Abstract
The membrane potential (V m) of beta-cells oscillates at glucose concentrations between ~6 and 25 mM, i.e. burst phases with action potentials alternate with silent interburst phases generating so-called slow waves. The slow waves drive oscillations of the cytosolic Ca2+ concentration ([Ca2+]c) and insulin secretion. The length of the bursts correlates with the amount of insulin release. Thus, the fraction of plateau phase (FOPP), i.e. the percentage of time with burst activity, is an excellent marker for beta-cell function and metabolic integrity. Extracellular voltage changes of mouse islets were measured using a microelectrode array (MEA) allowing the detection of burst and interburst phases. At a non-stimulating glucose concentration (3 mM) no electrical activity was detectable while bursting was continuous at 30 mM. The glucose concentration–response (determined as FOPP) curve revealed half-maximal stimulation at 12 ± 1 mM (Hill equation fit). The signal was sensitive to KATP channel modulators, e.g. tolbutamide or diazoxide. Simultaneous recordings of electrical activity and [Ca2+]c revealed congruent bursts and peaks, respectively. The extracellular recordings are in perfect agreement with more time-consuming intracellular electrical recordings. The results provide a 'proof-of-principle' for detection of beta-cell slow waves and determination of the FOPP using extracellular electrodes in a MEA-based system. The method is facile and provides the capability to study the effects of modulators of beta-cell function including possible anti-diabetic drugs in real time. Moreover, the method may be useful for checking the metabolic integrity of human donor islets prior to transplantation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The early work of Dean and Matthews [4] established that pancreatic beta-cells are electrically excitable. The beta-cell membrane potential (V m) exhibits slow waves of electrical activity [7], consisting of bursts of Ca2+ action potentials and silent interburst phases. This fluctuating electrical activity actuates slow oscillations of [Ca2+]c [18] and insulin secretion [2, 10]. The fraction of plateau phase (FOPP) is an excellent marker for intact beta-cell function and metabolism [7].
Before the introduction of the patch-clamp technique the determination of FOPP required measuring with sharp microelectrodes to elucidate electrical properties of beta-cells [7, 12, 13]. Both electrophysiological methods are technically demanding and are too time consuming to be used routinely. Moreover, it is difficult to detect V m oscillations of single or small beta-cell clusters with patch-clamping [6, 11, 15] although slow waves are invariably detectable when intact islets with electrically coupled cells are impaled by intracellular microelectrodes [6]. To circumvent this problem Göpel and co-workers developed a technique to patch-clamp whole islets [11], but the method is technically challenging and time consuming. Different patterns of [Ca2+]c oscillations between single cells and islets have been reported [9] emphasizing that measurement of oscillations from whole islets is more reliable and closer to the in vivo situation.
Microelectrode arrays (MEAs) present an alternative way to study the electrical activity of single cells or tissue slices and have been used successfully on cells from the heart, central and peripheral nervous system, and muscles [14, 20]. Data on the use of extracellular electrodes to record electrical activity from islets are scarce [3, 17] possibly because these attempts did not successfully resolve slow wave oscillations. Here we report on a non-invasive MEA-based system for the detection of glucose-induced slow waves in islets. This report is the 'proof-of-principle' for a method which can be extended for low-throughput to medium-throughput screening and thus allow the routine use of islets of Langerhans in industrial research. Additionally, the method is applicable to testing the metabolic status of human donor islets prior to transplantation.
Research design and methods
Islet preparation
Experiments were performed using intact islets of Langerhans isolated from adult C57Bl/6 N or NMRI mice (Janvier, France). The principles of laboratory animal care were followed according to German laws. Mice were euthanized by CO2 application. Islets were isolated by collagenase digestion of the pancreas and cultured up to 3 days in RPMI 1640 medium supplemented with 10% fetal calf serum, 100 U/ml penicillin and 100 μg/ml streptomycin.
Solution and chemicals
Measurements of [Ca2+]c, membrane potential changes by MEA (both 37°C), and patch-clamp recordings (34°C) were performed in a solution containing in millimolar: 140 NaCl, 5 KCl, 1.2 MgCl2, 2.5 CaCl2, 10 HEPES, pH 7.4, and glucose as indicated. Recordings of V m with microelectrodes were done in a bath solution composed of (in millimolar): 120 NaCl, 5 KCl, 2.5 CaCl2, 1.2 MgCl2, 24 NaHCO3, 15 glucose, aerated with 95% O2 and 5% CO2 to maintain a pH of 7.4 at 37°C.
RPMI 1640, fetal calf serum, penicillin/streptomycin, and fura-2 were provided by Invitrogen (Karlsruhe, Germany). All other chemicals were purchased from Sigma-Aldrich (Taufkirchen, Germany), Roth (Karlsruhe, Germany), or Merck (Darmstadt, Germany).
Recording setup
Extracellular membrane potential recordings were obtained with the microelectrode array (MEA) technique [20] using a MEA USB-1060 system with software MC-Rack (Multi Channel Systems (MCS), Reutlingen, Germany). Data were low-pass filtered at 70 or 100 Hz and sampled at 10 kHz. Titanium-nitride electrodes had a diameter of 30 μm (200/30-Ti; MCS). An islet was placed on one of the 59 electrodes by means of a glass holding pipette with a tip angle of 30° (Reproline, Rheinbach, Germany) and a micromanipulator (Eppendorf, Hamburg, Germany). Extracellular voltage changes were only recorded from the electrode where the islet was placed on (Fig. 1c) using the grounded bath electrode as reference.
Equipment and software for patch-clamp recordings and fluorescence measurements were from HEKA (Lambrecht, Germany) and from TILL photonics (Gräfelfing, Germany), respectively.
Electrophysiological experiments
Islets with a diameter of ~150 μm (Fig. 1a) were used for experiments after 1–3 days in culture. Islets were incubated in 3 mM glucose for at least 30 min prior to experiments. Single islets were then transferred to the MEA bath chamber (Fig. 1b, c), using a holding pipette with a negative pressure of about −15 mbar and fixed to an electrode with gentle mechanic pressure. The islet was continuously perifused with bath solution throughout the experiment. Recordings always started in 3 mM glucose which gave a base-line with no electrical activity. Oscillatory activity was usually followed for 30–45 min for each condition and evaluated at periods of steady-state oscillations for the last 5–10 min before a new manoeuvre started.
Patch-clamp and intracellular microelectrode recordings were performed according to Düfer et al. [8]. [Ca2+]c was determined as the ratio of fura-2 fluorescence at 360-nm and 380-nm excitation wavelength. The MEA was mounted on an inverted microscope (Zeiss, Stuttgart, Germany), and a pair of images was taken every 10 s. Because the glass of the MEA absorbed too much light at 340 nm, 360 nm excitation was used. The large difference in the ratios of the [Ca2+]c measurements between the experiments shown in Fig. 5 is presumably due to different absorption of light by the MEA since the islets were covered to a different extent by the electrode and/or conductor traces (cf. Fig. 1c).
Presentation of results
The recordings are representative of results from different islets; at least 3 different preparations were used for each series of experiments. Values are given as means ± SEM for the indicated number of experiments. Statistical significance was assessed by ANOVA followed by the Student–Newman–Keuls post-test. A value of p ≤ 0.05 was considered to be significant.
Results
To investigate whether a MEA-based setup can reliably detect the electrical activity of intact islets of Langerhans, extracellular recordings were compared to intracellular measurements obtained by traditional techniques.
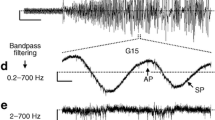
Figure 2a, c displays traces measured with the new MEA technique, while traces in Fig. 2b, d show intracellular recordings obtained with the perforated-patch technique. Figure 2a, b shows one burst of glucose-induced Ca2+ action potentials (APs) at an expanded time scale. Since the changes of V m shown in Fig. 2a are measured by extracellular electrodes, the AP voltage deflection is negative with reference to the grounded bath electrode. Thus, this recording reflects to a first approximation the negative of the first derivative of APs measured with intracellular electrodes which are by definition positive voltage deflections (Fig. 2b).
Extracellular recordings with the MEA technique (a, c) and intracellular recordings obtained using the perforated-patch technique (b, d) at 15 mM glucose. a, b Single burst of APs of beta-cells in an islet. Note that the AP voltage deflections are negative or positive when measured extracellularly or intracellularly. c, d Four consecutive oscillations in 15 mM glucose. Representative recordings for 8 extracellular and 5 intracellular measurements, respectively
Figure 2c, d shows that glucose-induced slow waves of mouse beta-cells exhibit similar patterns of electrical activity regardless of whether they are recorded by MEAs or intracellular techniques. Obviously the time course of the slow waves is different (note different time scales). This is presumably due to differences between individual islets. While the variation in individual burst and interburst times between different islets may be large, the relationship between both, i.e. the FOPP at a given glucose concentration, varies only in a narrow range. In addition, it may be that invasive electrodes induce a depolarising leakage current which makes the bursting faster. Figure 2c shows that the time resolution of extracellular recordings is sufficient to clearly discriminate burst and interburst phases and enable the determination and calculation of FOPP.
According to the consensus model of the stimulus-secretion coupling, electrical activity starts as soon as the ATP concentration close to the KATP channels is sufficiently increased to affect the channel activity [1, 19]. The glucose-induced ATP production of metabolically intact beta-cells leads to closure of KATP channels and as a consequence to the depolarization of V m [1, 7, 12]. Typically, a biphasic rise of V m with a first phase characterized by a very long burst followed by an extended silent period is observed. In the second phase the typical oscillations of V m occur. Figure 3a, b illustrates that this characteristic pattern of electrical activity is obtained with both, extracellular (MEA) and intracellular (microelectrodes) measurements. Again the burst duration in these examples is different, but a similar FOPP can be obtained in the second phase. Thus, the metabolic integrity of an islet can be estimated by extracellular measurements of changes in V m.
Electrical activity induced in beta-cells by an increase in glucose concentration from 3 to 15 mM. One representative of eight extracellular recordings (a) is compared to one of three intracellular microelectrode recordings (b). Note that in both measurements the first phase with a long burst is followed by a longer interburst. The second phase consists of more or less regular oscillations. In c the effect of 100 μM diazoxide and the additional application of 150 μM tolbutamide (indicated by horizontal bars) on an extracellular recording of an islet in 15 mM glucose is shown. One of eight experiments with similar results
Figure 3c shows the electrical activity (slow waves) of an islet in 15 mM glucose recorded with extracellular MEA electrodes which can be silenced by the addition of the KATP channel opener diazoxide (100 μM) and that APs reappear after addition of tolbutamide (150 μM).
Figure 4 shows the glucose dependency of the FOPP recorded with MEA electrodes. Traces in Fig. 4a, b show representative recordings in 10 mM and 15 mM glucose. The glucose concentration–response curve of the FOPP provides values of 0% (no activity) for 3 mM glucose (n = 10) or 100% (continuous spiking) for 30 mM glucose (n = 5). Consistent with values for intracellular recordings [12] the FOPP increased to 22 ± 3% (n = 5), 36 ± 4% (n = 5), 38 ± 3% (n = 8), and 56 ± 4% (n = 11) with 6, 8, 10, and 15 mM glucose, respectively. Fitting a Hill equation to these data (Fig. 4c) revealed half-maximal activity (50% FOPP) for 12 ± 1 mM glucose which is close to the value of 11 mM glucose measured with intracellular microelectrodes [12].
a, b Representative MEA recordings from islets in 10 or 15 mM glucose. c Glucose concentration–FOPP curve measured with MEA electrodes. Data are means ± SEM for 3 mM (n = 10), 6 mM (n = 5), 8 mM (n = 5), 10 mM (n = 8), 15 mM (n = 11), and 30 mM (n = 5) glucose, respectively. The data are fitted with the Hill equation (dotted line) giving a half-maximal glucose concentration of 12 ± 1 mM. All values are significantly different from each other (p ≤ 0.05) except those depicted as n.s.
In 1991 Santos and co-workers [18] have shown that simultaneous measurements of V m with intracellular electrodes and [Ca2+]c exhibit synchronous oscillations. Figure 5 compares electrical measurements by extracellular recording with [Ca2+]c. Two examples are given (Fig. 5a, b) respectively; the upper traces show glucose-induced electrical activity, and the lower traces are measurements of [Ca2+]c. Each burst of action potentials is accompanied by a peak in [Ca2+]c.
Discussion
Pancreatic beta-cells are unique as they exhibit oscillatory electrical activity known as slow waves in response to glucose [7]. The FOPP rises proportional to the glucose concentration within a range between ~6 and 25 mM with a half-maximum response of approximately 11 mM [12]. At concentrations > 25 mM continuous action potential firing occurs whereas at lower glucose concentrations (< 6 mM) beta-cells are electrically silent [7]. Extracellular MEA recordings from whole islets exhibit the same glucose dependence (Fig. 3). Thus, possible contamination from the electrical activity of alpha-cells that exhibit the opposite glucose dependence, i.e. activation at low and inhibition at high glucose [7], is negligible. This can be explicitly seen in Fig. 3a where the glucose concentration was changed from 3 to 15 mM. If alpha-cell electrical activity contributed to the background noise seen in 3 mM glucose, it should be silenced when the glucose concentration was increased (just before beta-cells were activated), but no change was detected in this and similar experiments.
The detection of islet cell electrical activity by extracellular electrodes has been reported so far by two groups [3, 17]. To our knowledge this is the first demonstration of glucose-induced slow waves and the first calculation of the glucose dependence of FOPP using MEA technology.
As [Ca2+]c [18] and importantly insulin secretion [2, 10] are coupled directly to the fraction of time beta-cells are bursting, FOPP provides an excellent marker for the actual insulin release from islets in response to glucose and/or test substances. Two examples (see Fig. 5) illustrate the close coupling between bursts measured with extracellular electrodes and [Ca2+]c. Every burst of action potentials produces a rise in [Ca2+]c as previously shown for simultaneous measurements with microelectrodes and Ca2+ imaging [18]. Thus, determining FOPP with an extracellular microelectrode array provides a facile means to estimate insulin release in real time in response to various stimuli. Compared to patch-clamp or Ca2+ measurements by fluorescence techniques the method has the advantage to be non-invasive. Other types of cells and tissue slices have been maintained on MEAs for long periods; [14] thus, the method offers the possibility of recording from the same islet multiple times at intervals of hours or days. Therefore, MEAs allow recording of both acute changes of beta-cell electrical activity and the chronic effects of metabolites or drugs. MEAs should be useful for investigating co-cultures, e.g. how co-culturing pig islets with spleen cells affects insulin secretion [21] or how bone marrow increases the survival time of human islets [16]. MEAs could be used to study co-cultures of macrophages and islets and determine the action of macrophage-released substances on beta-cell electrical activity.
Finally, MEA technology can be applied to human islets. A screening system allowing the simultaneous measurement of oscillating insulin secretion by up to 15 single islets has been published recently [5], but the required manual encapsulation of single islets and the use of specifically labelled insulin, insulin antibody, and a means of fluorescence detection make the assay technically difficult.
We have provided a "proof-of-principle" that FOPP can be measured using single islets and extracellular electrode arrays. These arrays have multiple electrodes which can be recorded from independently; thus, it is feasible to develop low-throughput to medium-throughput assay systems where the electrical activity of 50 or more islets can be measured simultaneously. This would provide an ideal tool to evaluate the metabolic state of primary islets of Langerhans and to test the effects of metabolites, cytokines, drugs, and co-cultured cells.
References
Ashcroft FM, Rorsman P (1989) Electrophysiology of the pancreatic beta-cell. Prog Biophys Mol Biol 54:87–143
Bergsten P, Grapengiesser E, Gylfe E, Tengholm A, Hellman B (1994) Synchronous oscillations of cytoplasmic Ca2+ and insulin release in glucose-stimulated pancreatic islets. J Biol Chem 269:8749–53
Bornat Y, Raoux M, Boutaib Y, Morin F, Charpentier C, Lang J, Renaud S (2010) Detection of electrical activity of pancreatic beta-cells using micro-electrode arrays. IEEE International Symposium on Electronic Design, Test & Applications 5:233–236
Dean PM, Matthews EK (1968) Electrical activity in pancreatic islet cells. Nature 219:389–90
Dishinger JF, Reid KR, Kennedy RT (2009) Quantitative monitoring of insulin secretion from single islets of Langerhans in parallel on a microfluidic chip. Anal Chem 81:3119–27
Drews G, Krämer C, Düfer M, Krippeit-Drews P (2000) Contrasting effects of alloxan on islets and single mouse pancreatic beta-cells. Biochem J 352(Pt 2):389–97
Drews G, Krippeit-Drews P, Düfer M (2010) Electrophysiology of islet cells. Adv Exp Med Biol 654:115–63
Düfer M, Haspel D, Krippeit-Drews P, Aguilar-Bryan L, Bryan J, Drews G (2004) Oscillations of membrane potential and cytosolic Ca2+ concentration in SUR1−/− beta cells. Diabetologia 47:488–98
Gilon P, Ravier MA, Jonas JC, Henquin JC (2002) Control mechanisms of the oscillations of insulin secretion in vitro and in vivo. Diabetes 51(Suppl 1):S144–51
Gilon P, Shepherd RM, Henquin JC (1993) Oscillations of secretion driven by oscillations of cytoplasmic Ca2+ as evidences in single pancreatic islets. J Biol Chem 268:22265–8
Göpel S, Kanno T, Barg S, Galvanovskis J, Rorsman P (1999) Voltage-gated and resting membrane currents recorded from B-cells in intact mouse pancreatic islets. J Physiol 521(Pt 3):717–28
Henquin JC (2009) Regulation of insulin secretion: a matter of phase control and amplitude modulation. Diabetologia 52:739–51
Henquin JC, Meissner HP (1984) Significance of ionic fluxes and changes in membrane potential for stimulus-secretion coupling in pancreatic B-cells. Experientia 40:1043–52
Hofmann F, Bading H (2006) Long term recordings with microelectrode arrays: studies of transcription-dependent neuronal plasticity and axonal regeneration. J Physiol Paris 99:125–32
Khan FA, Goforth PB, Zhang M, Satin LS (2001) Insulin activates ATP-sensitive K+ channels in pancreatic beta-cells through a phosphatidylinositol 3-kinase-dependent pathway. Diabetes 50:2192–8
Luo L, Badiavas E, Luo JZ, Maizel A (2007) Allogeneic bone marrow supports human islet beta cell survival and function over six months. Biochem Biophys Res Commun 361:859–64
Palti Y, David GB, Lachov E, Mida YH, Schatzberger R (1996) Islets of Langerhans generate wavelike electric activity modulated by glucose concentration. Diabetes 45:595–601
Santos RM, Rosario LM, Nadal A, Garcia-Sancho J, Soria B, Valdeolmillos M (1991) Widespread synchronous [Ca2+]i oscillations due to bursting electrical activity in single pancreatic islets. Pflügers Arch - Eur J Physiol 418:417–22
Schulze DU, Düfer M, Wieringa B, Krippeit-Drews P, Drews G (2007) An adenylate kinase is involved in KATP channel regulation of mouse pancreatic beta cells. Diabetologia 50:2126–34
Stett A, Egert U, Guenther E, Hofmann F, Meyer T, Nisch W, Haemmerle H (2003) Biological application of microelectrode arrays in drug discovery and basic research. Anal Bioanal Chem 377:486–95
You S, Rivereau AS, Gouin E, Sai P (2001) Co-incubation of pig islet cells with spleen cells from non-obese diabetic mice causes decreased insulin release by non-T-cell- and T-cell-mediated mechanisms. Clin Exp Immunol 125:25–31
Acknowledgement
This work was supported by grants from the Deutsche Forschungsgemeinschaft (G.D. and M.D.). We thank Professor Joseph Bryan for his comments on the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Pfeiffer, T., Kraushaar, U., Düfer, M. et al. Rapid functional evaluation of beta-cells by extracellular recording of membrane potential oscillations with microelectrode arrays. Pflugers Arch - Eur J Physiol 462, 835–840 (2011). https://doi.org/10.1007/s00424-011-1029-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00424-011-1029-z