution of alternative biosynthetic pathways for vitamin C following plastid acquisition in photosynthetic eukaryotes. eLife 4:e06369
Yabuta Y, Mieda T, Rapolu M, Nakamura A, Motoki T, Maruta T et al (2007) Light regulation of ascorbate biosynthesis is dependent on the photosynthetic electron transport chain but independent of sugars in Arabidopsis. J Exp Bot 58:2661–2671
Yin L, Wang S, Eltayeb AE, Uddin MI, Yamamoto Y, Tsuji W et al (2010) Overexpression of dehydroascorbate reductase, but not monodehydroascorbate reductase, confers tolerance to aluminum stress in transgenic tobacco. Planta 231:609–621
Zablackis E, York WS, Pauly M, Hantus S, Reiter WD, Chapple CC et al (1996) Substitution of l-fucose by l-galactose in cell walls of Arabidopsis mur1. Science 272:1808–1810
Zhang W, Lorence A, Gruszewski HA, Chevone BI, Nessler CL (2009) AMR1, an Arabidopsis gene that coordinately and negatively regulates the mannose/l-galactose ascorbic acid biosynthetic pathway. Plant Physiol 150:942–950
Zhang C, Liu J, Zhang Y, Cai X, Gong P, Zhang J et al (2011) Overexpression of SlGMEs leads to ascorbate accumulation with enhanced oxidative stress, cold, and salt tolerance in tomato. Plant Cell Rep 30:389–398
Zhang Z, Wang J, Zhang R, Huang R (2012) The ethylene response factor ERF protein AtERF98 enhances tolerance to salt through the transcriptional activation of ascorbic acid synthesis in Arabidopsis. Plant J 71:273–287
Zhang G-Y, Liu R-R, Zhang C-Q, Tang K-X, Sun M-F, Yan G-H, Liu Q-Q (2015a) Manipulation of the rice l-galactose pathway: evaluation of the effects of transgene overexpression on ascorbate accumulation and abiotic stress tolerance. PLoS ONE 10:e0125870
Zhang L, Ma G, Yamawaki K, Ikoma Y, Matsumoto H, Yoshioka T et al (2015b) Regulation of ascorbic acid metabolism by blue LED light irradiation in citrus juice sacs. Plant Sci 233:134–142
Zhou Y, Tao QC, Wang ZN, Fan R, Li Y, Sun XF, Tang KX (2012) Engineering ascorbic acid biosynthetic pathway in Arabidopsis leaves by single and double gene transformation. Biol Plant 56:451–457
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2016 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Bulley, S.M., Laing, W. (2016). Ascorbic Acid-Related Genes. In: Testolin, R., Huang, HW., Ferguson, A. (eds) The Kiwifruit Genome. Compendium of Plant Genomes. Springer, Cham. https://doi.org/10.1007/978-3-319-32274-2_13
Download citation
DOI: https://doi.org/10.1007/978-3-319-32274-2_13
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-32272-8
Online ISBN: 978-3-319-32274-2
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)