Abstract
Lithium, an inhibitor of glycogen synthase kinase 3 (GSK3), is widely used for the treatment of mood disorders. Side effects of lithium include nephrogenic diabetes insipidus, leading to renal water loss. Dehydration has in turn been shown to downregulate Klotho, which is required as co-receptor for the downregulation of 1,25(OH)2D3 formation by fibroblast growth factor 23 (FGF23). FGF23 decreases and 1,25(OH)2D3 stimulates renal tubular phosphate reabsorption. The present study explored whether lithium influences renal Klotho expression, FGF23 serum levels, 1,25(OH)2D3 formation, and renal phosphate excretion. To this end, mice were analyzed after a 14-day period of sham treatment or of treatment with lithium (200 mg/kg/day subcutaneously). Serum antidiuretic hormone (ADH), FGF23, and 1,25(OH)2D3 concentrations were determined by ELISA or EIA, renal Klotho protein abundance and GSK3 phosphorylation were analyzed by Western blotting, and serum phosphate and calcium concentration by photometry. Lithium treatment significantly increased renal GSK3 phosphorylation, enhanced serum ADH and FGF23 concentrations, downregulated renal Klotho expression, stimulated renal calcium and phosphate excretion, and decreased serum 1,25(OH)2D3 and phosphate concentrations. In conclusion, lithium treatment upregulates FGF23 formation, an effect paralleled by substantial decrease of serum 1,25(OH)2D3, and phosphate concentrations and thus possibly affecting tissue calcification.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Fibroblast growth factor 23 (FGF23), a hormone released mainly from osteoblasts [25, 47], is a powerful regulator of calcium phosphate metabolism [23, 51]. As previously shown [3, 4, 42, 51, 69, 71, 73, 75], FGF23 inhibits renal tubular phosphate reabsorption. Moreover, FGF23 downregulates renal 1α hydroxylase (Cyp27b1) and upregulates 25-hydroxyvitamin D 24-hydroxylase (Cyp24), effects leading to decreased formation and increased catabolism of 1,25-dihydroxyvitamin D3 (1,25(OH)2D3) [18, 26, 72, 75]. FGF23, thus, lowers serum 1,25(OH)2D3 levels [3, 4, 26, 62, 69, 71, 73, 75]. 1,25(OH)2D3 is a powerful regulator of renal and intestinal phosphate and calcium transport [10, 54, 55, 68]. FGF23 increases renal elimination of phosphate [3, 4, 69, 71, 73, 75]. FGF23 deficiency elevates serum phosphate, calcium, and 1,25(OH)2D3 levels, effects eventually leading to several severe disorders, such as vascular calcification, decrease of bone density, growth retardation, infertility, and a dramatic reduction of lifespan [15, 49, 67, 71, 72, 76].
To become effective, FGF23 requires Klotho as co-receptor [25, 36, 37]. Accordingly, mice with reduced Klotho expression similarly suffer from multiple age-related disorders with growth retardation, extensive soft tissue calcification, and decreased life span paralleled by osteopenia/osteoporosis, endothelial dysfunction, impaired angiogenesis, sinoatrial node dysfunction with sudden cardiac arrest, enhanced erythrocyte turnover, pulmonary emphysema, skin atrophy, hypogonadotropic hypogonadism, infertility, muscle dystrophy, hearing loss, neuron degeneration, Parkinson’s disease, cognition impairment, neoplasms, inflammation, and tissue fibrosis [5, 11, 17, 29, 35, 37–39, 50, 56, 57, 63, 74, 79, 82, 83]. Klotho deficiency is largely effective through strong increases in 1,25(OH)2D3 formation, inhibition of renal tubular phosphate transport, and subsequent elevation of serum phosphate levels [14, 24, 87]. Hyperphosphatemia predisposes to vascular calcification [20] and is recognized as predictor of mortality [81].
Recent observations revealed that renal Klotho expression is markedly downregulated by dehydration [80]. Drugs causing dehydration include lithium [21, 30], which is widely used in the treatment of bipolar disorders and Alzheimer’s disease [16, 28, 52]. Lithium treatment interferes with renal effects of antidiuretic hormone (ADH), thus causing nephrogenic diabetes insipidus [8, 31, 43, 65, 77]. Lithium is at least partially effective by inhibition of glycogen synthase kinase 3 (GSK3) [32], which in turn regulates aquaporin 2 water channels via adenylate cyclase or prostaglandin-E2 [60, 88]. GSK3 inhibition is similarly implicated in the effects of lithium on affective disorders and Alzheimer’s disease [6, 22, 27]. Potential side effects of lithium treatment may include hypercalcemia [1, 30, 40, 44, 48], which has been observed in 5–20 % of treated patients [40, 48]. The hypercalcemia has been attributed to hyperparathyroidism [1, 7, 9, 44]. Moreover, renal Ca2+ excretion may be compromised and hypercalcemia precipitated by volume depletion, which is expected to stimulate proximal tubular Na+ and, thus, Ca2+ reabsorption [66].
The present study explored the effect of lithium treatment on renal GSK3 phosphorylation and Klotho expression as well as on serum FGF23, 1,25(OH)2D3, calcium, and phosphate concentrations.
Methods
Mice
All animal experiments were conducted according to the German law for the welfare of animals and were approved by local authorities. Experiments were performed in female C57Bl6 mice at the age of 10 weeks. The mice had free access to water and control food (Ssniff, Soest, Germany). For lithium treatment, LiCl (Calbiochem, Merck GmbH, Germany) was dissolved in isotonic saline and administered subcutaneously at a dosage of 200 mg/kg/day for 14 days [28]. Control animals were treated with saline only.
Determination of serum and plasma concentrations
To collect blood specimens, animals were lightly anesthetized with diethyl ether (Roth, Karlsruhe, Germany) and about 50–200 μl of blood was withdrawn into heparinized capillaries by puncturing the retro-orbital plexus. Serum C-terminal-FGF23 (Immutopics International, CA, USA), ADH (AVP EIA kit, Phoenix Europe, Karlsruhe, Germany) and serum 1,25(OH)2D3 (IDS, Boldon, UK) concentrations were measured by ELISA or EIA. The serum phosphate and total Ca2+ concentrations were measured by a photometric method (FUJI FDC 3500i, Sysmex, Norsted, Germany). The free calcium concentration was analyzed by a blood gas analyzer (ABL 725, Radiometer, Copenhagen, Denmark) in heparinized plasma samples.
Measurement of urinary calcium and phosphate concentrations
To determine urinary parameters, the mice were placed individually in metabolic cages (Tecniplast, Hohenpeissenberg, Germany) from day 11–14 of lithium treatment. Twenty-four-hour collection of urine was performed. To assure quantitative urine collection, metabolic cages were siliconized, and urine was collected under water-saturated oil.
The urinary phosphate concentration was determined colorimetrically utilizing a commercial diagnostic kit (Roche Diagnostics, Mannheim, Germany). Urinary calcium was measured by flame photometry (Eppendorf, Hamburg, Germany).
Western blotting
To analyze Klotho and NaPi-IIa protein abundance and GSK3 phosphorylation in renal tissue, the kidneys were removed and immediately snap-frozen in liquid nitrogen. After homogenization in lysis buffer (54.6 mM HEPES; 2.69 mM Na4P2O7; 360 mM NaCl; 10 % (v/v) glycerol; 1 % (v/v) NP40 or RIPA lysis buffer (Cell Signaling, Frankfurt, Germany)) containing phosphatase and protease inhibitor cocktail tablet (Complete mini, Roche, Mannheim, Germany), the samples were incubated on ice for 30 min and then centrifuged at 14,000 rpm and 4 °C for 20 min. The supernatant was removed and used for Western blotting. Total protein (80 μg) was separated by SDS-PAGE, thereafter transferred to nitrocellulose membranes and blocked in 5 % nonfat milk/Tris-buffered saline/Tween-20 (TBST) at room temperature for 1 h. Membranes were probed overnight at 4 °C with polyclonal rat anti-Klotho antibody (1:1,000 in 5 % fat-free milk in TBST; kindly provided by Akiko Saito, Kyowa Hakko Kirin Co., Ltd., Japan), with rabbit anti-NaPi-IIa antibody ([13]; 1:3,000 in TBST with 5 % BSA), or with rabbit anti-phospho-GSK3ß (Cell Signaling; 1:1,000 in TBST supplemented with 5 % BSA). After incubation with horseradish peroxidase-conjugated anti-rat or anti-rabbit secondary antibodies (Cell Signaling, 1:2,000) for 1 h at room temperature, the bands were visualized with enhanced chemiluminescence reagents (Amersham, Freiburg, Germany). Membranes were also probed with GAPDH antibody as loading control. Densitometric analysis was performed using Quantity One software (Bio-Rad, Munich, Germany).
Real-time RT-PCR
UMR106 rat osteosarcoma cells were cultured in DMEM high glucose medium supplemented with 10 % FCS and 1 % penicillin/streptomycin under standard conditions. Cells were pretreated with 100 nM calcitriol (Sigma, Schnelldorf, Germany) to induce FGF23 expression [33]. After 24 h, cells were in addition treated either with lithium (2 mM) for another 24 h or with [Arg8]-vasopressin acetate salt (Sigma; 50 nM) for another 12 h, or with recombinant human Klotho protein (30 ng/ml) for another 12 h, or left untreated. Next, total RNA was isolated with TriFast RNA extraction reagent (Peqlab Biotechnologie GmbH, Erlangen, Germany) based on a chloroform extraction protocol. mRNA was transcribed with SuperScript III reverse transcriptase (Invitrogen, Karlsruhe, Germany) using an oligo-dT primer. Quantitative reverse-transcription polymerase chain reaction (RT-PCR) was performed on a Bio-Rad iCycler iQTM real-time PCR detection system (Bio-Rad Laboratories, München, Germany) using the following primers:
Tbp (TATA box-binding protein)
-
Forward (5′-3′): ACTCCTGCCACACCAGCC
-
Reverse (5′-3′): GGTCAAGTTTACAGCCAAGATTCA
Fgf23
-
Forward (5′-3′): TGGCCATGTAGACGGAACAC
-
Reverse (5′-3′): GGCCCCTATTATCACTACGGAG
The final volume of the PCR reaction mixture was 20 μl and contained 2 μl cDNA, 1 μM of each primer, 10 μl GoTaq qPCR master mix (Promega, Mannheim, Germany), and sterile water up to 20 μl. qPCR conditions were 95 °C for 3 min, followed by 40 cycles of 95 °C for 10 s, and 58 °C for 10 s. Calculated mRNA expression levels were normalized to the expression levels of Tbp of the same cDNA sample. Relative quantification of gene expression was performed using the ΔΔct method.
Statistics
Data are provided as means ± SEM; n represents the number of independent experiments. All data were tested for significance using unpaired Student t test unless otherwise stated. Only results with p < 0.05 were considered statistically significant.
Results
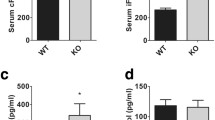
In a first series of experiments, the effect of lithium treatment on renal GSK3 phosphorylation was determined. To this end, 10-week-old female mice were treated with sham only or with LiCl (200 mg/kg/day s.c. for 14 days). As illustrated in Fig. 1, lithium treatment was followed by a significant increase in GSK3 phosphorylation. Since lithium treatment may lead to nephrogenic diabetes insipidus, a second series of experiments explored the serum concentration of ADH. As shown in Fig. 2a, lithium treatment was followed by an increase in ADH serum levels, an observation pointing to dehydration. Body weight, however, was not influenced by the 14-day lithium treatment (Fig. 2b).
Renal GSK3 phosphorylation in sham- and lithium-treated mice. Original Western blot showing the protein abundance of phosphorylated GSK3 and GAPDH (upper panel) and arithmetic means ± SEM (n = 4/group) of the phosphorylated GSK3 over GAPDH protein abundance ratio (lower panel) in renal tissue from sham- (white bar) and lithium-treated (black bar) wild type mice. *p < 0.05 indicates significant difference from untreated mice
Next, we explored the lithium effects on the Klotho/FGF23 hormonal axis, which regulates calcium and phosphate homeostasis. To study renal Klotho expression, kidneys were removed after the 14-day treatment with or without lithium and Klotho protein abundance determined by Western blotting. As illustrated in Fig. 3, lithium treatment was followed by a strong and statistically significant decrease of the Klotho over GAPDH ratio.
Renal Klotho expression in sham- and lithium-treated mice. Original Western blot showing renal expression of Klotho and GAPDH (upper panel) and arithmetic means ± SEM (n = 4/group) of the Klotho over GAPDH protein abundance ratio (lower panel) in renal tissue from sham- (white bar) and lithium-treated (black bar) wild type mice. *p < 0.05 indicates significant difference from untreated mice
In order to determine whether lithium regulates FGF23 release, serum FGF23 concentrations were determined by ELISA. As a result, lithium treatment was followed by a strong statistically significant increase in the serum FGF23 concentration (Fig. 4a). To study whether altered FGF23 release may result from an influence of ADH on FGF23 transcription, UMR106 osteoblast-like cells were treated with [Arg8]-vasopressin (50 nM) and FGF23 transcript levels determined by quantitative RT-PCR. As a result, [Arg8]-vasopressin significantly decreased FGF23 transcript levels (0.00522 ± 0.00100 arb. units (n = 15)) compared to untreated cells (0.01157 ± 0.00215 arb. units (n = 15); p < 0.05, u test). Thus, ADH is unlikely to account for enhanced FGF23 secretion in lithium-treated mice. Another series of experiments explored whether Klotho protein impacts on FGF23 transcription in UMR106 osteoblast-like cells. FGF23 transcript levels approached 0.01063 ± 0.00341 arb. units (n = 15) in Klotho protein-treated cells (30 ng/ml) and 0.00708 ± 0.00187 arb. units (n = 15) in untreated cells. Hence, Klotho protein did not significantly influence FGF23 expression. Exposure of UMR106 osteoblast-like cells to lithium (2 mM), however, stimulated FGF23 expression as revealed by quantitative RT-PCR. The FGF23 transcript level was 0.00769 ± 0.00098 arb. units (n = 15) in untreated and 0.01245 ± 0.00208 arb. units (n = 15) in lithium-treated UMR cells (p < 0.001, u test).
Serum FGF23 levels in sham- and lithium-treated mice. a Arithmetic means ± SEM (n = 10/group) of serum FGF23 levels in sham- (white bar) and lithium-treated (black bar) wild type mice. ***p < 0.001 indicates significant difference from untreated mice. b Original Western blot showing renal expression of NaPi-IIa (upper panel) and GAPDH (lower panel)
To study whether lithium treatment influences renal expression of the Na+/phosphate cotransporter NaPi-IIa, its expression was determined by Western blotting. As shown in Fig. 4b, lithium treatment did not significantly affect total renal NaPi-IIa protein abundance. Densitometric analysis yielded a renal NaPi-IIa abundance of 0.55 ± 0.04 arb. units (n = 6) in sham-treated and 0.59 ± 0.06 arb. units (n = 6) in lithium-treated animals.
As FGF23 and Klotho act in concert to downregulate 1α hydroxylase (Cyp27b1), the key enzyme in the synthesis of 1,25(OH)2D3, ELISA was employed to detect 1,25(OH)2D3 serum concentration. As shown in Fig. 5, lithium treatment indeed decreased the serum 1,25(OH)2D3 concentration.
Effects of 1,25(OH)2D3 include stimulation of intestinal and renal calcium and phosphate transport leading to a rise in both calcium and phosphate serum concentrations. Accordingly, serum calcium and phosphate concentrations were determined in sham- and lithium-treated animals by photometric methods. As illustrated in Figs. 6 and 7, lithium treatment indeed significantly decreased serum phosphate concentration and tended to decrease total serum calcium level. In line with this, lithium stimulated urinary calcium and phosphate excretion (Figs. 6 and 7). The plasma-free calcium levels were not different between sham-treated (0.93 ± 0.03 mM, n = 6) and lithium-treated mice (0.99 ± 0.04 mM, n = 5).
Serum phosphate and urinary phosphate excretion of sham- and lithium-treated mice. Arithmetic means ± SEM of serum phosphate concentration (n = 10/group; left) and urinary phosphate excretion (n = 6/group; right) in sham- (white bars) and lithium-treated (black bars) wild type mice. **p < 0.01 indicates significant difference from sham-treated mice
Serum calcium and urinary calcium excretion of sham- and lithium-treated mice. Arithmetic means ± SEM of total serum calcium concentration (n = 10/group; left) and urinary calcium excretion (n = 6/group; right) in sham- (white bars) and lithium-treated (black bars) wild type mice. ***p < 0.001 indicates significant difference from sham-treated mice
Discussion
The present observations disclose a novel powerful effect of lithium. A 14-day treatment with lithium resulted in a pronounced increase in FGF23 serum levels as well as in a statistically significant decrease of renal Klotho expression. These effects were paralleled by substantial statistically significant decreases of serum 1,25(OH)2D3 and phosphate concentrations. The hypophosphatemia is explained by the phosphaturia of the mice. Western blotting did not reveal a significant decrease of whole kidney NaPi-IIa protein abundance following lithium treatment. The finding does, however, not rule out that the protein abundance in the apical cell membrane is decreased by lithium treatment. Alternatively, lithium treatment affects the activity of the carrier.
The effect of lithium on FGF23 release may in part be due to GSK3 phosphorylation, a known effect of lithium [6, 19, 22, 27, 32, 60]. The impact of lithium on both FGF23 serum levels and renal Klotho expression could theoretically be in part due to polyuria and dehydration, reflected by increased serum antidiuretic hormone levels [77]. Dehydration has previously been shown to downregulate renal Klotho expression, an effect partially due to ADH [80]. It is noteworthy that serum FGF23 levels are similarly enhanced in gene-targeted mice lacking either SPAK [59] or OSR1 [58]. Both SPAK and OSR1 stimulate the renal tubular Na–Cl co-transporter, and lack of those kinases is expected to result in dehydration. However, according to the present study, the vasopressin analogue [Arg8]-vasopressin downregulated FGF23 transcript levels in UMR osteosarcoma cells, and, thus, ADH is unlikely to account for the enhanced FGF23 serum levels following lithium treatment.
FGF23 is well known to downregulate renal 1α hydroxylase and, thus, the formation of 1,25(OH)2D3 [18, 75]. For this effect, FGF23 requires Klotho as co-receptor [2, 37], which is upregulated by FGF23 [78]. Decreased Klotho expression would be expected to blunt the effects of FGF23 [2]. However, lithium treatment led to a decrease of 1,25(OH)2D3 serum levels. 1,25(OH)2D3 stimulates the release of FGF23 and the excessive 1,25(OH)2D3 formation in Klotho deficiency enhances FGF23 serum levels [53]. Following lithium treatment, FGF23 serum levels were increased despite the observed decrease of 1,25(OH)2D3 serum concentrations, which were expected to result in a decrease of FGF23 release [12, 64, 70, 86].
1,25(OH)2D3 is a powerful stimulator of both renal and intestinal phosphate transport [10]. In addition to its effect on 1,25(OH)2D3 formation, FGF23 reduces renal tubular phosphate reabsorption more directly by inhibiting Na+-coupled phosphate transport in proximal renal tubules [51, 75]. In view of the FGF23 effect on 1,25(OH)2D3 formation and renal tubular phosphate transport, increased FGF23 release is expected to generate phosphaturia and, thus, to decrease the serum phosphate concentration. Lithium treatment did not significantly change serum Ca2+ concentration. Lithium-induced hypercalcemia [1, 30, 40, 44, 48] is a known side effect observed in a fraction of lithium-treated patients [40, 48]. Lithium may upregulate the serum Ca2+ concentration by stimulating PTH release [1, 7, 9, 44], and it may decrease the serum Ca2+ concentration by lowering 1,25(OH)2D3 serum concentrations (Fig. 5). The eventual outcome may depend on the magnitude of the alterations in PTH and 1,25(OH)2D3 release.
As high serum phosphate concentrations lead to vascular calcification and are associated with accelerated aging and decreased life-span [61], the present observations may suggest that lithium is capable of counteracting vascular calcification, aging, and early death. As a matter of fact, lithium may attenuate tissue calcification [84]. Both Klotho [36] and FGF23 [5] are powerful regulators of aging. Lack of either Klotho [36] or FGF23 [75] accelerates the development of several age-related disorders and eventually leads to early death. It is tempting to speculate that lithium may counteract at least some of the multiple disorders observed in FGF23 deficiency.
FGF23 serum concentration similarly increases in renal insufficiency [34, 85]. Unlike renal insufficiency, however, lithium treatment lowers serum phosphate concentration. The hyperphosphatemia of renal insufficiency leads to vascular calcification [46], an effect counteracted by Klotho and FGF23 [41, 45].
In conclusion, lithium treatment led to upregulation of FGF23, thus decreasing serum 1,25(OH)2D3 and phosphate concentrations. At least in theory, the effects may counteract vascular calcification.
References
Albert U, De Cori D, Aguglia A, Barbaro F, Lanfranco F, Bogetto F, Maina G (2013) Lithium-associated hyperparathyroidism and hypercalcemia: a case–control cross-sectional study. J Affect Disord. doi:10.1016/j.jad.2013.06.046
Bai X, Dinghong Q, Miao D, Goltzman D, Karaplis AC (2009) Klotho ablation converts the biochemical and skeletal alterations in FGF23 (R176Q) transgenic mice to a Klotho-deficient phenotype. Am J Physiol Endocrinol Metab 296(1):E79–E88
Bai X, Miao D, Li J, Goltzman D, Karaplis AC (2004) Transgenic mice overexpressing human fibroblast growth factor 23 (R176Q) delineate a putative role for parathyroid hormone in renal phosphate wasting disorders. Endocrinology 145(11):5269–5279
Bai XY, Miao D, Goltzman D, Karaplis AC (2003) The autosomal dominant hypophosphatemic rickets R176Q mutation in fibroblast growth factor 23 resists proteolytic cleavage and enhances in vivo biological potency. J Biol Chem 278(11):9843–9849
Bernheim J, Benchetrit S (2011) The potential roles of FGF23 and Klotho in the prognosis of renal and cardiovascular diseases. Nephrol Dial Transplant 26(8):2433–2438
Bhat RV, Budd Haeberlein SL, Avila J (2004) Glycogen synthase kinase 3: a drug target for CNS therapies. J Neurochem 89(6):1313–1317
Birnbaum J, Klandorf H, Giuliano A, Van Herle A (1988) Lithium stimulates the release of human parathyroid hormone in vitro. J Clinical Endocrinol Metab 66(6):1187–1191
Bockenhauer D, Bichet DG (2013) Inherited secondary nephrogenic diabetes insipidus: concentrating on humans. Am J Physiol Renal Physiol 304(8):F1037–F1042
Broome JT, Solorzano CC (2011) Lithium use and primary hyperparathyroidism. Endocr Pract Off J Am Coll Endocrinol Am Assoc Clin Endocrinol 17(Suppl 1):31–35. doi:10.4158/EP10273.RA
Brown AJ, Finch J, Slatopolsky E (2002) Differential effects of 19-nor-1,25-dihydroxyvitamin D(2) and 1,25-dihydroxyvitamin D(3) on intestinal calcium and phosphate transport. J Lab Clin Med 139(5):279–284
Carpinelli MR, Wise AK, Burt RA (2011) Vitamin D-deficient diet rescues hearing loss in Klotho mice. Hear Res 275(1–2):105–109
Cross HR, Radda GK, Clarke K (1995) The role of Na+/K + ATPase activity during low flow ischemia in preventing myocardial injury: a 31P, 23Na, and 87Rb NMR spectroscopic study. Magn Reson Med 34(5):673–685
Custer M, Lotscher M, Biber J, Murer H, Kaissling B (1994) Expression of Na-P(i) cotransport in rat kidney: localization by RT-PCR and immunohistochemistry. Am J Physiol 266(5 Pt 2):F767–F774
Dermaku-Sopjani M, Sopjani M, Saxena A, Shojaiefard M, Bogatikov E, Alesutan I, Eichenmuller M, Lang F (2011) Downregulation of NaPi-IIa and NaPi-IIb Na-coupled phosphate transporters by coexpression of Klotho. Cell Physiol Biochem 28(2):251–258
Desjardins L, Liabeuf S, Renard C, Lenglet A, Lemke HD, Choukroun G, Drueke TB, Massy ZA, Group. EUTEW (2012) FGF23 is independently associated with vascular calcification but not bone mineral density in patients at various CKD stages. Osteoporos Int 23(7):2017–2025
Eldar-Finkelman H (2002) Glycogen synthase kinase 3: an emerging therapeutic target. Trends Mol Med 8(3):126–132
Fukuchi Y (2009) The aging lung and chronic obstructive pulmonary disease: similarity and difference. Proc Am Thorac Soc 6(7):570–572
Gattineni J, Twombley K, Goetz R, Mohammadi M, Baum M (2011) Regulation of serum 1,25(OH)2vitamin D3 levels by fibroblast growth factor 23 is mediated by FGF receptors 3 and 4. Am J Physiol Renal Physiol 301(2):F371–F377
George B, Vollenbroker B, Saleem MA, Huber TB, Pavenstadt H, Weide T (2011) GSK3beta inactivation in podocytes results in decreased phosphorylation of p70S6K accompanied by cytoskeletal rearrangements and inhibited motility. Am J Physiol Renal Physiol 300(5):F1152–F1162
Giachelli CM (2003) Vascular calcification: in vitro evidence for the role of inorganic phosphate. J Am Soc Nephrol 14(9 Suppl 4):S300–S304
Grunfeld JP, Rossier BC (2009) Lithium nephrotoxicity revisited. Nat Rev Nephrol 5(5):270–276. doi:10.1038/nrneph.2009.43
Hernandez F, Lucas JJ, Avila J (2013) GSK3 and tau: two convergence points in Alzheimer’s disease. J Alzheimers Dis 33(Suppl 1):S141–S144
Hori M, Shimizu Y, Fukumoto S (2011) Minireview: fibroblast growth factor 23 in phosphate homeostasis and bone metabolism. Endocrinology 152(1):4–10
Hu MC, Shi M, Zhang J, Pastor J, Nakatani T, Lanske B, Razzaque MS, Rosenblatt KP, Baum MG, K-o M, Moe OW (2010) Klotho: a novel phosphaturic substance acting as an autocrine enzyme in the renal proximal tubule. FASEB J 24(9):3438–3450
Hu MC, Shiizaki K, Kuro O, Moe OW (2013) Fibroblast growth factor 23 and Klotho: physiology and pathophysiology of an endocrine network of mineral metabolism. Annu Rev Physiol 75:503–533
Inoue Y, Segawa H, Kaneko I, Yamanaka S, Kusano K, Kawakami E, Furutani J, Ito M, Kuwahata M, Saito H, Fukushima N, Kato S, Kanayama HO, Miyamoto K (2005) Role of the vitamin D receptor in FGF23 action on phosphate metabolism. Biochem J 390(Pt 1):325–331
Jope RS (2011) Glycogen synthase kinase-3 in the etiology and treatment of mood disorders. Front Mol Neurosci 4:16
Kakefuda K, Oyagi A, Ishisaka M, Tsuruma K, Shimazawa M, Yokota K, Shirai Y, Horie K, Saito N, Takeda J, Hara H (2010) Diacylglycerol kinase beta knockout mice exhibit lithium-sensitive behavioral abnormalities. PLoS One 5(10):e13447
Kempe DS, Ackermann TF, Fischer SS, Koka S, Boini KM, Mahmud H, Foller M, Rosenblatt KP, Kuro O, Lang F (2009) Accelerated suicidal erythrocyte death in Klotho-deficient mice. Pflugers Arch 458(3):503–512
Khairallah W, Fawaz A, Brown EM, El-Hajj Fuleihan G (2007) Hypercalcemia and diabetes insipidus in a patient previously treated with lithium. Nat Clin Pract Nephrol 3(7):397–404. doi:10.1038/ncpneph0525
Kishore BK, Ecelbarger CM (2013) Lithium: a versatile tool to understand renal physiology. Am J Physiol Renal Physiol 304(9):F1139–F1149
Kjaersgaard G, Madsen K, Marcussen N, Christensen S, Walter S, Jensen BL (2012) Tissue injury after lithium treatment in human and rat postnatal kidney involves glycogen synthase kinase-3beta-positive epithelium. Am J Physiol Renal Physiol 302(4):F455–F465
Kolek OI, Hines ER, Jones MD, LeSueur LK, Lipko MA, Kiela PR, Collins JF, Haussler MR, Ghishan FK (2005) 1alpha,25-Dihydroxyvitamin D3 upregulates FGF23 gene expression in bone: the final link in a renal-gastrointestinal-skeletal axis that controls phosphate transport. Am J Physiol Gastrointest Liver Physiol 289(6):G1036–G1042. doi:10.1152/ajpgi.00243.2005
Komaba H, Fukagawa M (2012) The role of FGF23 in CKD–with or without Klotho. Nat Rev Nephrol 8(8):484–490
Kosakai A, Ito D, Nihei Y, Yamashita S, Okada Y, Takahashi K, Suzuki N (2011) Degeneration of mesencephalic dopaminergic neurons in Klotho mouse related to vitamin D exposure. Brain Res 1382:109–117
Kuro-o M (2010) Klotho. Pflugers Arch 459(2):333–343
Kuro-o M (2010) Overview of the FGF23-Klotho axis. Pediatr Nephrol 25(4):583–590
Kuro-o M, Matsumura Y, Aizawa H, Kawaguchi H, Suga T, Utsugi T, Ohyama Y, Kurabayashi M, Kaname T, Kume E, Iwasaki H, Iida A, Shiraki-Iida T, Nishikawa S, Nagai R, Nabeshima YI (1997) Mutation of the mouse Klotho gene leads to a syndrome resembling ageing. Nature 390(6655):45–51
Kuro O (2012) Klotho and betaKlotho. Adv Exp Med Biol 728:25–40
Lally J, Lee B, McDonald C (2013) Prevalence of hypercalcemia in patients on maintenance lithium therapy monitored in primary care. Irish Med J 106(1):15–17
Lang F, Ritz E, Voelkl J, Alesutan I (2013) Vascular calcification—is aldosterone a culprit? Nephrol Dial Transplant 28(5):1080–1084
Larsson T, Marsell R, Schipani E, Ohlsson C, Ljunggren O, Tenenhouse HS, Juppner H, Jonsson KB (2003) Transgenic mice expressing fibroblast growth factor 23 under the control of the a1(I) collagen promoter exhibit growth retardation, osteomalacia, and disturbed phosphate homeostatis. Endocrinology 145(3087):3094
Lee YJ, Lee JE, Choi HJ, Lim JS, Jung HJ, Baek MC, Frokiaer J, Nielsen S, Kwon TH (2011) E3 ubiquitin-protein ligases in rat kidney collecting duct: response to vasopressin stimulation and withdrawal. Am J Physiol Renal Physiol 301(4):F883–F896
Lehmann SW, Lee J (2013) Lithium-associated hypercalcemia and hyperparathyroidism in the elderly: what do we know? J Affect Disord 146(2):151–157. doi:10.1016/j.jad.2012.08.028
Lim K, Lu TS, Molostvov G, Lee C, Lam FT, Zehnder D, Hsiao LL (2012) Vascular Klotho deficiency potentiates the development of human artery calcification and mediates resistance to fibroblast growth factor 23. Circulation 125(18):2243–2255
London GM, Guerin AP, Marchais SJ, Metivier F, Pannier B, Adda H (2003) Arterial media calcification in end-stage renal disease: impact on all-cause and cardiovascular mortality. Nephrol Dial Transplant 18(9):1731–1740
Lu Y, Feng JQ (2011) FGF23 in skeletal modeling and remodeling. Curr Osteoporos Rep 9(2):103–108
Lumachi F, Cappelletti P, Tozzoli R, Basso SM, Luisetto G, Camozzi V (2012) Diagnosis of alterations of serum calcium metabolism. Med Chem 8(4):551–555
Mackenzie NC, MacRae VE (2011) The role of cellular senescence during vascular calcification: a key paradigm in aging research. Curr Aging Sci 4(2):128–136
Maltese G, Karalliedde J (2012) The putative role of the antiaging protein Klotho in cardiovascular and renal disease. Int J Hypertens 2012:757469
Marsell R, Jonsson KB (2010) The phosphate regulating hormone fibroblast growth factor-23. Acta Physiol (Oxf) 200(2):97–106
Martinez A, Castro A, Dorronsoro I, Alonso M (2002) Glycogen synthase kinase 3 (GSK-3) inhibitors as new promising drugs for diabetes, neurodegeneration, cancer, and inflammation. Med Res Rev 22(4):373–384
Masuyama R, Stockmans I, Torrekens S, Van Looveren R, Maes C, Carmeliet P, Bouillon R, Carmeliet G (2006) Vitamin D receptor in chondrocytes promotes osteoclastogenesis and regulates FGF23 production in osteoblasts. J Clin Invest 116(12):3150–3159
Miyamoto K, Segawa H, Ito M, Kuwahata M (2004) Physiological regulation of renal sodium-dependent phosphate cotransporters. Jpn J Physiol 54(2):93–102
Murer H, Hernando N, Forster I, Biber J (2000) Proximal tubular phosphate reabsorption: molecular mechanisms. Physiol Rev 80(4):1373–1409
Nikolov IG, Mozar A, Drueke TB, Massy ZA (2009) Impact of disturbances of calcium and phosphate metabolism on vascular calcification and clinical outcomes in patients with chronic kidney disease. Blood Purif 27(4):350–359
Ohnishi M, Razzaque MS (2010) Dietary and genetic evidence for phosphate toxicity accelerating mammalian aging. FASEB J 24(9):3562–3571
Pathare G, Foller M, Daryadel A, Mutig K, Bogatikov E, Fajol A, Almilaji A, Michael D, Stange G, Voelkl J, Wagner CA, Bachmann S, Lang F (2012) OSR1-sensitive renal tubular phosphate reabsorption. Kidney Blood Press Res 36(1):149–161
Pathare G, Foller M, Michael D, Walker B, Hierlmeier M, Mannheim JG, Pichler BJ, Lang F (2012) Enhanced FGF23 serum concentrations and phosphaturia in gene-targeted mice expressing WNK-resistant SPAK. Kidney Blood Press Res 36(1):355–364
Rao R (2012) Glycogen synthase kinase-3 regulation of urinary concentrating ability. Curr Opin Nephrol Hypertens 21(5):541–546
Rodriguez M, Martinez-Moreno JM, Rodriguez-Ortiz ME, Munoz-Castaneda JR, Almaden Y (2011) Vitamin D and vascular calcification in chronic kidney disease. Kidney Blood Press Res 34(4):261–268
Saito H, Kusano K, Kinosaki M, Ito H, Hirata M, Segawa H, Miyamoto K, Fukushima N (2003) Human fibroblast growth factor-23 mutants suppress Na + −dependent phosphate co-transport activity and 1alpha,25-dihydroxyvitamin D3 production. J Biol Chem 278(4):2206–2211
Saito Y, Nakamura T, Ohyama Y, Suzuki T, Iida A, Shiraki-Iida T, K-o M, Nabeshima Y, Kurabayashi M, Nagai R (2000) In vivo Klotho gene delivery protects against endothelial dysfunction in multiple risk factor syndrome. Biochem Biophys Res Commun 276(2):767–772
Sakoda H, Gotoh Y, Katagiri H, Kurokawa M, Ono H, Onishi Y, Anai M, Ogihara T, Fujishiro M, Fukushima Y, Abe M, Shojima N, Kikuchi M, Oka Y, Hirai H, Asano T (2003) Differing roles of Akt and serum- and glucocorticoid-regulated kinase in glucose metabolism, DNA synthesis, and oncogenic activity. J Biol Chem 278(28):25802–25807
Sanches TR, Volpini RA, Massola Shimizu MH, Braganca AC, Oshiro-Monreal F, Seguro AC, Andrade L (2012) Sildenafil reduces polyuria in rats with lithium-induced NDI. Am J Physiol Renal Physiol 302(1):F216–F225
Sandulache D, Grahammer F, Artunc F, Henke G, Hussain A, Nasir O, Mack A, Friedrich B, Vallon V, Wulff P, Kuhl D, Palmada M, Lang F (2006) Renal Ca2+ handling in sgk1 knockout mice. Pflugers Arch 452(4):444–452. doi:10.1007/s00424-005-0021-x
Schoppet M, Hofbauer LC, Brinskelle-Schmal N, Varennes A, Goudable J, Richard M, Hawa G, Chapurlat R, Szulc P (2012) Serum level of the phosphaturic factor FGF23 is associated with abdominal aortic calcification in men: the STRAMBO study. J Clin Endocrinol Metab 97(4):E575–E583
Segawa H, Kaneko I, Yamanaka S, Ito M, Kuwahata M, Inoue Y, Kato S, Miyamoto K (2004) Intestinal Na-P(i) cotransporter adaptation to dietary P(i) content in vitamin D receptor null mice. Am J Physiol Renal Physiol 287(1):F39–F47
Segawa H, Kawakami E, Kaneko I, Kuwahata M, Ito M, Kusano K, Saito H, Fukushima N, Miyamoto K (2003) Effect of hydrolysis-resistant FGF23-R179Q on dietary phosphate regulation of the renal type-II Na/Pi transporter. Pflugers Arch 446(5):585–592
Shaw M, Cohen P, Alessi DR (1997) Further evidence that the inhibition of glycogen synthase kinase-3beta by IGF-1 is mediated by PDK1/PKB-induced phosphorylation of Ser-9 and not by dephosphorylation of Tyr-216. FEBS Lett 416(3):307–311
Shimada T, Hasegawa H, Yamazaki Y, Muto T, Hino R, Takeuchi Y, Fujita T, Nakahara K, Fukumoto S, Yamashita T (2004) FGF-23 is a potent regulator of vitamin D metabolism and phosphate homeostasis. J Bone Miner Res 19(3):429–435
Shimada T, Kakitani M, Yamazaki Y, Hasegawa H, Takeuchi Y, Fujita T, Fukumoto S, Tomizuka K, Yamashita T (2004) Targeted ablation of Fgf23 demonstrates an essential physiological role of FGF23 in phosphate and vitamin D metabolism. J Clin Invest 113(4):561–568
Shimada T, Mizutani S, Muto T, Yoneya T, Hino R, Takeda S, Takeuchi Y, Fujita T, Fukumoto S, Yamashita T (2001) Cloning and characterization of FGF23 as a causative factor of tumor-induced osteomalacia. Proc Natl Acad Sci U S A 98(11):6500–6505
Shimada T, Takeshita Y, Murohara T, Sasaki K, Egami K, Shintani S, Katsuda Y, Ikeda H, Nabeshima Y, Imaizumi T (2004) Angiogenesis and vasculogenesis are impaired in the precocious-aging Klotho mouse. Circulation 110(9):1148–1155
Shimada T, Yamazaki Y, Takahashi M, Hasegawa H, Urakawa I, Oshima T, Ono K, Kakitani M, Tomizuka K, Fujita T, Fukumoto S, Yamashita T (2005) Vitamin D receptor-independent FGF23 actions in regulating phosphate and vitamin D metabolism. Am J Physiol Renal Physiol 289(5):F1088–F1095
Sitara D, Razzaque MS, Hesse M, Yoganathan S, Taguchi T, Erben RG, Juppner H, Lanske B (2004) Homozygous ablation of fibroblast growth factor-23 results in hyperphosphatemia and impaired skeletogenesis, and reverses hypophosphatemia in Phex-deficient mice. Matrix Biol 23(7):421–432
Sugawara M, Hashimoto K, Ota Z (1988) Involvement of prostaglandin E2, cAMP, and vasopressin in lithium-induced polyuria. Am J Physiol 254(6 Pt 2):R863–R869
Takenaka T, Watanabe Y, Inoue T, Miyazaki T, Suzuki H (2013) Fibroblast growth factor 23 enhances renal Klotho abundance. Pflugers Arch 465(7):935–943
Takeshita K, Fujimori T, Kurotaki Y, Honjo H, Tsujikawa H, Yasui K, Lee JK, Kamiya K, Kitaichi K, Yamamoto K, Ito M, Kondo T, Iino S, Inden Y, Hirai M, Murohara T, Kodama I, Nabeshima Y (2004) Sinoatrial node dysfunction and early unexpected death of mice with a defect of Klotho gene expression. Circulation 109(14):1776–1782
Tang C, Pathare G, Michael D, Fajol A, Eichenmuller M, Lang F (2011) Downregulation of Klotho expression by dehydration. Am J Physiol Renal Physiol 301(4):F745–F750
Tonelli M, Sacks F, Pfeffer M, Gao Z, Curhan G (2005) Relation between serum phosphate level and cardiovascular event rate in people with coronary disease. Circulation 112(17):2627–2633
Torres PU, Prie D, Molina-Bletry V, Beck L, Silve C, Friedlander G (2007) Klotho: an antiaging protein involved in mineral and vitamin D metabolism. Kidney Int 71(8):730–737
Tuohimaa P (2009) Vitamin D and aging. J Steroid Biochem Mol Biol 114(1–2):78–84
Tyber MA (1974) Treatment of the painful shoulder syndrome with amitriptyline and lithium carbonate. Can Med Assoc J 111(2):137–140
Unsal A, Kose BS, Koc Y, Basturk T, Sakaci T, Ahbap E, Sinangil A (2012) Relationship of fibroblast growth factor 23 with left ventricle mass index and coronary calcification in chronic renal disease. Kidney Blood Press Res 36(1):55–64
Wyatt AW, Hussain A, Amann K, Klingel K, Kandolf R, Artunc F, Grahammer F, Huang DY, Vallon V, Kuhl D, Lang F (2006) DOCA-induced phosphorylation of glycogen synthase kinase 3beta. Cell Physiol Biochem 17(3–4):137–144
Yoshida T, Fujimori T, Nabeshima Y (2002) Mediation of unusually high concentrations of 1,25-dihydroxyvitamin D in homozygous Klotho mutant mice by increased expression of renal 1alpha-hydroxylase gene. Endocrinology 143(2):683–689
Zhang Y, Pop IL, Carlson NG, Kishore BK (2012) Genetic deletion of the P2Y2 receptor offers significant resistance to development of lithium-induced polyuria accompanied by alterations in PGE2 signaling. Am J Physiol Renal Physiol 302(1):F70–F77
Acknowledgments
The authors acknowledge the technical assistance of E. Faber and the meticulous preparation of the manuscript by A. Soleimanpour and L. Subasic. The study was supported by the Deutsche Forschungsgemeinschaft (La 315/15-1 and Fo 695/1-1).
Disclosures
All authors disclose that they do not have any potential conflict of interest (e.g., consultancies, stock ownership, equity interests, patent-licensing arrangements, lack of access to data, or lack of control of the decision to publish).
Author information
Authors and Affiliations
Corresponding author
Additional information
Hajar Fakhri and Ganesh Pathare contributed equally and, thus, share first authorship
Rights and permissions
About this article
Cite this article
Fakhri, H., Pathare, G., Fajol, A. et al. Regulation of mineral metabolism by lithium. Pflugers Arch - Eur J Physiol 466, 467–475 (2014). https://doi.org/10.1007/s00424-013-1340-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00424-013-1340-y