Abstract
Background
Proximal early gastric cancer is a good indication for totally laparoscopic proximal gastrectomy (TLPG) with double-tract reconstruction (DTR). However, when most of the dietary intake passes through the escape route of the jejunum, the functional benefits of proximal gastrectomy might be similar to those after total gastrectomy. Our DTR procedure was improved for easy passage through the remnant stomach. The purposes of this study were to present a novel technique for intracorporeal DTR using linear staplers after TLPG and to investigate surgical outcomes.
Methods
DTR was performed using linear staplers only. A side-to-side jejunogastrostomy with twisting of both the remnant stomach and the anal jejunum was performed for the purpose of passing meals through the remnant stomach (an oblique jejunogastrostomy technique). The ten patients who underwent TLPG with DTR from January 2011 to August 2016 in Hokkaido University Hospital were retrospectively reviewed. Their clinicopathological characteristics and surgical and postoperative outcomes were collected and analyzed.
Results
The median duration of operation was 285 (range 146–440) min. No patients required blood transfusions. The number of dissected lymph nodes was 32 (range 22–56). There were no intraoperative complications, and no cases were converted to open surgery. All the patients were pT1N0M0 stage IA. No anastomotic leakage or complications were detected. Postoperative gastrography after reconstruction showed that contrast medium flowed mainly to the remnant stomach. The average percentage body weight loss was 14.0 ± 7.1% at 10 months. The average percentage decrease in serum hemoglobin was 5.4 ± 10.4% at 12 months.
Conclusions
This novel technique for intracorporeal DTR provided a considerable advantage by the passage of dietary intake to the remnant stomach after LPG.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Gastric cancer is the most common form of cancer in East Asian countries [1]. The reported incidence of early gastric cancer (EGC) has increased as a result of improved surveillance by the national cancer screening program in Japan [2]. It has also been found that Helicobacter pylori infection is associated with gastric cancer. H. pylori eradication therapy by antibiotic treatment decreases the incidence of distal gastric cancer [3]. On the other hand, the incidence of proximal gastric cancer in Korea has gradually increased from 5.3 to 14.0% [4]. In recent decades, the oncological safety of minimally invasive surgery for the treatment of EGC has been established [5]. Laparoscopic gastrectomy with regional lymph node dissection has been used in the treatment of EGC with low mortality and morbidity and improved patient quality of life [6]. We have performed totally laparoscopic gastrectomy for EGC, including totally laparoscopic distal gastrectomy (TLDG), totally laparoscopic proximal gastrectomy (TLPG), and totally laparoscopic total gastrectomy (TLTG) with intracorporeal anastomosis, using a laparoscopic linear stapler [7].
Proximal gastrectomy for EGC was a significant improvement over total gastrectomy in terms of maintaining physiological function and quality of life, such as weight loss, the necessity for additional meals, dumping [8], and maintaining high hemoglobin levels [9, 10]. Proximal EGC is a good indication for TLPG when functional preservation or minimal invasiveness is taken into consideration. However, total gastrectomy has still been widely performed as a standard treatment for early upper-third gastric cancer to achieve a tumor-free resection margin and extended lymph node dissection [11]. The oncological safety and functional benefits of proximal gastrectomy have been reported in several studies. They concluded that the long-term overall survival of patients who underwent proximal gastrectomy for proximal EGC was similar to that of those who underwent total gastrectomy [12,13,14]. Among the various reconstructions of laparoscopic proximal gastrectomy, such as esophagogastrostomy [15], jejunal interposition [16], and double-tract reconstruction (DTR) [17], we have performed TLPG with DTR for EGC. Aikou et al. [18] first reported DTR after proximal gastrectomy and the original gastrojejunostomy method called the N-shaped double-tract method. One of the reasons why we chose DTR was its technical similarity to the DTR with Roux-en-Y reconstruction for laparoscopic total gastrectomy, which has been established as a standard laparoscopic reconstruction procedure. However, there is some concern that, with DTR, most of the dietary intake might escape into the jejunum. To prevent this disadvantageous phenomenon, we have developed a newly devised DTR that allows dietary intake to pass easily through the remnant stomach.
The purposes of this study were to present a novel technique for intracorporeal DTR using linear staplers after proximal gastrectomy and to investigate the short- and mid-term outcomes of LPG-DTR.
Patients and methods
Patient selection
Between January 2011 and August 2016, ten patients diagnosed with EGC preoperatively underwent LPG with DTR at Hokkaido University Hospital, Sapporo, Japan. Preoperative assessments were carried out by endoscopy, computed tomography (CT), and endoscopic ultrasound. The eligibility criteria of the patients were (1) gastric cancer invaded within the submucosal layer (cT1), (2) suspected to have no lymph node metastases (cN0), and (3) tumor located more than a 5-cm distance from the angular region and the remnant stomach would be more than half the size of the preoperative stomach. Patients who had a previous history of upper gastrointestinal surgery were excluded from DTR. Informed consent was obtained from all patients. Patients’ characteristics including age, sex, body mass index (BMI), ASA physical status classification system (American Society of Anesthesiologists (ASA)), history of abdominal surgery and endoscopic submucosal dissection (ESD), and chronic disease status were recorded. Operation time, blood loss, time to resume a soft diet, postoperative hospital stay, postoperative complications, and pathological findings were also analyzed. Postoperative morbidity was evaluated using the Clavien-Dindo classification [19]. Among early complications, the main complications were investigated, including intraperitoneal or digestive tract hemorrhage, anastomotic leakage, bowel or anastomosis obstruction, and abdominal infection. Specimens were evaluated according to the Japanese classification of gastric carcinoma established by the Japanese Research Society for Gastric Cancer [20]. Follow-up was conducted to evaluate late complications, including reflux esophagitis and anastomotic stenosis, the changes of body weight and hemoglobin concentration, and the rates of recurrence and survival.
Procedures for gastrectomy
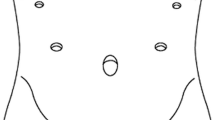
The patients are placed in the supine reverse Trendelenburg position with the legs apart under general anesthesia. The operator is positioned between the legs of the patient, the assistant is on the left side, and the scopist is on the opposite side. Five trocars (Xcel® Ethicon Endo-Surgery, Cincinnati, OH, USA) are used, and a 12-mm paraumbilical port is subsequently extended to 3.0 cm when extracting specimens. After carbon dioxide pneumoperitoneum is established at a pressure of 10 mmHg, a laparoscope (3CCD Video System SX-2, Olympus, Tokyo, Japan) is introduced through this port, and four other trocars (three 12-mm trocars and one 5-mm trocar) are placed (Fig. 1). We dissect lymph nodes and coagulate vessels using laparoscopic coagulation shears (SONOSURG-X®, Olympus Medical Systems or Harmonic Ace®, Ethicon Endo-Surgery) and a vessel sealing system (Ligasure Maryland Jaw™, Medtronic, Mansfield, MA, USA). The basic extent of lymph node dissection in the present series was D1+ dissection of lymph node nos. 1, 2, 3a, 4sa, 4sb, 7, 8a, 9, and 11p; lymph node regions and dissection were decided according to the Japanese classification of gastric carcinoma published by the Japanese Gastric Cancer Association [21]. The lymph nodes of nos. 4d, 5, and 6 with the right gastroepiploic vessels and right gastric vessels are preserved. The bottom of the esophagus and the distal 1/3 to 1/2 stomach are transected by 60-mm endoscopic linear staplers (Powered Echelon 60®, Ethicon Endo-Surgery). The proximal and distal free margins are more than 2 cm.
Procedures for double-tract reconstruction
DTR after TLPG is performed using only linear staplers. The jejunum is transected by a 60-mm endoscopic linear stapler at a point 20 cm distant from the ligament of Treitz. For esophagojejunostomy, we use functional end-to-end technique [7, 22]. Thereafter, 10-mm transverse incisions are created at the antimesenteric wall of the jejunum 20 cm below the esophagojejunostomy (Fig. 2a), and 10-mm incisions are also created at the greater curvature side of the remnant stomach (Fig. 2b). Jaws of a 60-mm linear stapler (Powered Echelon 60®, Ethicon Endo-Surgery) are inserted into the holes. Then, both the stomach and jejunum are twisted posteriorly, and the posterior wall of the remnant stomach and the posterior wall of the jejunum are put together. An oblique side-to-side jejunogastrostomy from the antimesenteric wall to the posterior wall is performed (Fig. 2 c, d). The entry hole for this stapler is closed with a running suture (3–0 Vicryl, Ethicon). In this way, the jejunum returns the torsion of the jejunum to the counter-clockwise direction and rides on the remnant stomach (Fig. 2e). Finally, a side-to-side jejunojejunostomy is made between the jejunum 20 cm below the jejunogastrostomy and the proximal jejunum. The jejunojejunostomy is pulled toward the cranial side without sacrificing the small intestine (Fig. 3). Thereby, the distal jejunum from the jejunogastrostomy can easily bend to the ventral side, and the exit route becomes narrow (Online Resource 1). We called this jejunogastrostomy with twisting of both the remnant stomach and the anal jejunum and the jejunojejunostomy pulled toward the cranial side an oblique jejunogastrostomy technique.
a A purple line is drawn on the antimesenteric border of the jejunum. c Black squares show the entry holes of the linear stapler made on the greater curvature of the remnant stomach and the antimesenteric wall of the jejunum. The dotted line is drawn at the antimesenteric border of the jejunum. d Both the stomach and jejunum are twisted posteriorly. The arrows indicate the twisting direction for the remnant stomach and the jejunum. Then, a side-to-side jejunogastrostomy is performed between the posterior wall of the remnant stomach and the jejunum. b Intraoperative photo of the side-to-side jejunogastrostomy performed between the posterior wall of the remnant stomach and the jejunum. e The jejunum rides on the remnant stomach by returning the torsion of the jejunum to the counter-clockwise direction, because the twisted jejunum with the support of the mesentery returns to the original position. The jejunum of the anastomotic site is lifted to the ventral side by the remnant stomach that enters behind. ST stomach, J jejunum
a The distal jejunum from the jejunogastrostomy can be lifted up to the cranial side, because the esophagojejunostomy and jejunojejunostomy are placed a short distance from each other without sacrificing jejunum and dividing the mesentery. The white arrow shows the site of esophagojejunostomy. The white arrow head shows the jejunojejunostomy site. ST stomach, J jejunum. b The schema shows an overview of the double-tract reconstruction before jejunojejunostomy is performed. The gray triangle shows the anastomotic sites. The position of the proximal jejunum (black arrow head) near the ligament of Treitz is able to be pulled toward the cranial side by not sacrificing part of the jejunum. c The schema shows the overview of the double-tract reconstruction. The jejunum is bent toward the cranial side just distal from the gastrojejunostomy (black arrow) because it is lifted up by the jejunojejunostomy located in the cranial side
Ethics and consent
The Hokkaido University Hospital Ethics Committee approved this study in 2016 (No. 016-0194). The consent form stated the aim of the study on the web site of our hospital and the participants’ right to decline to participate or opt out at any time. Informed consent was obtained from all individual participants included in the study. The ethics committee/IRB approved this consent procedure.
Results
Clinical characteristics of patients
Ten patients (seven males, three females; median age 70 (range 55–77) years; median BMI 23.7 (range 18.3–28.7) kg/m2) were included. Six patients had previously undergone endoscopic submucosal dissection (ESD). Two patients had undergone previous abdominal operations, including one appendectomy and one colorectal cancer operation. The patients’ clinical characteristics are presented in Table 1.
Operative and pathological data
The operative and pathological data are summarized in Table 2. The median duration of operation was 285 (range 146–440) min. No patients required blood transfusions. The number of dissected lymph nodes was 32 (range 22–56). There were no intraoperative complications, and no cases were converted to open surgery. Cancer had invaded the submucosa in all the ten patients. All patients were pT1N0M0 stage IA. Histological types included five well-differentiated cases, three moderately differentiated cases, and two poorly differentiated cases. The free margins of the tumor were no less than 2 cm from the distal end (2.1–8.5 cm).
Postoperative outcomes
The operative and pathological data are summarized in Table 3. The median time to resume a soft diet postoperatively was 3 (range 3–5) days, and the median postoperative hospital stay was 13 (range 9–16) days. No patients required second surgery. No anastomotic leakage and no complications were seen. Recurrence was not observed in any patient after LPG over a median follow-up period of 10.7 (range 1–37.6) months. Some patients underwent endoscopy follow-up and upper gastrointestinal series examinations. They showed the wide entry hole of the gastrojejunostomy and the narrow route of the distal jejunum. No patients complained of dumping syndrome, while two patients complained of moderate reflux symptoms of heartburn, but they soon recovered with proton-pump inhibitor treatment. The average percentage body weight loss was 14.0% (SD ± 7.1%). The average percentage decrease in serum hemoglobin was 5.4% (SD ± 10.4%). The median follow-up periods for these two were 10 and 12 months, respectively.
Discussion
Three representative reconstruction procedures after proximal gastrectomy have been reported: esophagogastrostomy, jejunal pouch interposition reconstruction, and DTR. DTR was thought to be the best reconstruction procedure with respect to anastomosis-related late complications, especially postoperative reflux esophagitis. Esophagogastrostomy is simpler than the other procedures because it includes only one anastomosis, and it is the most popular and the classical reconstruction [23]. However, a relatively high incidence (27.4 to 67.4%) of postoperative anastomosis-related complications such as reflux esophagitis has been reported [13, 14, 24, 25]. Jejunal (pouch) interposition reconstruction is the second most common reconstruction [23]. Some papers [26,27,28] reported that proximal gastrectomy with jejunal interposition had a high incidence (10.2, 9.1 and 31.8%) of anastomotic stricture. Kinoshita et al. [27] speculated about the reason for the stricture. They suggested that the small amount of bile reflux to the interposed jejunum and tension to the interposed jejunum cause stenosis. In four studies [17, 29,30,31] of laparoscopic proximal gastrectomy with DTR, three reported postoperative complications. In the literature, there were no anastomosis-related complications. The morbidity rates were 9.5, 11.6, and 25%. Symptoms related to reflux esophagitis occurred in 0, 4.7, and 4.8%. The present results for postoperative complications were similar to these reports. As to long-term function, changes of serum hemoglobin and body weight after gastrectomy were reported. The present results for body weight loss and decrease in serum hemoglobin were not worse than those for the body weight loss (14.1–30.0%) [32,33,34,35,36] and decrease in serum hemoglobin (9.7%) [33] at 12 months after total gastrectomy. Ahn et al. [17] first reported 43 cases of DTR after LPG, and they performed a Roux-en-Y esophagojejunostomy by intracorporeal anastomosis with a circular stapler by a mini-laparotomy, and side-to-side gastrojejunostomy was performed in an extracorporeal fashion using two linear staplers. Nomura et al. [29] used circular staplers to complete the esophagojejunostomies. In the remaining two reports, linear staplers were used to complete all anastomoses, which is similar to the present procedure. Hong et al. [30] first reported TLPG with DTR with a triangular stapling technique (delta-shaped anastomosis). Linear staplers can make a larger-size anastomosis than a circular stapler [37], and thus, the occurrence of anastomotic stenosis can be reduced. With extracorporeal anastomosis, it was sometimes difficult to move the intestinal tracts just under the mini-laparotomy, especially in obese patients. We selected DTR to be able to perform intracorporeal totally laparoscopic surgery and to have less anastomosis-related problems, such as reflux esophagitis and anastomotic stenosis.
DTR was first reported by Aikou et al. [18] in terms of gaining the smooth transfer of larger foods through the duodenal route. There are some concerns with DTR that the functional benefits of proximal gastrectomy, such as preserving the antrum, might be lost when most of the dietary intake passes through the escape route of the jejunum. In order to overcome this disadvantage, we modified the intracorporeal anastomosis of jejunogastrostomy in the traditional DTR by narrowing the exit route of the jejunum to induce meals to pass into the remnant stomach. Aikou et al. [18] also reported the original jejunogastrostomy method after proximal gastrectomy called the N-shaped method, due to the flow of larger quantities of food into the antrum of the residual stomach. The residual stomach was twisted 180 degrees anteriorly beforehand, the twisted residual stomach was restored to its usual position, and the jejunum was then shaped like the letter “N” around the gastrojejunostomy. Seeing this anastomotic technique, we modified it to be suitable for intracorporeal laparoscopic reconstruction. The anastomotic method of jejunogastrostomy with twisting of only the stomach created an anastomotic site in which anastomosis ischemia was prevented. On the other hand, with twisting of both the stomach and the jejunum, the dietary intake could easily flow into the remnant stomach because the anastomotic site of the jejunogastrostomy was made at the posterior wall by the jejunum riding on the stomach and faced slightly more vertically against the flow of the meal, returning the torsion of the jejunum to the counter-clockwise direction. There appear to be two reasons for this: (1) the twisted jejunum returns to the original position because the rim of the jejunum was fixed by the mesentery and the remnant stomach was relatively free to move with the lymph dissection, and (2) without sacrificing several centimeters of the small intestine, the distal jejunum from the jejunogastrostomy could be bent forward and lifted up to the cranial side. The jejunojejunostomy was placed a short distance from the caudal side of the esophagojejunostomy. Thereby, the jejunogastrostomy and jejunojejunostomy were placed at almost the same level as the remnant stomach. Our innovation was to create the anastomosis by twisting a small amount of the small intestine, bending the small intestine of the outflow path into a U-shaped appearance without sacrificing the small intestine. This enables food to enter the residual stomach more easily, because the bent distal jejunum reduces the outflow of the contents. Hong et al. [30] reported a delta-shaped intracorporeal jejunogastrostomy in DTR in a procedure similar to ours. However, it was difficult to narrow the exit routes of the jejunum by twisting only the remnant stomach.
The second weak point is that the DTR needs more anastomotic sites than other reconstructive procedures for the proximal gastrectomy. However, every anastomosis for DTR used the same anastomotic procedure, which had been established as the intracorporeal reconstruction of laparoscopic gastrectomy. The esophagojejunostomy and jejunojejunostomy for DTR could be performed in the same procedure as Roux-en-Y reconstruction after laparoscopic total gastrectomy [7]. The jejunogastrostomy for DTR could be performed in the same fashion as the delta-shaped anastomosis after laparoscopic distal gastrectomy [38]. In contrast, intracorporeal esophagogastrostomy and jejunal interposed reconstruction are not well known even by experienced laparoscopic surgeons, because these procedures are not necessary for either total or distal gastrectomy. The total operation time was similar between TLPG with DTR and that with Roux-en-Y reconstruction in our department, although DTR requires one more reconstruction than Roux-en-Y reconstruction. The reconstruction time of DTR (median time 108 min) was approximately 20 min longer than that of Roux-en-Y reconstruction (median time 87.5 min) in our department when comparing the same periods (data not shown). As mentioned above, we consider that DTR is a better reconstructive procedure for TLPG, because the procedure can be more easily introduced.
The present study had several limitations. Subjective symptoms were not evaluated using a questionnaire, and long-term functional benefits were not assessed. Gastric emptying was not examined in all the patients. Despite this being a retrospective study of a small number of cases, our technique appears to provide feasible results.
Conclusion
Our novel oblique jejunogastrostomy technique for DTR appeared to have the considerable advantage of passage of dietary intake to the remnant stomach, as well as reasonable complications rates without any anastomosis-related problems. Further studies to evaluate subjective function and symptoms are needed.
References
Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A (2015) Global cancer statistics, 2012. CA Cancer J Clin 65(2):87–108. doi:10.3322/caac.21262
Sano T, Hollowood A (2006) Early gastric cancer: diagnosis and less invasive treatments. Scand J Surg 95(4):249–255
Asaka M, Mabe K, Matsushima R, Tsuda M (2015) Helicobacter pylori eradication to eliminate gastric cancer: the Japanese strategy. Gastroenterol Clin N Am 44(3):639–648. doi:10.1016/j.gtc.2015.05.010
Jeong O, Park YK (2011) Clinicopathological features and surgical treatment of gastric cancer in South Korea: the results of 2009 nationwide survey on surgically treated gastric cancer patients. J Gastric Cancer 11(2):69–77. doi:10.5230/jgc.2011.11.2.69
Kim YI, Kim SY, Cho SJ, Park JH, Choi IJ, Lee YJ, Lee EK, Kook MC, Kim CG, Ryu KW, Kim YW (2014) Long-term metformin use reduces gastric cancer risk in type 2 diabetics without insulin treatment: a nationwide cohort study. Aliment Pharmacol Ther 39(8):854–863. doi:10.1111/apt.12660
Kawamura H, Okada K, Isizu H, Masuko H, Yamagami H, Honma S, Ueki S, Noguchi K, Kondo Y (2008) Laparoscopic gastrectomy for early gastric cancer targeting as a less invasive procedure. Surg Endosc 22(1):81–85. doi:10.1007/s00464-007-9373-y
Ebihara Y, Okushiba S, Kawarada Y, Kitashiro S, Katoh H (2013) Outcome of functional end-to-end esophagojejunostomy in totally laparoscopic total gastrectomy. Langenbeck's archives of surgery / Deutsche Gesellschaft fur Chirurgie 398(3):475–479. doi:10.1007/s00423-013-1051-z
Takiguchi N, Takahashi M, Ikeda M, Inagawa S, Ueda S, Nobuoka T, Ota M, Iwasaki Y, Uchida N, Kodera Y, Nakada K (2015) Long-term quality-of-life comparison of total gastrectomy and proximal gastrectomy by postgastrectomy syndrome assessment scale (PGSAS-45): a nationwide multi-institutional study. Gastric Cancer 18(2):407–416. doi:10.1007/s10120-014-0377-8
Nozaki I, Hato S, Kobatake T, Ohta K, Kubo Y, Kurita A (2013) Long-term outcome after proximal gastrectomy with jejunal interposition for gastric cancer compared with total gastrectomy. World J Surg 37(3):558–564. doi:10.1007/s00268-012-1894-4
Ichikawa D, Komatsu S, Kubota T, Okamoto K, Shiozaki A, Fujiwara H, Otsuji E (2014) Long-term outcomes of patients who underwent limited proximal gastrectomy. Gastric Cancer 17(1):141–145. doi:10.1007/s10120-013-0257-7
Papachristou DN, Fortner JG (1980) Adenocarcinoma of the gastric cardia. The choice of gastrectomy. Ann Surg 192(1):58–64
Yoo CH, Sohn BH, Han WK, Pae WK (2004) Long-term results of proximal and total gastrectomy for adenocarcinoma of the upper third of the stomach. Cancer Res Treat 36(1):50–55. doi:10.4143/crt.2004.36.1.50
Wen L, Chen XZ, Wu B, Chen XL, Wang L, Yang K, Zhang B, Chen ZX, Chen JP, Zhou ZG, Li CM, Hu JK (2012) Total vs. proximal gastrectomy for proximal gastric cancer: a systematic review and meta-analysis. Hepato-Gastroenterology 59(114):633–640. doi:10.5754/hge11834
Pu YW, Gong W, Wu YY, Chen Q, He TF, Xing CG (2013) Proximal gastrectomy versus total gastrectomy for proximal gastric carcinoma. A meta-analysis on postoperative complications, 5-year survival, and recurrence rate. Saudi Med J 34(12):1223–1228
Uyama I, Ogiwara H, Takahara T, Kikuchi K, Iida S (1995) Laparoscopic and minilaparotomy proximal gastrectomy and esophagogastrostomy: technique and case report. Surgical laparoscopy & endoscopy 5(6):487–491
Uyama I, Sugioka A, Fujita J, Komori Y, Matsui H, Hasumi A (2000) Completely laparoscopic proximal gastrectomy with jejunal interposition and lymphadenectomy. J Am Coll Surg 191(1):114–119
Ahn SH, do Jung H, Son SY, Lee CM, do Park J, Kim HH (2014) Laparoscopic double-tract proximal gastrectomy for proximal early gastric cancer. Gastric Cancer 17(3):562–570. doi:10.1007/s10120-013-0303-5
Aikou T, Natsugoe S, Shimazu H, Nishi M (1988) Antrum preserving double tract method for reconstruction following proximal gastrectomy. Jpn J Surg 18(1):114–115
Dindo D, Demartines N, Clavien PA (2004) Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 240(2):205–213
Nakajima T (2002) Gastric cancer treatment guidelines in Japan. Gastric Cancer 5(1):1–5. doi:10.1007/s101200200000
Japanese Gastric Cancer A (1998) Japanese classification of gastric carcinoma—2nd English edition. Gastric Cancer 1(1):10–24. doi:10.1007/s101209800016
Matsui H, Uyama I, Sugioka A, Fujita J, Komori Y, Ochiai M, Hasumi A (2002) Linear stapling forms improved anastomoses during esophagojejunostomy after a total gastrectomy. Am J Surg 184(1):58–60
Kumagai K, Shimizu K, Yokoyama N, Aida S, Arima S, Aikou T, Japanese Society for the Study of Postoperative Morbidity after Gastrectomy (2012) Questionnaire survey regarding the current status and controversial issues concerning reconstruction after gastrectomy in Japan. Surg Today 42(5):411–418. doi:10.1007/s00595-012-0159-z
Katsoulis IE, Robotis JF, Kouraklis G, Yannopoulos PA (2006) What is the difference between proximal and total gastrectomy regarding postoperative bile reflux into the oesophagus? Dig Surg 23(5–6):325–330. doi:10.1159/000097948
An JY, Youn HG, Choi MG, Noh JH, Sohn TS, Kim S (2008) The difficult choice between total and proximal gastrectomy in proximal early gastric cancer. Am J Surg 196(4):587–591. doi:10.1016/j.amjsurg.2007.09.040
Katai H, Morita S, Saka M, Taniguchi H, Fukagawa T (2010) Long-term outcome after proximal gastrectomy with jejunal interposition for suspected early cancer in the upper third of the stomach. Br J Surg 97(4):558–562. doi:10.1002/bjs.6944
Kinoshita T, Gotohda N, Kato Y, Takahashi S, Konishi M, Kinoshita T (2013) Laparoscopic proximal gastrectomy with jejunal interposition for gastric cancer in the proximal third of the stomach: a retrospective comparison with open surgery. Surg Endosc 27(1):146–153. doi:10.1007/s00464-012-2401-6
Nakamura M, Nakamori M, Ojima T, Katsuda M, Iida T, Hayata K, Matsumura S, Kato T, Kitadani J, Iwahashi M, Yamaue H (2014) Reconstruction after proximal gastrectomy for early gastric cancer in the upper third of the stomach: an analysis of our 13-year experience. Surgery 156(1):57–63. doi:10.1016/j.surg.2014.02.015
Nomura E, Lee SW, Kawai M, Yamazaki M, Nabeshima K, Nakamura K, Uchiyama K (2014) Functional outcomes by reconstruction technique following laparoscopic proximal gastrectomy for gastric cancer: double tract versus jejunal interposition. World J Surg Oncol 12:20. doi:10.1186/1477-7819-12-20
Hong J, Qian L, Wang YP, Wang J, Hua LC, Hao HK (2015) A novel method of delta-shaped intracorporeal double-tract reconstruction in totally laparoscopic proximal gastrectomy. Surg Endosc. doi:10.1007/s00464-015-4490-5
Yang K, Bang HJ, Almadani ME, Dy-Abalajon DM, Kim YN, Roh KH, Lim SH, Son T, Kim HI, Noh SH, Hyung WJ (2016) Laparoscopic proximal gastrectomy with double-tract reconstruction by intracorporeal anastomosis with linear staplers. J Am Coll Surg. doi:10.1016/j.jamcollsurg.2016.01.002
Davis JL, Selby LV, Chou JF, Schattner M, Ilson DH, Capanu M, Brennan MF, Coit DG, Strong VE (2016) Patterns and predictors of weight loss after gastrectomy for cancer. Ann Surg Oncol 23(5):1639–1645. doi:10.1245/s10434-015-5065-3
Ohashi M, Morita S, Fukagawa T, Oda I, Kushima R, Katai H (2015) Functional advantages of proximal gastrectomy with jejunal interposition over total gastrectomy with Roux-en-Y esophagojejunostomy for early gastric cancer. World J Surg 39(11):2726–2733. doi:10.1007/s00268-015-3180-8
Nishigori T, Okabe H, Tsunoda S, Shinohara H, Obama K, Hosogi H, Hisamori S, Miyazaki K, Nakayama T, Sakai Y (2017) Superiority of laparoscopic proximal gastrectomy with hand-sewn esophagogastrostomy over total gastrectomy in improving postoperative body weight loss and quality of life. Surg Endosc. doi:10.1007/s00464-016-5403-y
Iwahashi M, Nakamori M, Nakamura M, Naka T, Ojima T, Iida T, Katsuda M, Ueda K, Yamaue H (2009) Evaluation of double tract reconstruction after total gastrectomy in patients with gastric cancer: prospective randomized controlled trial. World J Surg 33(9):1882–1888. doi:10.1007/s00268-009-0109-0
Nomura E, Lee SW, Tokuhara T, Nitta T, Kawai M, Uchiyama K (2013) Functional outcomes according to the size of the gastric remnant and the type of reconstruction following distal gastrectomy for gastric cancer: an investigation including total gastrectomy. Jpn J Clin Oncol 43(12):1195–1202. doi:10.1093/jjco/hyt141
Zhou D, Liu QX, Deng XF, Min JX, Dai JG (2015) Comparison of two different mechanical esophagogastric anastomosis in esophageal cancer patients: a meta-analysis. J Cardiothorac Surg 10:67. doi:10.1186/s13019-015-0271-4
Kanaya S, Gomi T, Momoi H, Tamaki N, Isobe H, Katayama T, Wada Y, Ohtoshi M (2002) Delta-shaped anastomosis in totally laparoscopic Billroth I gastrectomy: new technique of intraabdominal gastroduodenostomy. J Am Coll Surg 195(2):284–287
Author information
Authors and Affiliations
Contributions
Study conception and design: Kimitaka Tanaka, Yuma Ebihara, Yo Kurashima, and Satoshi Hirano; acquisition of data: Kimitaka Tanaka, Yuma Ebihara, and Yo Kurashima; drafting of manuscript: Kimitaka, Tanaka, Yuma Ebihara, and Satoshi Hirano; and critical revision of manuscript: Yuma Ebihara, Yo Kurashima, Yoshitsugu Nakanishi, Toshimichi Asano, Takehiro Noji, Soichi Murakami, Toru Nakamura, Takahiro Tsuchikawa, Keisuke Okamura, Toshiaki Shichinohe, and Satoshi Hirano
Corresponding author
Ethics declarations
Funding
This study received no funding.
Conflict of interest
The authors declare that they have no conflicts of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Electronic supplementary material
ESM 1
(MP4 58,318 kb)
Rights and permissions
About this article
Cite this article
Tanaka, K., Ebihara, Y., Kurashima, Y. et al. Laparoscopic proximal gastrectomy with oblique jejunogastrostomy. Langenbecks Arch Surg 402, 995–1002 (2017). https://doi.org/10.1007/s00423-017-1587-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00423-017-1587-4