Abstract
Background
Construction of an esophagojejunostomy is still a challenging procedure in totally laparoscopic total gastrectomy (TLTG), and there is no standard anastomosing method. The aims of this study were to describe our TLTG with the overlap method using a linear stapler and report surgical outcomes.
Methods
From January 2015 to April 2016, 50 patients underwent TLTG using the overlap method for gastric cancer. The procedures were performed by a single surgeon, and the patients’ medical records were reviewed. Their clinicopathologic characteristics, operation time, date of flatus, hospital stay, morbidity, and mortality were analyzed.
Results
The median age and body mass index were 56 years and 23.5, respectively. Stage 1A tumors were the most common. Mean operating time was 144.6 min, and no cases required changing to open laparotomy during surgery. On average, flatus occurred 3.5 days after surgery, and patients were discharged 6.8 days after surgery. No patient experienced anastomosis leakage, stricture, duodenal stump leakage, luminal bleeding, pancreatic fistula, or wound problems. There were two cases of intra-abdominal bleeding that required additional surgery. Intra-abdominal fluid collection and mechanical ileus occurred in two patients, respectively, and were successfully managed with conservative treatment.
Conclusions
We reported favorable surgical outcomes of TLTG using the overlap method. It is a feasible and safe option for treatment of gastric cancer.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Since the introduction of laparoscopy-assisted distal gastrectomy by Kitano [1], laparoscopic gastrectomy has become an effective surgical option in the treatment of early gastric cancer [2, 3]. Favorable outcomes, including better cosmetic appearance, shorter hospital stay, reduced postoperative pain, and improved quality of life, have been reported [2, 4]. Based on a nationwide survey of surgically treated gastric cancer patients by the Korean Gastric Cancer Association in 2014 (data not published), about half of all gastric cancer surgeries were laparoscopic gastrectomies and intracorporeal anastomosis become increasingly popular for laparoscopic distal gastrectomy in Korea. However, laparoscopic total gastrectomy is not as widely practiced as laparoscopic distal gastrectomy owing to its technical difficulties, especially the construction of an esophagojejunostomy. In laparoscopic total gastrectomy, many surgeons prefer to use a circular stapler, similar to conventional open total gastrectomy. However, the placement of purse-string sutures and insertion of the anvil can be technically challenging in a laparoscopic procedure. Despite evidence of favorable results using a linear stapler in laparoscopic total gastrectomy [5–7], there is still no standard anastomosing method for laparoscopic total gastrectomy. Inaba et al. [6] introduced the overlap method, which has some advantages compared with functional anastomosis, including less mesenteric division and consequently less tension around the anastomosis, which is one of the most important risk factors leading to anastomosis leakage. Recently, Kitagami et al. reported successful results for 100 cases of laparoscopic total gastrectomy using the overlap method in Japan [8–10] and a report from the USA also reported the feasibility and safety of it in advanced gastric cancer [11]. However, there are no data from another country, and evidence of safety and surgical outcomes is still lacking.
The present study introduced our totally laparoscopic total gastrectomy (TLTG) using the overlap method and reported the early outcomes for 50 patients who underwent the procedure. Our objective was to demonstrate that TLTG using the overlap method is a feasible and safe procedure for gastric cancer.
Materials and methods
From January 2015 to April 2016, a total of 50 patients underwent TLTG using the overlap method for gastric cancer by the same surgeon (I.S. Lee) at the Asan Medical Center. After the institutional review board approved the study, their medical records were reviewed to determine demographic and clinicopathologic characteristics, including age at operation, sex, body mass index (BMI), American Society of Anesthesiologists (ASA) score, tumor location, size, and depth of invasion, number of metastatic and harvested lymph nodes, TNM stage based on the American Joint Committee on Cancer (AJCC) Staging Manual 7th edition, operation time, date of flatus, hospital stay, morbidity, and mortality. All patients visited the outpatient clinic for follow-up at 2 weeks, and 3 and 6 months after surgery, and underwent esophagogastroduodenoscopy and abdominopelvic computed tomography during the follow-up period. Follow-up was conducted to August 2016, and median period was 7.8 months (range 2.3–19.3 months). Patients who had tumors with Siewert type 2 esophagogastric junction adenocarcinoma and those with suspected serosa infiltration, revealed during the preoperative exam, were excluded.
Surgical technique
We used five ports for the procedure. Using an open technique, a 12-mm main port was placed below the umbilicus for laparoscope entry and specimen delivery. On both sides of the upper abdomen, 12- and 5-mm trocars were inserted for manipulation (Fig. 1). To retract the liver, a triangle method was used [12].
In the reverse Trendelenburg position, surgery commenced with division of the gastrohepatic ligament and clearance of structures around the esophagus. We then performed an omentum-preserving total gastrectomy with D1+ lymph node dissection in cases of early gastric cancer, and total omentectomy with D2 lymph node dissection for advanced tumors.
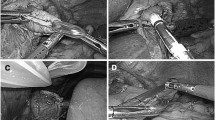
After removal of soft tissue and vagus nerves around the esophagus and verification that the stomach was fully mobilized, the esophagus was transected, using a linear stapler with a cartridge for 1.5–2.25 mm thickness, from the right to the left side. A hole was made on the left side of the esophagus to insert a limb of the stapler. Three tagging sutures were added to the remaining esophageal stump, one of which was placed in the midportion of the staple line. The other two sutures were placed at the 1- and 5-o’clock positions of the esophageal opening, to make it easy to manipulate during anastomosis and to avoid slippage of any layer of the esophageal wall. A specimen was removed with an endobag, a proximal resection margin of which was obtained to check for the presence of tumor cells on frozen biopsy. During the pathologic examination, jejunal preparation commenced and the mesentery was divided in the abdominal cavity. Using a linear stapler, the jejunum was transected at a point 40 cm distal to the ligament of Treitz. A small enterotomy was made on the anti-mesenteric side of the efferent jejunum. A limb of a 45-mm linear stapler was inserted into the efferent loop and was drawn up to the esophagus. The other limb of the stapler was introduced into the left side of esophagus to construct a Roux-en-Y side-to-side esophagojejunal anastomosis (Fig. 2). The common opening was approximated with four sutures and finally closed with an additional 60-mm linear stapler (Fig. 3). A jejunojejunal side-to-side anastomosis was made at about 40–50 cm below the esophagojejunostomy, similar to the overlap method. Finally, we added sutures between the mesentery of the jejunum to prevent internal herniation. If some tension was suspected around the anastomosis, one suture was added between the esophagojejunostomy and the right crus muscle. A drain was placed through the right 5-mm port into the abdominal cavity, and the other port sites were closed. There was no case to place a feeding jejunostomy.
Results
The study included 29 males and 21 females. The median age at surgery and BMI were 56 years and 23.5, respectively. In the preoperative examination, an ASA score of 2 was the most common, followed by 1 and 3. About three quarters of the sample had a tumor in the upper third of the stomach. The mean tumor size and median number of harvested lymph nodes were 3.6 cm and 31, respectively. Cancer invaded the mucosa in 20 (40.0%), submucosa in 22 (44.0%), proper muscle in 2 (4.0%), and subserosa in 6 (12.0%) patients. Tumors with TNM stage 1A were the most common (Table 1).
Most patients started to drink water 24 h after operation, and a liquid diet was permitted on postoperative day 3. After passing flatus, a soft diet was begun. Two patients who had suspected anastomosis instability underwent fluoroscopic examination before diet.
Mean operating time was 144.6 min, and no patients required conversion to open laparotomy during operation. Flatus occurred at 3.5 days after surgery. On average, patients were discharged 6.8 days after surgery. There were no anastomosis-related complications, including leakage or stricture. No patients experienced luminal bleeding, pancreatic fistula, or wound problems. Two patients had intra-abdominal bleeding that required surgery. Intra-abdominal fluid collection occurred in two patients and resolved without intervention. Two patient experienced mechanical ileus after discharge, which was successfully managed with conservative treatment (Table 2). All patients who are candidate for adjuvant chemotherapy received treatment after postoperative 4–5 weeks. There was no recurrence or mortality observed during the follow-up period.
Discussion
Compared with a circular stapler, construction of an esophagojejunostomy using a linear stapler can simplify the procedure, including insertion of an intracorporeal or transoral anvil and placement of purse-string sutures. A linear stapler does not require an additional mini-laparotomy to insert devices to make an anastomosis. On the other hand, functional end-to-end anastomosis methods using a linear stapler require a longer segment of the esophagus and more extensive division of the jejunal mesentery. The overlap method can reduce these limitations of both procedures.
Previous Japanese studies reported the feasibility and favorable surgical outcomes of the overlap method [6, 9, 10]. Our procedure differed from their methods in some points. First, we did not use a nasogastric tube as a guide to the lumen in the esophageal stump. Instead, we used tagging sutures that included all layers of the esophagus. This can avoid unnecessary esophageal injury during laparoscopic manipulation or slippage of any layer of the esophagus and make identification of the lumen easy. Secondly, we closed the common entry of the esophagojejunostomy with a linear stapler instead of suturing, except in two patients whose esophageal stump in the peritoneal cavity was short and closure of the anastomosis by stapling was impossible. Among the 50 patients in this study, none complained of stricture-related symptoms, such as recurrent nausea and vomiting during the follow-up period. Thirty-eight patients underwent routine postoperative esophagogastroduodenoscopy at 6 months after operation, and no anastomosis strictures were observed. Thirdly, we manipulated and divided the jejunal mesentery in the peritoneal cavity in the laparoscopic view. In obese patients with a thick abdominal wall and short mesentery, a larger incision is usually required for extracorporeal manipulation. However, in the laparoscopic magnified vision, it is not difficult to trace the vasculature and divide the mesentery. Finally, we did not fix the efferent jejunum to the duodenal stump to prevent passage disturbance by kinking of the lifted jejunal loop. Instead, we obtained more length from the afferent loop and used 40 cm from the Treitz ligament to perform side-to-side jejunojejunostomy. If the remaining afferent limb was short, it would have pulled the efferent loop in the retroperitoneal direction, leading to efferent loop narrowing or angulation.
Compared with other studies, we reported more favorable surgical outcomes, including shorter operation times, hospital stays, and a lower morbidity rate. It is difficult to directly compare our results with others because there were differences in patient characteristics, the distribution of clinical and/or pathologic stages of gastric cancer, and the presence of combined resection. However, there were no serious complications related to the anastomosis or duodenal stump. This suggests that the overlap method is a feasible and safe laparoscopic option for the treatment of gastric cancer.
This study had several limitations. We presented the results of a single surgeon in a high-volume facility. This is not a comparative study of our methods with other procedures. We did not perform the overlap method for Siewert type 2 esophagogastric junction adenocarcinoma or tumors invading serosa. Despite these limitations, the present study is valuable because it is the first report from an Asian country other than Japan. In addition, we re-evaluated the safety and favorable outcomes of TLTG using the overlap method to treat gastric cancer.
References
Kitano S, Iso Y, Moriyama M, Sugimachi K (1994) Laparoscopy-assisted Billroth I gastrectomy. Surg Laparosc Endosc 4:146–148
Kim HH, Hyung WJ, Cho GS, Kim MC, Han SU, Kim W, Ryu SW, Lee HJ, Song KY (2010) Morbidity and mortality of laparoscopic gastrectomy versus open gastrectomy for gastric cancer: an interim report—a phase III multicenter, prospective, randomized Trial (KLASS Trial). Ann Surg 251:417–420
Nikawa T, Ikemoto M, Watanabe C, Kitano T, Kano M, Yoshimoto M, Towatari T, Katunuma N, Shizuka F, Kishi K (2002) A cysteine protease inhibitor prevents suspension-induced declines in bone weight and strength in rats. J Physiol Anthropol Appl Hum Sci 21:51–57
Kim YW, Baik YH, Yun YH, Nam BH, Kim DH, Choi IJ, Bae JM (2008) Improved quality of life outcomes after laparoscopy-assisted distal gastrectomy for early gastric cancer: results of a prospective randomized clinical trial. Ann Surg 248:721–727
Bracale U, Marzano E, Nastro P, Barone M, Cuccurullo D, Cutini G, Corcione F, Pignata G (2010) Side-to-side esophagojejunostomy during totally laparoscopic total gastrectomy for malignant disease: a multicenter study. Surg Endosc 24:2475–2479
Inaba K, Satoh S, Ishida Y, Taniguchi K, Isogaki J, Kanaya S, Uyama I (2010) Overlap method: novel intracorporeal esophagojejunostomy after laparoscopic total gastrectomy. J Am Coll Surg 211:e25–e29
Lee IS, Kim TH, Kim KC, Yook JH, Kim BS (2012) Modified techniques and early outcomes of totally laparoscopic total gastrectomy with side-to-side esophagojejunostomy. J Laparoendosc Adv Surg Tech A 22:876–880
Tsujimoto H, Uyama I, Yaguchi Y, Kumano I, Takahata R, Matsumoto Y, Yoshida K, Horiguchi H, Aosasa S, Ono S, Yamamoto J, Hase K (2012) Outcome of overlap anastomosis using a linear stapler after laparoscopic total and proximal gastrectomy. Langenbecks Arch Surg 397:833–840
Morimoto M, Kitagami H, Hayakawa T, Tanaka M, Matsuo Y, Takeyama H (2014) The overlap method is a safe and feasible for esophagojejunostomy after laparoscopic-assisted total gastrectomy. World J Surg Oncol 12:392
Kitagami H, Morimoto M, Nakamura K, Watanabe T, Kurashima Y, Nonoyama K, Watanabe K, Fujihata S, Yasuda A, Yamamoto M, Shimizu Y, Tanaka M (2016) Technique of Roux-en-Y reconstruction using overlap method after laparoscopic total gastrectomy for gastric cancer: 100 consecutively successful cases. Surg Endosc 30:4086–4091
Treitl D, Hochwald SN, Bao PQ, Unger JM, Ben-David K (2016) Laparoscopic total gastrectomy with D2 lymphadenectomy and side-to-side stapled esophagojejunostomy. J Gastrointest Surg 20:1523–1529
Lee IS, Kim TH, Yook JH, Kim HS, Kim BS, Kim BS (2012) A triangle method: simple suture retraction for the left lobe of the liver during laparoscopic gastric surgery. J Laparoendosc Adv Surg Tech A 22:989–991
Acknowledgements
We thank Mr. Chang-Geun Heo, Chun-Myoung Ji, and Du-Gi Pin for help with this article.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosures
Drs. Tae-Gyun Lee, In-Seob Lee, Jeong-Hwan Yook, and Byung-Sik Kim have no conflicts of interest or financial ties to disclose.
Rights and permissions
About this article
Cite this article
Lee, TG., Lee, IS., Yook, JH. et al. Totally laparoscopic total gastrectomy using the overlap method; early outcomes of 50 consecutive cases. Surg Endosc 31, 3186–3190 (2017). https://doi.org/10.1007/s00464-016-5343-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00464-016-5343-6