Abstract
To evaluate the relationship between lactate release and [lac]art and to investigate the influence of the catecholamines on the lactate release, 14 healthy men [age 25±3 (SE) year] were studied by superimposing cycle on forearm exercise, both at 65% of their maximal power reached in respective incremental tests. Handgrip exercise was performed for 30 min at 65% of peak power. In addition, between the tenth and the 22nd minute, cycling with the same intensity was superimposed. The increase in venous lactate concentration ([lac]ven) (rest: 1.3±0.4 mmol·l−1; 3rd min: 3.9±0.8 mmol·l−1) begins with the forearm exercise, whereas arterial lactate concentration ([lac]art) remains almost unchanged. Once cycling has been added to forearm exercise (COMB), [lac]art increases with a concomitant increase in [lac]ven (12th min: [lac]art, 3.2±1.3 mmol·l−1; [lac]ven, 5.7±2.2 mmol·l−1). A correlation between oxygen tension (PvO2) and [lac]ven cannot be detected. There is a significant correlation between [lac]art and norepinephrine ([NE]) (y=0.25x+1.2; r=0.815; p<0.01) but no correlation between lactate release and epinephrine ([EPI]) at moderate intensity. Our main conclusion is that lactate release from exercising muscles at moderate intensities is neither dependent on PvO2 nor on [EPI] in the blood.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Lactate concentration [lac] is often used as an indicator to determine exercise intensity [33, 43, 44, 47]. For a long time, the occurrence of lactate was attributed to an insufficient oxygen supply to the working muscles [8, 46]. But experiments with isolated muscles have shown that [lac] seems to be dependent on exercise intensity but independent of oxygen concentration [9]. This was shown in experiments on humans during one-legged or forearm exercise. There was no relation between lactate release and PO2 in the blood [27, 36]. This indicates that the increase in [lac] during exercise is not triggered by an insufficient oxygen supply to the working muscles [4, 5, 9].
Another hypothesis was developed linking the epinephrine concentration [EPI] and [lac] because of a parallel rise during incremental exercise [26]. Evidences for a direct effect of epinephrine on lactate release came from infusion experiments in animals and humans. Stainsby et al. [42], for example, have concluded that lactate release during progressive work with isolated muscles can be elevated by an epinephrine infusion. This was demonstrated during cycling exercise as well [25, 45]. Those findings suggest the possibility of a causal relationship between the increase of plasma catecholamines and [lac]. On the other hand, results from studies with β-blockade are inconsistent [6, 16, 21, 23]. In some studies, there was no effect on lactate release [16, 21], and in others, lactate release was reduced [6]. But it is clear from studies of isolated muscles that [lac] can increase without the presence of epinephrine [9]. In that cited study, muscle lactate concentration was linearly related to exercise intensity.
During moderate exercise in humans, [lac] increases without a significant increase of [EPI] [4, 25]. In case exercise intensity is high enough, arterial [lac] reaches a plateau and remains almost constant until the end of exercise. But several authors reported that under such conditions, lactate release from the working muscle decreases [39] in spite of an increased [EPI] [6, 39]. Saltin et al. [39] pointed out that a net uptake of lactate in the working muscle might occur. The same lactate-release pattern is reported from exercise with small muscle groups. Jorfeldt and Wahren [22] reported that medium intensity forearm exercise leads to a sharp increase of lactate release in the first 10 min but then gradually diminishes. Under those exercise conditions, [EPI] does not increase significantly.
Alterations in the tone of the sympathetic nerve activity and circulating catecholamines are influenced by exercising muscle mass. Hence, we performed a study to distinguish between muscular and systemic effects on lactate concentration in blood. For this purpose, we chose an experimental design where exercise of a small muscle group was combined with exercise of a large muscle group since a small muscle group would not trigger sympathetic nervous activity. Additionally, exercise with a small muscle group causes negligible changes in the composition of arterial blood [29]. Exercise with the small muscle group preceding the period of combined exercise with a large muscle group served to supply us with information on muscular events [29, 35]. Adding whole body exercise to it submerges the small muscle group with systemic effects, which are mediated by the large muscle group.
On the basis of these previous observations and the above-mentioned considerations, we hypothesized that the elevated blood muscle lactate release during submaximal exercise was not associated to changes in PvO2 or [EPI].
Materials and methods
Fourteen healthy male subjects, aged 22–28 years, volunteered to participate in this study. They were informed about potential risks and discomfort associated with the experiment and gave their consent to participate. All subjects were physically active and under no medication. They performed combined continuous forearm and cycle exercise, both at 65% of their maximal performance capacity. Maximal performances were evaluated before the combined exercise during incremental exercise tests—pretest—of the respective muscle group.
Pretest protocols
Forearm exercise
Power output of the small muscle group in the distal forearm was evoked by handgrip exercise lifting defined weights over a maximum distance of 4 cm. The vertical movement of the weight was analyzed online measuring contraction velocity and distance. The forearm performance was measured in an incremental test starting with an initial weight of 2.5 kg. The weight was increased every 3 min by 2.5 kg until subjective fatigue. The contraction frequency was 24 times per minute. The subject's elbow rested on an arm pad supporting the horizontal position to ensure that only the muscles of the distal forearm were recruited during work.
Cycle exercise
After a 1-h break following the forearm pretest, the subjects started with the cycle exercise pretest. Maximal power on the cycle ergometer (Lode Excalibur Sport, Groningen, The Netherlands) was also evaluated during an incremental exercise test. The test began with 100 Ws Increments of 16.7 W were added every minute. Power was increased until subjective exhaustion. The average cadence was 80 cycles per minute.
Experimental protocol
Three days after the pretests, the subjects performed the combination test. Subjects were instructed not to perform physical exercise the day before the experiment. The subjects were seated on the cycle ergometer with their right arm attached to the handgrip ergometer. A teflon catheter (Introcan, B. Braun, Melsungen, Germany) was inserted into a deep antecubital vein. This ensured that the blood sampled during exercise was from the active forearm muscles [22]. To minimize circulatory effects deriving from skin blood flow into the antecubital vein, the whole right forearm was cooled by means of a fan throughout the entire experiment. The left forearm including the hand was heated with two heating pads. A teflon catheter was inserted into a dorsal vein of the left hand for sampling arterialized venous blood [3, 24].
Heart rate was recorded using a heart rate monitor (Sporttester Polar, Finland). Forearm blood flow (LBF) was measured by venous occlusion plethysmography (Periquant 3800, Gutmann, Germany). The subjects' left earlobe was hyperemized (Finalgon, Thomae, Biberach a. d. Riss, Germany) for additional blood samples. After the subjects had been prepared for the test, resting blood samples were taken. Limb perfusion and heart rate were registered simultaneously (t=−3 min). At t=0, the experiment started with dynamic contractions (contraction frequency was 24 times per minute) of the small muscle group (HAND1) at 65% of peak performance. HAND1 continued for 10 min. Thereafter, the subjects started to cycle at 65% of peak performance, while handgrip exercise continued so that exercise of the small and large muscle group were combined (COMB). COMB lasted for 12 min, whereas handgrip exercise continued for another 8 min after cessation of cycle ergometry (HAND2).
Arterialized and venous blood were sampled at rest and then every 3 min during exercise for the analysis of [lac], blood gases, as well as acid and base in a heparinized, (20 μl Liquemin N 2500, Hoffmann La Roche, Grenzach-Wyhlen, Germany) 10-ml syringe (LUER, B. Braun). In addition, another 2 ml of venous blood (anticoagulant EDTA) was sampled for the determination of catecholamine concentrations ([EPI] and [NE]). Arterialized blood from the hyperemized earlobe was also taken every 3 min for [lac]art determination. After each blood sampling routine, the handgrip exercise was interrupted for less than 5 s to proceed with the LBF measurement.
Analyses
Syringes were analyzed in a random order to rule out systematic changes due to storage patterns. PO2, Hemoglobin concentration ([Hb]) and hemoxyglobin (HbO2) (ABL 330, Hemoximeter OSM3, Radiometer, Copenhagen, Denmark) were measured.
For lactate analysis, an enzyme-electrode (Biosen 5030L Envitec, Wismar, Germany) was used. The aliquots of plasma were stored at −20°C for later catecholamine detection. High-pressure liquid chromatography (Knauer, Berlin, Germany; Column, Lichtopher 60, RP Select B, 5 μm/ 250×4 mm, Merck, Germany) was used for further analysis. Catecholamine concentrations were determined by means of an electrochemical detector (2143-RPE, Pharmacia LKB, Freiburg, Germany) and computed as nanomole per liter.
The oxygen uptake and lactate release were calculated according to the Fick principle.
Statistical evaluation
Results are presented as means±SD or standard error of the mean (SEM). Analysis of variance for repeated measures (parametric) was performed to evaluate significant differences between repeated mean values. A Scheffé’s test was used for post hoc testing. When appropriate, linear regressions were calculated and correlations of variables were determined. The level of significance was chosen to be p<0.05.
Results
The average weight lifted was 12.64±3.8 kg corresponding to 2.02 W. Power during cycling exercise was 252±29.1 W, both corresponding to 65% of the respective incremental tests.
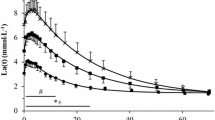
At rest [lac]art and [lac]ven did not differ significantly. At the onset of the handgrip exercise, [lac]ven rises within the first 3 min (p<0.05). As [lac]art remains almost constant, a-v difflac, presented in the lower part of Fig. 1, shows the accumulation of lactate in venous blood (a-v difflac, rest: −0.2±0.1 mmol·l−1; 3rd min: −2.6±0.6 mmol·l−1, p<0.01). With the onset of cycle exercise, venous and arterial lactate concentrations rise simultaneously. However, [lac]ven remains higher than [lac]art throughout the test. [lac]ven reaches a plateau after the 15th minute. During HAND2 [lac] decreases in arterialized and venous blood. At the end of the experiment both [lac]art and [lac]ven are higher than at the beginning (p<0.05). The rise in arterial and venous [lac] at the start of COMB leads to a slight increase of the a-v difflac (Fig. 1). The net lactate release increases in the first 3 min (p<0.01) and then decreases thereafter almost to preexercise values (Fig. 2). For comparison, the time course of lactate release during sole handgrip exercise under similar conditions is shown (unpublished data). The PvO2 does not correlate with the [lac]ven, neither during HAND nor during COMB (data not shown). The resting values of [NE] and [EPI] of the present study are similar to values already reported in the literature. [EPI] and [NE] remain constant during HAND1 and increase significantly during COMB [rest: EPI, 0.2±0.14; NE, 4.5±1.6 nmol·l−1; COMB: EPI, 2.6±1.8; NE, 26. 5±10.4 nmol·l−1 (p<0.05)] (Fig. 3). In Fig. 4, the relationship between [NE] and [lac]art during HAND1 and COMB is shown. There is a significant correlation between [NE] and [lac]art (p<0.01), as well as between [lac]art and [EPI] (y=1.2+0.24x, r=0.804; data not shown). However, there is no correlation between a-v difflac and [EPI] (Fig. 5) during work at moderate intensity. The changes in the a-v diffO2 and the PvO2 are not significant during the whole exercise period (Fig. 6). Forearm blood flow rises from 3.0±1.8 to 32.0±18.3 ml·100 ml tissue−1·min−1 (p<0.01), 3 min after the start of the handgrip exercise. The blood flow decreases slightly but insignificantly during COMB and recovers until the end of experiment. From the a-v diffO2 and the perfusion rates (Q), the local oxygen consumption (VO2) was calculated (Fig. 6). From the onset of HAND1, the local VO2 increases rapidly (p<0.01) and does not change significantly throughout the experiment.
Time courses of venous and arterial lactate concentration ([lac]ven, [lac]art) and arteriovenous difference (a-v difflac) during HAND1, COMB, and HAND2. Values are means±SD. Asterisks denote values that are significantly different from resting values (P<0.05). Plus symbols denote values that are significantly different from the values at the end of HAND1 (P<0.05)
Time course of net lactate release during HAND1, COMB, and HAND2. Values are means±SEM (filled symbols). Asterisks denote values that are significantly different from resting values. Note: HAND2 n=11, n=9, n=8. In addition, the time course of net lactate release during forearm exercise under similar conditions is included (open symbols). n=8 (unpublished data)
Discussion
The purpose of our study was to investigate the changes in blood lactate concentration at the site of a solely working small muscle group. Additionally, we wanted to determine the effects of catecholamines on [lac] during superimposed continuous submaximal cycling exercise.
With our experimental setup, we have shown that the time course of net lactate release from the exercising muscle group at moderate intensity neither depends on PO2 nor on [EPI]; this conclusion is valid for any chosen timeframe of our experimental periods. The altered ratio of active muscle mass to distribution volume causes higher systemic [lac] during COMB. Additionally, the increase in [NE] might lead to a reduced perfusion of lactate-eliminating organs and, therefore, might contribute to a rising systemic [lac].
Methodological considerations
Choice of the experimental setup
The isolated forearm exercise gave us some indications about the metabolic changes occurring in working muscles [35] because the systemic effects mediated by the sympathetic nervous system during forearm contractions are negligible [29]. Due to the systemic impact caused by the bicycle work, the sympathetic nervous system might then enhance or at least affect the observed metabolic changes in the small muscle group during COMB.
Forster et al. [13] have shown that acid–base values and [lac] in arterialized blood from a hand vein are comparable to arterial blood. Today, this technique is a commonly accepted method [3, 24].
Similarity of exercise intensities: small vs large muscle group
The subjects had to perform handgrip and cycle exercise, both at 65% of the peak performance. We were able to find out in our lab in previous experiments that subjects can sustain this workload for at least 1 h in both exercise modes. Several studies using forearm or leg muscles at the same relative exercise intensity have shown similar lactate-release patterns [17, 22, 40]. Direct comparison of prolonged leg and arm exercise in the same subjects resulted in a similar time course of lactate release [21]. Therefore, it is reasonable to assume that the lactate release at the chosen exercise intensities of the forearm and the leg muscles are comparable.
The relation between oxygen deficit, PO2, and lactate concentration
The sharp rise in [lac]ven during HAND1, the isolated exercise of the small muscle group, is due to lactate efflux from this muscle group, as [lac]art remained unchanged. The amount of lactate released into the venous blood is too small to cause an increase in [lac]art because the distribution volume during one-armed exercise is too large to register a systemic increase in [lac].
The lactate released from the forearm during the whole 30 min of exercise is by far more than what could be explained by the oxygen deficit developed during the transition from rest to exercise during the first minutes. The steady state in oxygen uptake is reached in the third minute of exercise. If we assume a linear increase within the first 3 min, the oxygen-deficit would cumulate to approximate 60 ml O2 per liter of tissue. This corresponds to 9.5 mmol l−1 lactate. In our study, the total lactate release from the third to the 30th minute was about 18 mmol l−1 lactate. Furthermore, in this calculation, we have not considered two aspects: first, the breakdown of phosphocreatine and, second, we have assumed a linear time course of VO2, which, in reality, is mono-exponential [15]. Both would further reduce the calculated amount of produced lactate. Thus, the assumptions to calculate the oxygen deficit reflect an overestimation of the lactate produced during the transition from rest to exercise. However, the total amount of lactate released exceeds the amount being produced during the first 3 min, showing that lactate production is going on during steady-state exercise but may decrease over time [39]. This might be reflected by the diminishing lactate release, which was not dependent on PvO2 neither during HAND1 nor during COMB.
The relation between muscle mass and lactate concentration
The present data show again that the amount of recruited muscle mass has a substantial influence on lactate kinetics in the arterial blood. The concentration of a substance in the blood depends on its amount and the volume where the substance will be distributed. During the first 3 min of the forearm contraction at 65% of the peak power, there is a steep increase in [lac]ven, which is followed by a steady decrease toward resting values. This is different from the arterial lactate time course during cycle ergometry. It was shown in other studies that [lac]art increases at the beginning and then remains almost constant at the respective intensity [28]. There are some aspects that have to be considered while comparing exercise with a small and a large muscle group: (1) the alteration in the ratio between working and resting muscle mass and (2) the alteration between the mass of the working muscle and the distribution volume [19, 37]. At the same exercise intensity and the same lactate production rate per unit muscle mass, the total amount of substance produced must rise. Consequently, [lac]art increases as the volume of distribution remains constant. One underlying reason for the decrease in a-v difflac might be the influence of increasing [lac]art on the diffusion gradient across the sarcolemma. The recirculating lactate reduces the diffusion gradient, which results in an increased intracellular [lac]. As the sarcolemma is easily permeable for water [30], an increase in intracellular [lac] should result in an increase in blood osmolality. Since the incline in a-v diffosmo is small and transient (data not shown), an accumulation of intracellular lactate during COMB seems to be improbable. The decrease in lactate release over time from muscles working at moderate intensity was already shown by Jorfeldt and Wahren [22], who investigated forearm muscles, and by Brooks et al. [6], Bergman et al. [2] and Sjogaard et al. [40] during cycling exercise. This seems to be caused by a reduction in intracellular [lac] during prolonged exercise with moderate intensity [34]. In this study, lactate release is not affected by the added cycling exercise. The lactate-release pattern over time resembled that of the solely exercising small muscle group (Fig. 2) [40] and during cycling ergometry [6]. The curve fits almost perfectly to the lactate release from the forearm, published by Jorfeldt and Wahren [22]. In the present study, there was an almost linear decrease of lactate release as seen in Jorfeldt’s work, despite the start of COMB. The relative decrease was similar as well.
The impact of the sympathetic nervous system on blood lactate concentration
Exercise at submaximal intensities with a large muscle group stimulates the sympathetic nervous system. This is indicated by rising catecholamine concentrations during COMB. Several studies have shown a strong correlation between blood lactate and [EPI] during graded and steady-state exercise [6, 26, 31, 41] as well as during exercise at high altitude [4, 5]. These data suggest that glycogenolysis and glycolysis are stimulated by β-adrenergic agonism, resulting in an enhanced lactate production with a subsequent rise in blood [lac] [17, 20, 23, 31]. However, in our experiment, a-v difflac and lactate release decline with rising [EPI]. A plot of these parameters does not reveal any correlation. This pleads against a role of epinephrine as a major factor causing lactate release from the working muscle at moderate intensities. This is consistent with results of van Hall et al. [17], who found that the circulating [EPI] did not correlate with the rate of glycogen breakdown during exercise at moderate intensity. Glycogen breakdown was unchanged by β-blockade [18]. This is in line with authors who were also unable to show any differences in muscle lactate-release pattern and systemic lactate concentrations during moderate exercise intensity after β-blockade [16, 21]. Our findings seem to be in contrast to others [25, 45]. With epinephrine infusion, they found an increased [lac] in muscle and blood [45], but they reached [EPI] usually found during exhausting exercise. With the maximum [EPI] reached in our experiments, there was no significant change in muscle and blood lactate in their study as well. Unfortunately, this was at rest and at the beginning of the exercise; thus, the conditions are not easy to compare. On the other hand, [lac]art in the present study was higher than the [lac] after epinephrine infusion [25, 45]. This indicates that high [lac] can be reached without high [EPI]. The consequence is that, during exercise of moderate intensity, epinephrine is not the main factor for an elevated lactate release [16, 21].
Nevertheless, [lac]art correlates well with [EPI] and [NE] during HAND1 and COMB. The first correlation might indicate that lactate originates from other tissues like fat [10, 12, 14], lung [7], or even sweat glands [11]. The second might indicate increased [lac] caused by reduced blood flow in lactate-eliminating organs, like resting muscles [32, 38] or liver [1].
Conclusion
At moderate exercise intensity, lactate release from the forearm muscle is not affected by superimposing extra muscle exercise. A β-adrenergic effect on lactate release cannot be detected. Lactate release from the working muscle decreases with continuing exercise. In spite of the reduced release, the increasing [lac]art is the result of a change in the ratio of exercising muscle mass vs distribution volume. This might be the result from an attenuated elimination of lactate in resting organs due to a reduced blood flow, e.g., toward splanchnic organs and resting muscles. Further investigations have to reveal the quantitative contribution of the different factors influencing blood [lac]. But it is evident that arterial [lac] does not simply reflect muscle metabolism.
References
Ahlborg G, Wahren J, Felig P (1986) Splanchnic and peripheral glucose and lactate metabolism during and after prolonged arm exercise. J Clin Invest 77:690–699
Bergman BC, Wolfel EE, Butterfield GE, Lopaschuk GD, Casazza GA, Horning MA, Brooks GA (1999) Active muscle and whole body lactate kinetics after endurance training in men. J Appl Physiol 87:1684–1696
Bohnert B, Ward SA, Whipp BJ (1998) Effects of prior arm exercise on pulmonary gas exchange kinetics during high-intensity leg exercise in humans. Exp Physiol 83:557–570
Brooks GA, Butterfield GE, Wolfe RR, Groves BM, Mazzeo RS, Sutton JR, Wolfel EE, Reeves JT (1991) Decreased reliance on lactate during exercise after acclimatization to 4,300 m. J Appl Physiol 71:333–341
Brooks GA, Wolfel EE, Groves BM, Bender PR, Butterfield GE, Cymerman, Mazzeo RS, Sutton JR, Wolfe RR, Reeves JT (1992) Muscle accounts for glucose disposal but not blood lactate appearance during exercise after acclimatization to 4,300 m. J Appl Physiol 72:2435–2445
Brooks GA, Wolfel EE, Butterfield GE, Cymerman A, Roberts AC, Mazzeo RS, Reeves JT (1998) Poor relationship between arterial [lactate] and leg net release during exercise at 4,300 m altitude. Am J Physiol 275:R1192–R1201
Brooks GA, Henderson GC, Horning MA, Lehmann SL, Bergman BC, Wolfel EE (2004) Pyruvate–lactate shuttling in vivo. 9th Annual Congress, ECSS, Clermont Ferrand, Book of Abstracts 150
Bylund-Fellenius AC, Walker PM, Elander A, Holm S, Holm J, Schersten (1981) Energy metabolism in relation to oxygen partial pressure in human skeletal muscle during exercise. Biochem J 200:247–255
Connett RJ, Gayeski TE, Honig CR (1984) Lactate accumulation in fully aerobic, working, dog gracilis muscle. Am J Physiol 246:H120–H128
Coppack SW, Fisher RM, Gibbons GF, Humphreys SM, McDonough MJ, Potts JL, Frayn KN (1990) Postprandial substrate deposition in human forearm and adipose tissues in vivo. Clin Sci (Lond) 79:339–348
de Boer J, Plijter-Groendijk H, Visser KR, Mook GA, Korf J (1994) Continuous monitoring of lactate during exercise in humans using subcutaneous and transcutaneous microdialysis. Eur J Appl Physiol Occup Physiol 69:281–286
Faintrenie G, Geloen A (1998) Alpha-1 adrenergic stimulation of glucose uptake in rat white adipocytes. J Pharmacol Exp Ther 286:607–610
Forster HV, Dempsey JA, Thomson J, Vidruk E, DoPico GA (1972) Estimation of arterial PO2, PCO2, pH, and lactate from arterialized venous blood. J Appl Physiol 32:134–137
Frayn KN, Coppack SW, Humphreys SM, Whyte PL (1989) Metabolic characteristics of human adipose tissue in vivo. Clin Sci 76:509–516
Grassi B, Gladden LB, Stary CM, Wagner PD, Hogan MC (1998) Peripheral O2 diffusion does not affect V(O2)on-kinetics in isolated in situ canine muscle. J Appl Physiol 85:1404–1412
Gullestad L, Hallen J, Sejersted OM (1993) Variable effects of beta-adrenoceptor blockade on muscle blood flow during exercise. Acta Physiol Scand 149:257–271
Hall G van, Wagenmakers AJM, Saltin B (1996) Muscle glycogen content and K+ exchange during prolonged dynamic exercise. In: Amino acids, ammonia and exercise in man. Doctoral Thesis University Maastricht, pp 55–67
Hartling OJ, Schibye B, Klausen K, Lund LO, Trap-Jansen J (1986) Metabolism of exercising skeletal muscle during beta 1-selective adrenoceptor blockade. Clin Physiol 6:431–438
Hooker SP, Wells CL, Manore MM, Philip SA, Martin N (1990) Differences in epinephrine and substrate responses between arm and leg exercise. Med Sci Sports Exerc 22:779–784
Hughson RL, Green HJ, Sharratt MT (1995) Gas exchange, blood lactate, and plasma catecholamines during incremental exercise in hypoxia and normoxia. J Appl Physiol 79:1134–1141
Jensen-Urstad M, Ahlborg G, Sahlin K (1993) High lactate and NH3 release during arm vs. leg exercise is not due to beta-adrenoceptor stimulation. J Appl Physiol 74:2860–2867
Jorfeldt L, Wahren J (1970) Human forearm muscle metabolism during exercise. Scand J Clin Lab Invest 26:73–78
Kaiser P, Tesch PA, Thorsson A, Karlsson J, Kaijser L (1985) Skeletal muscle glycolysis during submaximal exercise following acute beta-adrenergic blockade in man. Acta Physiol Scand 123:285–291
Kerckhoffs DA, Arner P, Bolinder J (1998) Lipolysis and lactate production in human skeletal muscle and adipose tissue following glucose ingestion. Clin Sci (Lond) 94:71–77
Kreisman SH, Ah-Mew N, Arsenault M, Nessim SJ, Halter JB, Vranic M, Marliss EB (2000) Epinephrine infusion during moderate intensity exercise increases glucose production and uptake. Am J Physiol Endocrinol Metab 278:E949–E957
Lehmann M, Schmid P, Keul J (1985) Plasma catecholamine and blood lactate cumulation during incremental exhaustive exercise. Int J Sports Med 6:78–81
Maassen N (1996) Mechanism of fatigue in small muscle groups. In: Steinacker JM, Ward SA (eds) The physiology and pathophysiology of exercise tolerance. Plenum Press New York London, pp 31–36
Maassen N, Busse MW (1989) The relationship between lactic acid and work load: a measure for endurance capacity or an indicator of carbohydrate deficiency? Eur J Appl Physiol Occup Physiol 58:728–737
Maassen N, Schneider G (1997) Mechanism of fatigue in small muscle groups. Int J Sports Med 18(Suppl 4):S320–S321
Macknight ADC, Leaf A (1977) Regulation of cellular volume. Physiol Rev 57:510–573
Mazzeo RS (1989) Influence of plasma catecholamines on the lactate threshold during graded exercise. J Appl Physiol 67:1319–1322
McAllister RM (1998) Adaptations in control of blood flow with training: splanchnic and renal blood flows. Med Sci Sports Exerc 30:375–381
Nicholson RM, Sleivert GG (2001) Indices of lactate threshold and their relationship with 10-km running velocity. Med Sci Sports Exerc 33(2):339–342, Feb
Phillips SM, Green HJ, Tarnopolsky MA, Heigenhauser GJ, Grant SM (1996) Progressive effect of endurance training on metabolic adaptations in working skeletal muscle. Am J Physiol 270:E265–E272
Raynaud J, Douguet D, Legros P, Capderou A, Raffestin B, Durand J (1986) Time course of muscular blood metabolites during forearm rhythmic exercise in hypoxia. J Appl Physiol 60:1203–1208
Richardson RS, Noyszewski EA, Leigh JS, Wagner PD (1998) Lactate efflux from exercising human skeletal muscle: role of intracellular PO2. J Appl Physiol 85:627–634
Richter EA, Kiens B, Saltin B, Christensen NJ, Savard G (1988) Skeletal muscle glucose uptake during dynamic exercise in humans: role of muscle mass. Am J Physiol 254:E555–E561
Rowell LB, Blackmon JR, Kenny MA, Escourrou P (1984) Splanchnic vasomotor and metabolic adjustments to hypoxia and exercise in humans. Am J Physiol 247:H251–H258
Saltin B, Nazar K, Costill DL, Stein E, Jansson E, Essen B, Gollnick (1976) The nature of the training response; peripheral and central adaptations of one-legged exercise. Acta Physiol Scand 96:289–305
Sjogaard G, Adams RP, Saltin B (1985) Water and ion shifts in skeletal muscle of humans with intense dynamic knee extension. Am J Physiol 248:R190–R196
Stainsby WN, Sumners C, Andrew GM (1984) Plasma catecholamines and their effect on blood lactate and muscle lactate output. J Appl Physiol 57:321–325
Stainsby WN, Sumners C, Eitzman PD (1985) Effects of catecholamines on lactic acid output during progressive working contractions. J Appl Physiol 59:1809–1814
Stegmann H, Kindermann W, Schnabel A (1981) Lactate kinetics and individual anaerobic threshold. Int J Sports Med 2:160–165
Wasserman K (1964) Detecting the threshold of anaerobic metabolism in cardiac patients during exercise. Am J Cardiol 14:844
Watt MJ, Howlett KF, Febbraio MA, Spriet LL, Hargreaves M (2001) Adrenaline increases skeletal muscle glycogenolysis, pyruvate dehydrogenase activation and carbohydrate oxidation during moderate exercise in humans. J Physiol 534:269–278
Whipp BJ, Davis JA, Torres F, Wasserman K (1981) A test to determine parameters of aerobic function during exercise. J Appl Physiol 50:217–221
Wiswell RA, Jaque SV, Marcell TJ, Hawkins SA, Tarpenning KM, Constantino N, Hyslop DM (2000) Maximal aerobic power, lactate threshold, and running performance in master athletes. Med Sci Sports Exerc 32:1165–1170
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chudalla, R., Baerwalde, S., Schneider, G. et al. Local and systemic effects on blood lactate concentration during exercise with small and large muscle groups. Pflugers Arch - Eur J Physiol 452, 690–697 (2006). https://doi.org/10.1007/s00424-006-0082-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00424-006-0082-5