Abstract
Purpose
The aim of this study was to investigate lactate recovery kinetics after high-intensity exercises.
Methods
Six competitive middle-distance runners performed 500-, 1000-, and 1500-m trials at 90 % of their current maximal speed over 1500 m. Each event was followed by a passive recovery to obtain blood lactate recovery curves (BLRC). BLRC were fitted by the bi-exponential time function: La(t) = La(0) + A 1(1–e −γ1t) + A 2(1–e −γ2t), where La(0) is the blood lactate concentration at exercise completion, and γ 1 and γ 2 enlighten the lactate exchange ability between the previously active muscles and the blood and the overall lactate removal ability, respectively. Applications of the model provided parameters related to lactate release, removal and accumulation rates at exercise completion, and net amount of lactate released during recovery.
Results
The increase of running distance was accompanied by (1) a continuous decrease in γ 1 (p < 0.05), (2) a primary decrease (p < 0.05) and then a stabilization of γ 2, and (3) a constant increase in blood concentrations (p < 0.05) and whole body accumulation of lactate (p < 0.05). Estimated net lactate release, removal and accumulation rates at exercise completion, as well as the net amount of lactate released during recovery were not significantly altered by distance.
Conclusion
Alterations of lactate exchange and removal abilities have presumably been compensated by an increase in muscle-to-blood lactate gradient and blood lactate concentrations, respectively, so that estimated lactate release, removal and accumulation rates remained almost stable as distance increased.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
During high-intensity exercise, glycogenolysis and glycolysis are greatly activated, leading to concomitant lactate accumulation and pH decrease in active muscles and subsequently in blood (Marcinek et al. 2010; Osnes and Hermansen 1972). However, several studies suggest that at high concentrations, lactate and H+ may potentially impair muscle function and energetics (Bangsbo et al. 1996; Costa Leite et al. 2007; Favero et al. 1997; Hollidge-Horvat et al. 1999; Jubrias et al. 2003; Nelson and Fitts 2014). Therefore, it may appear crucial for the exercising muscles to delay lactate accumulation and intracellular pH decline to preserve muscle function, although this point remains controversial (Allen and Westerblad 2004; Nielsen et al. 2001; Pedersen et al. 2004).
The possible deleterious effect of important lactate and proton accumulations has provided support for the idea that lactate and H+ extrusion from myocytes might be determinant in delaying muscle dysfunction. In accordance with this line of reasoning, previous studies have shown that the ability to exchange lactate from muscles to blood was related to performance during high-intensity exercise (Messonnier et al. 2002, 2007).
Lactate removal constitutes an alternate and complementary way to delay muscle, and more generally, body lactate accumulation. It is well-established that lactate can be used as fuel by skeletal muscles (Bergman et al. 1999; Brooks 1986, 2000, 2002, 2009; Donovan and Brooks 1983; Emhoff et al. 2013; Messonnier et al. 2013; Miller et al. 2002; Stanley et al. 1986). Interestingly, previous studies have suggested that performance during high-intensity exercise may be related to the subject’s overall ability to remove lactate (Messonnier et al. 1997, 2002; Thomas et al. 2005). In agreement with these studies and the lactate shuttle concept (Brooks 2002), Thomas et al. (2004) underlined that the ability to remove lactate was related to muscle oxidative capacity and fatigue index, suggesting that oxidation might play an important role in the lactate removal processes, even in the context of high-intensity exercise.
High-intensity exercises have been described to induce unsteady state conditions: muscle and blood lactate concentrations and pH are constantly changing (Marcinek et al. 2010; Osnes and Hermansen 1972). However, lactate transport and removal may be affected by these alterations of milieu intérieur. For instance, the changes in muscle and blood lactate concentrations (Juel 1997; Roth and Brooks 1990) as well as local blood flow (Pilegaard et al. 1995) may affect lactate transport. Besides, mitochondrial respiration, which is the ultimate step of lactate removal by oxidation, has been shown to be inhibited by acidosis (Jubrias et al. 2003). Taken together, these latter results suggest that the lactate exchange and removal abilities may decrease during short high-intensity exercise and/or during subsequent recovery. In accordance with this latter inference, Freund et al. (1989) observed lower lactate exchange and removal abilities after 6 min than after 3 min of high-intensity exercise. However, no study has investigated, to date and to our knowledge, the alterations of lactate kinetics parameters measured during recovery after high-intensity exercises of various distances. Furthermore, beside to the classic lactate exchange and removal abilities, the lactate release, removal, and whole body accumulation rates estimated at exercise completion by applications of the bi-compartmental model of the lactate distribution space have never been considered. In the present study, they will be reported and discussed for the first time.
In this context, the aim of the present study was to investigate lactate recovery kinetics in response to high-intensity 500-, 1000-, and 1500-m running trials. Concisely, we hypothesized significant lactate accumulation and consistent alterations of lactate exchange and removal abilities during recovery when running distance increased.
Methods
Subjects
Six male middle-distance runners volunteered to participate in this study. Their mean age, body mass, and height (±standard error) were 21 ± 1 years, 62.1 ± 3.7 kg, and 177 ± 3 cm, respectively. Their current best performance over 1500 m (measured during a competition during the last month before the experiments) was 246 s ± 7 s (4′06″ ± 7″, average speed of 22.0 ± 0.6 km h−1) that corresponded to 84 % of the world record over the distance 3′26″ (26.2 km h−1). The experiment was conducted in accordance with the Declaration of Helsinki regarding the use of human subjects and was approved by the Local Ethics Committee (no. 20146). Subjects were fully informed about the nature, the potential risks involved and the potential benefits of the study and gave their written consent to participate.
Study design
The experimental protocol consisted of three exercise sessions performed on a treadmill (Gymrol super 2500, HEF-Techmachine, Andrézieux-Bouthéon, France). Sessions 1 and 2 were separated by at least 1 week, whereas sessions 2 and 3 were separated by at least 2 days. Subjects were asked to refrain from any intense physical exercise 36 h prior to each exercise session but to continue their habitual daily living activities.
Session 1: incremental exercise up to exhaustion
At rest, 20 μL of blood was sampled from the fingertip for the determination of blood lactate concentration. Thereafter, the subjects performed an incremental exercise test until volitional exhaustion. This test consisted of 3-min exercise stages interspersed by 0.5-min rest periods. Initial speed was set at 10.5 km h−1. Running speed was subsequently increased by 1.5 km h−1 every stage. Expired gases were collected during the last 30 s of exercise at each stage and the rest periods allowed blood sampling. Heart rate was recorded continuously throughout exercise session. This session was used for individual determination of maximal oxygen uptake, maximal aerobic speed, maximal heart rate, and blood lactate concentration (vs. running velocity) curve.
Sessions 2 and 3: 500-, 1000-, and 1500-m running trials
At their arrival, a heart rate monitor was placed on subjects’ chest to record heart rate throughout the session. A resting blood lactate sample was then withdrawn from the earlobe. After a self-selected warm-up (~30 to 40 min) followed by a resting period (~10 min), another blood lactate sample was taken. Then, subjects performed 500 or 1000 m at the same speed corresponding to 90 % of their current best performance over 1500 m obtained in official competition. The 1500-m test was performed the same day 2 h after the 500 m. This procedure (the 500 m performed 2 h before the 1500 m) allowed the use of the 500-m test as a preparation for the subsequent intense exercise (1500 m). The same procedure of warm-up was used before 1500 m than before 500 and 1000 m. The 1000- and 500/1500-m tests were performed at least 2 days apart. The order of the exercise sessions between the 1000 m on the one hand, and the 500 and 1500 m on the other hand, was randomly determined. Exercises were followed by a seated passive recovery of 70, 80, or 90 min for the 500-, 1000-, and 1500-m events, respectively. During recovery, 20 µL blood samples were taken from the earlobe at the following times: 0 (exercise completion), 0.5, 1, 1.5, 2, 2.5, 3, 3.5, 4, 4.5, 5, 6, 8, 10, 12, 15, 20, 30, 40, 50, 60, and 70 min and also at 80 and 90 min when applicable. These sessions allowed to obtain individual blood lactate recovery curves.
Ventilatory and metabolic measurements
After passing through a two-way low resistance mouthpiece (model 2700, Hans Rudolph, Kansas City, MO), and a low dead-space mixing chamber, the expired gases were collected in Douglas bags (HP Production, Saint-Etienne, France). The O2 and CO2 fractions were measured in ambient air and in Douglas bags by a gas analyzer (Transrack type 2240) pre-calibrated on precision-analyzed gas mixtures. The expired air volumes, measured using a Tissot spirometer (Gymrol, Roche-la-Molière, France), and the O2 and CO2 fractions were recorded to calculate \(\dot{V}\)O2. Maximal oxygen uptake (\(\dot{V}\)O2max) was reached when \(\dot{V}\)O2 displayed a plateau as speed continued to increase. If the plateau in \(\dot{V}\)O2 was not observed, the highest \(\dot{V}\)O2 recorded was considered to be \(\dot{V}\)O2max if two of the three following criteria were fulfilled: a respiratory exchange ratio exceeding 1.1, a blood lactate concentration higher than 9 mmol L−1, and the achievement of a theoretical maximal heart rate (220 − age ± 10 beats min−1).
Maximal aerobic speed
Maximal aerobic speed corresponded to the speed at which \(\dot{V}\)O2max was reached. It was determined by linear interpolation from the \(\dot{V}\)O2 vs. running speed curve.
Heart rate
Heart rate was recorded using a three-lead electrocardiogram (session 1) and a heart rate monitor (Polar RS 400, Polar Electro, Kemple, Finland) (sessions 2 and 3).
Blood lactate concentration
Arterialized capillary blood (20 μL) sampled from fingertips (session 1) or earlobes (sessions 2 and 3) was immediately placed in tubes containing saponin (for hemolysis) and 180 µL of dilution liquid. Tubes were then stored at 4 °C until analysis. Lactate concentration was determined enzymatically in the whole blood using a YSI 2300 analyzer (YSI Inc., Yellow Springs, OH, USA).
Lactate kinetics after 500, 1000, and 1500 m
Tracer studies constitute the reference method to determine lactate kinetics parameters during exercise [e.g., (Brooks et al. 1991; Messonnier et al. 2013; Miller et al. 2002)]. However, to be fully applicable and reliable, tracer techniques require almost steady states in blood lactate and tracer concentrations, a near equilibrium in concentrations between the compartments of the body (e.g., between active muscles and blood) and several blood samples during exercise. These requirements are not fulfilled during high-intensity exercise. Therefore, for the present study, we have chosen to use the bi-compartmental model of lactate distribution space following high-intensity exercise (Freund and Zouloumian 1981a, b) which is appropriate for studying unsteady state conditions. In that model, lactate accumulated in the body at exercise completion is used as its own tracer for studying its kinetics during the subsequent recovery. Hence, individual blood lactate recovery curves were fitted to the bi-exponential time function:
where La(0) and La(t) (mmol L−1) are blood lactate concentrations measured at exercise completion and at a given time into recovery, respectively. Concentration parameters A 1 and A 2 (mmol L−1) are the amplitudes of the exponential functions. A 1 describes the amplitude of concentration variations due to the appearance of lactate in blood during recovery, so that the A 1 value depends directly on the muscle-to-blood lactate gradient. The velocity constants γ 1 (min−1) represent the ability to exchange lactate between the previously active muscles and the blood, and γ 2 (min−1) denotes the overall ability to remove lactate from the body (Freund et al. 1986; Freund and Zouloumian 1981a). The blood lactate recovery curves were fitted to Eq. (1) by iterative non-linear regression on the KaleidaGraph 4.0 software (Synergy Software, Reading, PA, USA) to determine the values of A 1, A 2, γ 1, and γ 2, La(0) being an experimental measurement. Although determined during recovery, it is important to note that the alterations in γ 1 and γ 2 reflect very closely the adjustments of the lactate kinetic parameters implemented during exercise. Interestingly, the values of γ 2 have been shown to be very close to and to follow the same pattern in function of work rate and in response to endurance training than those of the fractional lactate turnover rate measured during low- to moderate-intensity exercises using tracers (Freund et al. 1989; Messonnier et al. 2001). Moreover, the values of γ 1 are consistent with the values of rate constants of lactate efflux from sarcolemmal vesicles obtained from human skeletal muscles (Juel 1997).
Applications of the bi-compartmental model of lactate distribution space allow predictions of the net lactate release rate during recovery (NLRR, mmol min−1), the net amount of lactate released during recovery (NALR, mmol), the amount of lactate accumulated in the body at exercise completion (QLaA, mmol), the lactate removal rate at exercise completion [LRR(0), mmol min−1], and the lactate accumulation rate during each fraction of 500 m (LAR, mmol min−1). The metabolic clearance rate of lactate during recovery (MCRR, mL kg−1 min−1) and the lactate disappearance rate at exercise completion [Rd R(0), mg kg−1 min−1] were also estimated. Methods and equations are detailed in the Appendix (vide infra).
Statistics
Means (±standard errors) were calculated by standard methods. The Kolmogorov–Smirnov test was used to refute the assumption of normality. Significant differences between the three distances were identified using Friedman’s two-way ANOVA. The Wilcoxon rank test allowed us to determine the differences. Correlations between variables were studied by means of simple linear regression techniques. The statistical significance threshold was set at p < 0.05.
Results
Incremental exercise up to exhaustion
Maximal heart rate and oxygen uptake measured at exhaustion were 191 ± 3 beats min−1 and 4.13 ± 0.19 L min−1 (65.7 ± 2.3 mL min−1 kg−1), respectively. Maximal aerobic speed was 21.8 ± 0.4 km h−1. At exhaustion, blood lactate concentrations reached 12.1 ± 1.1 mmol L−1.
Lactate recovery kinetics after 500, 1000, and 1500 m
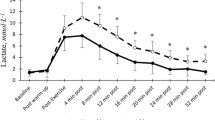
The different distances were run at 93 ± 1 % of maximal aerobic speed. The 500-, 1000-, and 1500-m trials lasted 91 ± 2 s (1′31″ ± 2″), 182 ± 5 s (3′02″ ± 5″), and 273 ± 7 s (4′33″ ± 7″), respectively. Blood lactate concentrations at rest before each trial were not significantly different (1.53 ± 0.14, 1.43 ± 0.13, and 1.37 ± 0.09 mmol L−1 for 500, 1000, and 1500 m, respectively). Blood lactate concentrations at the end of the warm-up were not significantly different (1.68 ± 0.09, 1.54 ± 0.26, and 1.65 ± 0.18 mmol L−1 for 500, 1000, and 1500 m, respectively). Figure 1 shows the time courses of blood lactate concentrations during recovery following 500, 1000, and 1500 m at 90 % of the current personal record. After all trials, blood lactate concentrations displayed the classic biphasic pattern (Fig. 1) and were very well fitted by the bi-exponential model (r 2 > 0.99). Blood lactate levels were significantly different between the three distances from the end of exercise until the 12th minute of recovery (Fig. 1).
Time courses of blood lactate concentrations during recovery after 500 m (circle), 1000 m (square), and 1500 m (cross). *Significantly different between 500 and 1000 m (p < 0.05), +significantly different between 500 and 1500 m (p < 0.05), and #significantly different between 1000 and 1500 m (p < 0.05)
La(0) raised as distance increased. From 3.08 ± 0.19 mmol L−1 at the end of the 500-m trial, La(0) increased to 4.90 ± 0.36 mmol L−1 after 1000 m (p < 0.05) and further to 7.05 ± 0.54 mmol L−1 (p < 0.05) after 1500 m (Fig. 2a). Lapeak followed the same pattern as La(0): it increased significantly with distance (4.12 ± 0.34, 6.43 ± 0.52, and 8.51 ± 0.86 mmol L−1 for 500, 1000, and 1500 m, respectively; p < 0.05). Moreover, the time at which Lapeak was reached (tLapeak) increased with distance (1.3 ± 0.1, 2.3 ± 0.2, and 3.3 ± 0.4 min for 500, 1000, and 1500 m, respectively; p < 0.05).
a La(0): the blood lactate concentration at the end of exercise, b A 1: the concentration parameter representing the amplitude of the first exponential term, c γ 1: the velocity constant representing the ability to exchange lactate from the previously active muscle to the blood, and d γ 2: the velocity constant representing the ability to remove lactate from the body after 500-, 1000-, and 1500-m running, *significantly different from 500 m (p < 0.05). +Significantly different from 1000 m (p < 0.05)
A 1 increased with running distance. From 1.7 ± 0.3 mmol L−1 after 500 m, it increased to 2.8 ± 0.4 mmol L−1 after 1000 m (p < 0.05) and further (although not significantly) to 3.3 ± 0.4 mmol L−1 after 1500 m (Fig. 2b). A 2 decreased as distance increased. From −3.3 ± 0.4 mmol L−1 at the end of the 500-m trial, A 2 decreased to −6.4 ± 0.7 mmol L−1 after 1000 m (p < 0.05) and further to −9.3 ± 0.9 mmol L−1 after 1500 m (p < 0.05).
γ 1 decreased non-linearly from 1.376 ± 0.199 min−1 after 500 m to 0.712 ± 0.033 min−1 after 1000 m (p < 0.05) and further to 0.559 ± 0.074 min−1 after 1500 m (p < 0.05; Fig. 2c). γ 2 significantly decreased (p < 0.05) between 500 m (0.0824 ± 0.0046 min−1) and 1000 m (0.0604 ± 0.0089 min−1). However, the 8 % decrease of γ 2 between the 1000- and 1500-m trials (0.0556 ± 0.0112 min−1) was not statistically significant (Fig. 2d).
NLRR(0) was not different after 500 m (30.5 ± 5.5 mmol min−1), 1000 m (28.0 ± 4.3 mmol min−1), or 1500 m (24.8 ± 5.3 mmol min−1; NS; Fig. 3a). NALRmax was not different among trials (31.9 ± 3.7, 38.8 ± 4.9, and 42.8 ± 4.7 mmol after 500, 1000, and 1500 m, respectively; Fig. 3b). The amount of lactate accumulated (QLaA) calculated at the end of exercise increased almost linearly with distance (137 ± 6, 219 ± 8, and 300 ± 25 mmol for 500, 1000, and 1500 m, respectively; Fig. 3c).
a NLRR(0): the net lactate release rate at the end of exercise, b NALRmax: the maximal net amount of lactate released, c QLaA: the amount of lactate accumulated at the end of exercise, and d LRR(0): the lactate removal rate at the end of exercise after 500-, 1000-, and 1500-m running. *Significantly different from 500 m (p < 0.05). +Significantly different from 1000 m (p < 0.05)
LRR(0) (11.2 ± 0.6, 13.1 ± 1.7, and 15.9 ± 2.9 mmol min−1 after 500, 1000, and 1500 m, respectively; Fig. 3d) and LAR (56 ± 6, 55 ± 6, and 55 ± 13 mmol min−1 after 500, 1000, and 1500 m, respectively) were not significantly different among trials.
MCRR was significantly higher after 500 m (41 ± 2 mL kg−1 min−1) than after 1000 m (p < 0.05). No differences were observed in MCRR after 1000 and 1500 m (30 ± 4 and 28 ± 6 mL kg−1 min−1, respectively, NS). R d R at the end of exercise [Rd R(0)] was not different among trials (16.3 ± 0.9, 18.7 ± 2.4, and 22.3 ± 2.9 mg kg−1 min−1 after 500, 1000, and 1500 m, respectively).
Discussion
The purpose of this study was to investigate alterations in parameters of lactate recovery kinetics in response to 500-, 1000-, and 1500-m high-intensity running trials. The main findings of the present study were that the increase of running distance induced (1) decrease of the lactate exchange ability, (2) decrease and then relative stabilization of the lactate removal ability during recovery, and (3) regular increases in the amount of lactate accumulated in the body and blood lactate concentrations at exercise completion. Estimated lactate release, removal and accumulation rates at exercise completion, and the net amount of lactate released during recovery were not significantly altered by running distance.
Lactate exchange ability
The decrease of γ 1 observed in the present study (Fig. 2c) testified to an important decline in the lactate exchange ability with running distance, particularly in response to the second 500 m. This decrease in γ 1 means that a smaller fraction of muscular lactate is released into the bloodstream per unit of time after 1000 m than after 500 m, and after 1500 m than after 1000 m. In other words, the efficiency with which lactate is released from the previously active muscles into the blood diminished with the increase of running distance. The lactate exchange ability depends (1) on muscle perfusion which itself depends on capillary density (Messonnier et al. 2002) and recruitment (Coggins et al. 2001) and on local blood flow (Pilegaard et al. 1995) and (2) on lactate transport capacity (Dubouchaud et al. 1999; Eydoux et al. 2000; Juel 1997; Roth and Brooks 1990) which itself depends on sarcolemmal MCT1 and MCT4 (lactate/H+ co-transporters) content (Dubouchaud et al. 2000) as well as on the functionality of the carriers (Juel 1997). During high-intensity submaximal exercises, reduction in muscle perfusion (i.e., alterations in muscle blood flow and capillary recruitment) is unexpected (Mortensen et al. 2008). On the other hand, the drop of lactate exchange ability can be more likely attributed either to (1) a decrease in the carriers content, (2) alterations in the functionality of transporters, and (3) a combination of these possibilities. Consistent with a possible decrease in the sarcolemmal lactate carriers content, Bishop et al. (2007) have reported a decrease in lactate/H+ co-transporters MCT1 and MCT4 during short high-intensity exercise that could induce impairment in the lactate exchange ability. Consistent with the possible alteration in the functionality of the transporters, Juel (1997) reported that for a same muscle-to-blood lactate gradient, the net lactate efflux from giant sarcolemmal vesicles decreased when lactate concentrations increased, suggesting a deleterious role of the concentrations per se on the efficiency with which the transporters work. These data led him to the conclusion that lactate accumulated externally may affect lactate extrusion, probably due to a higher lactate/lactate exchange, resulting in fewer carriers transporting lactate out (Juel 1997). In accordance with the results of Juel and his interpretation, we observed in the present study an inverse relationship between La(0) and γ 1, suggesting that blood lactate concentrations may contribute to dampen the lactate exchange ability (Fig. 4).
Net lactate release rate
A decrease of lactate exchange ability is expected to create a hindrance in lactate efflux during exercise. However, the estimated net lactate release rate at the end of exercise [i.e., NLRR(0)] was not significantly different after 500, 1000, or 1500 m (Fig. 3a). This lack of significant alteration in NLRR(0) might be explained by an increase during exercise of the muscle-to-blood lactate gradient which determines, in addition to the exchange ability, lactate flux between muscle and blood. In other words, the decrease in lactate exchange ability was compensated by an increase in muscle-to-blood lactate gradient, so that the estimated net lactate release rate remained almost constant after 500, 1000, and 1500 m. In accordance with this inference, A 1 values, which depend on the muscle-to-blood lactate gradient, tended to increase with distance (Fig. 2b). The present results and the above line of reasoning are in accordance with the previous works showing that the net lactate release rate increased and then plateaued during high-intensity exercise, while the muscle-to-blood lactate gradient obviously increased, indicating disturbances in the lactate exchange ability (Juel et al. 1990).
Net amount of lactate released during recovery
NALRmax did not increase significantly with running distance. This means that despite an obvious increase in muscle lactate concentrations with distance, the quantity of lactate that left the previously active muscles during recovery did not change drastically. This suggests that a higher proportion of accumulated lactate remained inside the previously active muscles during the subsequent recovery.
Lactate removal
The results of the present study demonstrated that after an initial period of decrease, there is a relative maintenance of the removal ability. Specifically, γ 2 was lower after 1000 m than after 500 m, but was not further altered after 1500 m. A possible hypothesis to explain the decrease in the lactate removal ability would be that acidosis associated with lactate accumulation (Marcinek et al. 2010) had altered cellular respiration (Jubrias et al. 2003) and consequently lactate removal (Thomas et al. 2004). However, this hypothesis is not consistent with the fact that γ 2 decreased only between the 500- and 1000-m trials when lactate levels were relatively modest. Furthermore, the lack of further decrease in γ 2 after 1500 m might indicate that lactate accumulation was not sufficient in the present study to alter substantially acid/base balance and, therefore, mitochondrial respiration. Thus, it appears clear that the underlying mechanisms that explain the observed changes in the lactate removal ability remain unknown and deserve to be specifically investigated in future studies.
We also estimated lactate removal rate at exercise completion. LRR(0) was not significantly different after 500, 1000, or 1500 m. The maintenance of LRR(0) with exercise duration, while γ 2 decreased, can be attributed to the concomitant elevation of blood lactate concentrations [(i.e., La(0)] when running distance increased. Indeed, the effect of lactate concentrations per se on lactate disappearance rate had been clearly shown by experiments using a lactate clamp procedure which consistently demonstrated that the lactate disappearance rate increased when blood lactate concentrations were increased by the lactate clamp (Messonnier et al. 2013; Miller et al. 2002). In other words, the decrease of γ 2 observed in the present study has been compensated by an increase in blood lactate concentrations, thus maintaining LRR(0).
The lactate removal ability expresses the efficiency with which lactate is removed from the body. Therefore, the physiological meaning of γ 2 is very close to the one of the lactate metabolic clearance rate (MCR) obtained via tracer methods. Interestingly, an MCR during recovery (MCRR, mL−1 kg−1 min−1) can be derived from γ 2 (see Eq. 6). Remarkably, the MCRR values obtained in the present study are in line with those of the literature (Freund et al. 1989; Messonnier et al. 2001) and with those of MCR when the relative exercise intensity is taken into account (Fig. 5). We can also estimate the lactate disappearance rate at exercise completion [Rd R(0), mg kg−1 min−1]. Interestingly, Rd R(0) values are very close to those obtained in the literature (Messonnier et al. 2013; Miller et al. 2002).

Redrawn from Messonnier et al. (2013)
Metabolic clearance rate of lactate as a function of relative rates (\(\dot{V}\)O2max) elicited in the present and previous studies.
Blood and whole body lactate accumulation
Because at exercise completion, the net lactate release rate [NLRR(0)] was similar among trials and the lactate removal rate [LRR(0)] was (1) lower than NLRR(0) and (2) also not different among trials, it is, therefore, not surprising that La(0) increased almost linearly with running distance. Moreover, the fact that LAR was similar during each fraction of 500 m, while LRR(0) remained unchanged among trials, argue in favor of an almost constant lactate production rate. At first sight, it would seem that the levels of lactate and well-known concomitant decrease in pH were not strong enough in the present study to dampen significantly muscle energetics and especially lactate production during exercise, as it has been experimentally demonstrated and modeled (Hollidge-Horvat et al. 1999; Korzeniewski and Zoladz 2015).
Limitations
We are aware that the present study involved a relatively low number of subjects. However, we do not believe that this constitutes a major limitation in the present case. Our position lies on the fact that (1) the trials were very different, so that the alterations in lactate concentrations and kinetics parameters among trials were important and much higher than the intra-individual variability and (2) each subject was compared to himself that also contributes to reduce a possible bias.
Besides, we would like to underline that in the present study, we used the 500-, 1000-, and 1500-m trials at 90 % of maximal competition speed as a model to investigate the changes in lactate kinetics after high-intensity exercises and not in the purpose to understand what may happen during a 1500-m race. In other words, the present results cannot be extended to what happens during a 1500-m race, where speed is not constant and exercise is exhaustive.
Perspectives
Because the lactate exchange and removal abilities are related to performance (Bret et al. 2003; Messonnier et al. 1997, 2002) and since both γ 1 and γ 2 decline with running distance, this raises the question of knowing whether an optimal running strategy exists to delay or reduce these decreases, maintain work capacity and ultimately improve performance. Thus, in a near future, lactate recovery kinetics in response to high-intensity exercises using different running strategy would be warranted.
Conclusions
In summary, alterations in lactate kinetics occurred in response to high-intensity 500-, 1000-, and 1500-m running trials. These changes included: (1) a continued decrease in lactate exchange ability (γ 1) and (2) an initial decrease and then a stabilization of the lactate removal ability (γ 2). Despite these alterations, the net lactate release [NLRR(0)], removal [LRR(0)], and whole body accumulation (LAR) rates were not significantly modified by running distance, so that lactate concentration [La(0)] and whole body accumulation (QLaA) increased almost linearly with running distance. It was also deduced from these results that the lactate production rate remained approximately constant. Finally, the fact that the net amount of lactate released during subsequent recovery was unaltered by running distance, while an increase in muscle lactate concentrations was expected, suggested that a higher proportion of muscle lactate remained inside the previously active muscles during the subsequent recovery with the increase in running distance.
Abbreviations
- A 1 and A 2 :
-
Concentration parameters (amplitudes of the exponential functions)
- BLRC:
-
Blood lactate recovery curves
- La(0):
-
Blood lactate concentration at exercise completion
- La(t):
-
Blood lactate concentration at time t
- Lapeak :
-
Maximal blood lactate concentration during recovery
- Lawarm-up :
-
Blood lactate concentration at the end of the warm-up
- LAR:
-
Lactate accumulation rate
- LRR:
-
Lactate removal rate
- MCR:
-
Metabolic clearance rate of lactate
- MCRR :
-
Metabolic clearance rate of lactate during recovery
- NALR:
-
Net amount of lactate released
- NLRR:
-
Net lactate release rate
- QLaA:
-
Amount of lactate accumulated in the body at exercise completion
- QLaR:
-
Amount of lactate removed from the end of exercise to Lapeak
- Rd R :
-
Lactate disappearance rate
- tLapeak :
-
Time to reach the maximal lactate concentration during recovery
- V M :
-
Lactate distribution volume of muscles previously involved in exercise
- \(\dot{V}\)O2max :
-
Maximal oxygen uptake
- V S :
-
Volume of compartment S (V TLS − V M)
- V TLS :
-
Volume of the total lactate distribution space
- γ 1 :
-
Velocity constant enlighten lactate exchange ability
- γ 2 :
-
Velocity constant enlighten lactate removal ability
References
Allen D, Westerblad H (2004) Physiology. Lactic acid—the latest performance-enhancing drug. Science 305:1112–1113
Bangsbo J, Madsen K, Kiens B, Richter EA (1996) Effect of muscle acidity on muscle metabolism and fatigue during intense exercise in man. J Physiol 495(Pt 2):587–596
Bergman BC, Wolfel EE, Butterfield GE, Lopaschuk GD, Casazza GA, Horning MA, Brooks GA (1999) Active muscle and whole body lactate kinetics after endurance training in men. J Appl Physiol 87:1684–1696
Bishop D, Edge J, Thomas C, Mercier J (2007) High-intensity exercise acutely decreases the membrane content of MCT1 and MCT4 and buffer capacity in human skeletal muscle. J Appl Physiol 102:616–621
Bret C, Messonnier L, Nouck Nouck JM, Freund H, Dufour AB, Lacour JR (2003) Differences in lactate exchange and removal abilities in athletes specialised in different track running events (100 to 1500 m). Int J Sports Med 24:108–113
Brooks GA (1986) The lactate shuttle during exercise and recovery. Med Sci Sports Exerc 18:360–368
Brooks GA (2000) Intra- and extra-cellular lactate shuttles. Med Sci Sports Exerc 32:790–799
Brooks GA (2002) Lactate shuttle—between but not within cells? J Physiol 541:333–334
Brooks GA (2009) Cell–cell and intracellular lactate shuttles. J Physiol 587:5591–5600
Brooks GA, Butterfield GE, Wolfe RR, Groves BM, Mazzeo RS, Sutton JR, Wolfel EE, Reeves JT (1991) Decreased reliance on lactate during exercise after acclimatization to 4300 m. J Appl Physiol 71:333–341
Coggins M, Lindner J, Rattigan S, Jahn L, Fasy E, Kaul S, Barrett E (2001) Physiologic hyperinsulinemia enhances human skeletal muscle perfusion by capillary recruitment. Diabetes 50:2682–2690
Costa Leite T, Da Silva D, Guimaraes Coelho R, Zancan P, Sola-Penna M (2007) Lactate favours the dissociation of skeletal muscle 6-phosphofructo-1-kinase tetramers down-regulating the enzyme and muscle glycolysis. Biochem J 408:123–130
Donovan CM, Brooks GA (1983) Endurance training affects lactate clearance, not lactate production. Am J Physiol 244:E83–E92
Dubouchaud H, Eydoux N, Granier P, Prefaut C, Mercier J (1999) Lactate transport activity in rat skeletal muscle sarcolemmal vesicles after acute exhaustive exercise. J Appl Physiol 87:955–961
Dubouchaud H, Butterfield GE, Wolfel EE, Bergman BC, Brooks GA (2000) Endurance training, expression, and physiology of LDH, MCT1, and MCT4 in human skeletal muscle. Am J Physiol Endocrinol Metab 278:E571–E579
Emhoff CA, Messonnier LA, Horning MA, Fattor JA, Carlson TJ, Brooks GA (2013) Direct and indirect lactate oxidation in trained and untrained men. J Appl Physiol 1985 115:829–838
Eydoux N, Py G, Lambert K, Dubouchaud H, Prefaut C, Mercier J (2000) Training does not protect against exhaustive exercise-induced lactate transport capacity alterations. Am J Physiol Endocrinol Metab 278:E1045–E1052
Favero TG, Zable AC, Colter D, Abramson JJ (1997) Lactate inhibits Ca(2+)-activated Ca(2+)-channel activity from skeletal muscle sarcoplasmic reticulum. J Appl Physiol 82:447–452
Freund H, Zouloumian P (1981a) Lactate after exercise in man: I. Evolution kinetics in arterial blood. Eur J Appl Physiol Occup Physiol 46:121–133
Freund H, Zouloumian P (1981b) Lactate after exercise in man: IV. Physiological observations and model predictions. Eur J Appl Physiol Occup Physiol 46:161–176
Freund H, Oyono-Enguéllé S, Heitz A, Marbach J, Ott C, Zouloumian P, Lampert E (1986) Work rate-dependent lactate kinetics after exercise in humans. J Appl Physiol 61:932–939
Freund H, Oyono-Enguéllé S, Heitz A, Marbach J, Ott C, Gartner M (1989) Effect of exercise duration on lactate kinetics after short muscular exercise. Eur J Appl Physiol Occup Physiol 58:534–542
Hollidge-Horvat MG, Parolin ML, Wong D, Jones NL, Heigenhauser GJ (1999) Effect of induced metabolic acidosis on human skeletal muscle metabolism during exercise. Am J Physiol 277:E647–E658
Jubrias SA, Crowther GJ, Shankland EG, Gronka RK, Conley KE (2003) Acidosis inhibits oxidative phosphorylation in contracting human skeletal muscle in vivo. J Physiol 553:589–599
Juel C (1997) Lactate-proton cotransport in skeletal muscle. Physiol Rev 77:321–358
Juel C, Bangsbo J, Graham T, Saltin B (1990) Lactate and potassium fluxes from human skeletal muscle during and after intense, dynamic, knee extensor exercise. Acta Physiol Scand 140:147–159
Korzeniewski B, Zoladz JA (2015) Possible mechanisms underlying slow component of VO2 on-kinetics in skeletal muscle. J Appl Physiol 1985 118:1240–1249
Maciejewski H, Bourdin M, Lacour JR, Denis C, Moyen B, Messonnier L (2013) Lactate accumulation in response to supramaximal exercise in rowers. Scand J Med Sci Sports 23:585–592
Marcinek DJ, Kushmerick MJ, Conley KE (2010) Lactic acidosis in vivo: testing the link between lactate generation and H+ accumulation in ischemic mouse muscle. J Appl Physiol 108:1479–1486
Messonnier L, Freund H, Bourdin M, Belli A, Lacour JR (1997) Lactate exchange and removal abilities in rowing performance. Med Sci Sports Exerc 29:396–401
Messonnier L, Freund H, Feasson L, Prieur F, Castells J, Denis C, Linossier MT, Geyssant A, Lacour JR (2001) Blood lactate exchange and removal abilities after relative high-intensity exercise: effects of training in normoxia and hypoxia. Eur J Appl Physiol 84:403–412
Messonnier L, Freund H, Denis C, Dormois D, Dufour AB, Lacour JR (2002) Time to exhaustion at VO(2)max is related to the lactate exchange and removal abilities. Int J Sports Med 23:433–438
Messonnier L, Kristensen M, Juel C, Denis C (2007) Importance of pH regulation and lactate/H+ transport capacity for work production during supramaximal exercise in humans. J Appl Physiol 102:1936–1944
Messonnier LA, Emhoff CA, Fattor JA, Horning MA, Carlson TJ, Brooks GA (2013) Lactate kinetics at the lactate threshold in trained and untrained men. J Appl Physiol 114:1593–1602
Miller BF, Fattor JA, Jacobs KA, Horning MA, Navazio F, Lindinger MI, Brooks GA (2002) Lactate and glucose interactions during rest and exercise in men: effect of exogenous lactate infusion. J Physiol 544:963–975
Mortensen SP, Damsgaard R, Dawson EA, Secher NH, Gonzalez-Alonso J (2008) Restrictions in systemic and locomotor skeletal muscle perfusion, oxygen supply and VO2 during high-intensity whole-body exercise in humans. J Physiol 586:2621–2635
Nelson CR, Fitts RH (2014) Effects of low cell pH and elevated inorganic phosphate on the pCa-force relationship in single muscle fibers at near-physiological temperatures. Am J Physiol Cell Physiol 306:C670–C678
Nielsen OB, de Paoli F, Overgaard K (2001) Protective effects of lactic acid on force production in rat skeletal muscle. J Physiol 536:161–166
Osnes JB, Hermansen L (1972) Acid–base balance after maximal exercise of short duration. J Appl Physiol 32:59–63
Pedersen TH, Nielsen OB, Lamb GD, Stephenson DG (2004) Intracellular acidosis enhances the excitability of working muscle. Science 305:1144–1147
Pilegaard H, Bangsbo J, Henningsen P, Juel C, Richter EA (1995) Effect of blood flow on muscle lactate release studied in perfused rat hindlimb. Am J Physiol 269:E1044–E1051
Roth DA, Brooks GA (1990) Lactate transport is mediated by a membrane-bound carrier in rat skeletal muscle sarcolemmal vesicles. Arch Biochem Biophys 279:377–385
Stanley WC, Gertz EW, Wisneski JA, Neese RA, Morris DL, Brooks GA (1986) Lactate extraction during net lactate release in legs of humans during exercise. J Appl Physiol 1985 60:1116–1120
Thomas C, Sirvent P, Perrey S, Raynaud E, Mercier J (2004) Relationships between maximal muscle oxidative capacity and blood lactate removal after supramaximal exercise and fatigue indexes in humans. J Appl Physiol 97:2132–2138
Thomas C, Perrey S, Lambert K, Hugon G, Mornet D, Mercier J (2005) Monocarboxylate transporters, blood lactate removal after supramaximal exercise, and fatigue indexes in humans. J Appl Physiol 98:804–809
Acknowledgments
The authors would like to thank Josiane Castells for her technical assistance, all the athletes for their voluntary participation in this study, and the coaches, the clubs, and Pierre Bavazzano for their support. This project was supported by the Université Savoie Mont Blanc.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
No conflicts of interest, financial or otherwise, are declared by the authors.
Additional information
Communicated by Jean-René Lacour.
Appendix
Appendix
An application of the bi-compartmental model of lactate distribution space allows the prediction of the net lactate release rate (NLRR, mmol min−1) during recovery using the following equation:
where V S is the volume of compartment S [i.e., 250 mL kg−1 body mass; V S = V TLS (volume of the total lactate distribution space) − V M (lactate distribution volume of muscles previously involved in exercise)], µ is the net muscular release rate of lactate at t → ∞ and was set at 0.12 mmol min−1 (Bret et al. 2003; Freund and Zouloumian 1981b; Maciejewski et al. 2013), and d 2 is the efficiency of lactate utilization in compartment S. The application of the model gives realistic prediction when d 2 is close to γ 2. Therefore, to approximate NLRR, we set, as previously (Bret et al. 2003; Freund and Zouloumian 1981b; Maciejewski et al. 2013), d 2 = γ 2 − 0.005. The integral of Eq. (2) gives an estimation of the net amount of lactate released during recovery (NALR, mmol) from the previously active muscles to the blood (Bret et al. 2003). The maximal value (NALRmax) will be considered (Maciejewski et al. 2013).
Recently, Maciejewski et al. (2013) have developed a method to estimate the amount of lactate accumulated in the body at exercise completion (QLaA, mmol):
where QLaA at Lapeak is the quantity of lactate accumulated at Lapeak which is calculated as the following:
where Lapeak represents the maximal blood lactate concentration during recovery, and V TLS represents the volume of the total lactate distribution space (i.e., 500 mL kg−1).
QLaR represents the amount of lactate removed from the end of exercise to Lapeak, which is calculated as
where tLapeak is the time to reach the maximal lactate concentration during recovery. For more information about this method, we refer the reader to Maciejewski et al. (2013).
Lactate removal rate at the end of each exercise [LRR(0), mmol min−1] was estimated from the following equation:
A calculation of the lactate accumulation rate (LAR, mmol min−1) during each fraction of 500 m was derived from QLaA values according to the following equation:
where ΔQLaA is the difference in QLaA between two distances and t is the time to complete 500 m. For the first 500 m, ΔQLaA = QLaA − (Lawarm-up × V TLS), where Lawarm-up is the blood lactate concentration at the end of the warm-up.
As done in the past (Freund et al. 1986; Messonnier et al. 2001), a metabolic clearance rate of lactate during recovery (MCRR, mL kg−1 min−1) can be derived from γ 2 values according to the following equation:
A calculation of the lactate disappearance rate at exercise completion (Rd R(0), mg kg−1 min−1) can also be derived from Eq. (8) according to the following equation:
Rights and permissions
About this article
Cite this article
Chatel, B., Bret, C., Edouard, P. et al. Lactate recovery kinetics in response to high-intensity exercises. Eur J Appl Physiol 116, 1455–1465 (2016). https://doi.org/10.1007/s00421-016-3420-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00421-016-3420-0