Abstract
Background
Due to a tremendous organ shortage, livers from donors with extended criteria are increasingly considered for transplantation. Pathologists are more and more requested to evaluate these livers histopathologically using frozen sections at high urgency for acceptability.
Methods
This article reviews the current knowledge on pre-transplant histology in liver transplantation. Prerequisites and conditions for proper pre-transplant evaluation of donor liver tissue are discussed as well as frozen section evaluation and reporting. Data sources include the relevant medical literature, web sites specialized in organ transplantation, and the authors’ experiences in liver transplant centers.
Conclusions
Pre-transplant histopathological evaluation is a time-effective, accurate, and reliable tool to assess liver quality from candidate deceased donors. Pre-transplant biopsies are of value in the selection of donor livers for transplantation, especially in case of extended criteria donors, and should be performed more frequently in order to avoid unnecessary loss of organs suitable for transplantation and transplantation of inappropriate organs. Correlation of histopathological findings with clinical conditions is essential and requires excellent communication between pathologists, surgeons, and the other members of the transplant team.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Donor risk factors that correlate with poor allograft function have been identified and are in part reflected by allograft histology at procurement [1–7]. The risk may manifest as impaired allograft function or donor transmitted disease.
Pre-transplant biopsies from deceased liver donors are not mandatory for decision making in the process of transplantation and thus protocol biopsies are conducted only in a few transplant programs. If transplantability is not clearly given on the basis of clinical, laboratory, or visual criteria, an additional frozen section biopsy is performed of the potential graft and the sample is histologically interpreted by a pathologist. A final decision is made based on both the grafts clinical and histological information and the medical state of the intended recipient. The most common reason for discharging livers either during or after recovery is steatosis [8, 9].
As the demand for organs increases, livers from extended criteria donors are increasingly considered for transplantation. Even though a biopsy is not a prerequisite for the utilization of such organs, pathologists are more and more asked to evaluate these potential grafts.
Tissue acquisition
As donor liver biopsies are not officially required for the decision analysis for organ utilization, biopsies are performed on demand either at procurement or at the recipient institution. If the judgment of the procurement surgeon upon allograft visualization leads to a biopsy, a wedge biopsy can be obtained prior to the initiation of cold perfusion. If the macroscopic appearance of the organ casts doubts on transplantability by the recipient surgeon, he may perform the biopsy (Fig. 1). Our preference as well as expert-driven consent is a subcapsular wedge biopsy (1.5 cm2), but in literature, also core biopsies with a 2.0-cm-long needle are reported (http://tpis.upmc.com). For diffuse processes, the site of biopsy does not affect histology; a single biopsy adequately represents the histologic characteristics as a whole [10–12]. Biopsy material should be sufficient to eliminate artifacts due to sampling of the capsule and should contain liver tissue beyond the 0.5-cm subcapsular region in order to better represent the extent of fibrosis. When mass lesions are sampled, a separate biopsy of the remaining liver is recommended for evaluation of chronic liver disease or other potential contraindications to transplantation. The biopsy should be submitted unfixed. Gauze or saline should not be used as they can induce artifacts that may lead to incorrect biopsy interpretation.
Pathologic work-up
Because of the limited period of time between organ retrieval and transplantation histopathologic evaluation is typically performed on frozen section specimens using hematoxylin-eosin staining. Few centers optionally use additional stainings to evaluate steatosis (Sudan–III staining, toluidine blue staining, or oil red O staining) [13]. These special stainings are time-consuming and depend on technical expertise, which may not be available during night hours.
The biopsy tissue is best processed immediately at the institution where organ donation is performed, but respective service is provided in most hospitals only during regular working hours. Only few pathology centers in Europe provide a 24/7 frozen section service for evaluation of donor organs. Hence, outside regular working hours, the biopsy needs to be transported to such a center. During transportation, the biopsy should be kept cooled.
The frozen section material should be fixed afterwards, embedded in paraffin, and several sections should be cut. The stainings routinely applied to liver biopsies vary according to local protocols, but should include hematoxylin-eosin (H&E), a staining for connective tissue (e.g., modified Gomori’s staining) and an iron staining (e.g., Prussian blue) [14]. Also, periodic acid-Schiff staining with or without diastase digestion (D-PAS or PAS) is helpful, e.g., for improved definition of steatosis and biliary disease.
Histopathological reports should provide information about the extent of steatosis, as well as the extent of fibrosis, inflammation, necrosis, and any other relevant finding [15]. Steatosis can be microvesicular or macrovesicular. Macrovesicular steatosis is histologically defined as a single fat vacuole within an individual hepatocyte that displaces the nucleus to the periphery. Microvesicular steatosis is characterized by a cytoplasmatic accumulation of one to several small vacuoli that are smaller than the nucleus [16]. Macrovesicular steatosis often occurs simultaneously with microvesicular steatosis. Macrovesicular steatosis is traditionally classified as mild (<30 %), moderate (30–60 %), or severe (>60 %) including the percentage of fat content in the histopathological report (best by 10 % steps). Macrovesicular steatosis is most often centrilobular but can become panlobular in severe steatosis.
Pathologists traditionally evaluate steatosis using the percentage of hepatocytes containing lipid droplets, yet they may also estimate the percentage of parenchyma with macrovesicular fat determined in an integral manner. The latter method is reasonable to use for pre-transplant histology, as establishing the border between hepatocytes is difficult and time-consuming in frozen section material. Furthermore, the extent of macrovesicular steatosis, which is important for transplantability, is about the same assessed by both methods.
Surgical evaluation of donor livers
In deceased donors, neither biochemical nor clinical parameters correlate well with the degree of steatosis [17]. For surgeons, the most commonly used methods to establish liver quality is visual inspection and palpation at organ procurement. It is a well-known practical experience that there can be a discrepancy between the surgical assessment of fat content in donor livers and the histological results, but only few publications have addressed this issue [9, 13, 18]. Liver color and morphological degree of steatosis do not necessarily correlate; yellowish color changes lead to overestimation of the fat content [19]. In a study with 36 donor livers, a significant number of potential donor organs (n = 6, 16.7 %) that were discarded might have been used for transplantation according to histological criteria. Another study [9] showed that in situations where pathology service was not available for biopsy evaluation, the clinical judgment of experienced liver surgeons differed substantially from the final histological analysis performed after liver was discarded. In nine discarded organs (6 because of extreme steatosis and 3 because of cirrhosis), the clinical diagnosis of the procurement surgeon matched the pathologist’s evaluation only in one single case. Eight organs could have been transplanted. Even three cases of suspected cirrhosis were not confirmed histologically. As a consequence, the authors claim for intraoperative biopsies in all cases of liver donation. However, realistically, a pathology service is not available at all times in all places. Therefore, it has been suggested to improve access to histopathology by using a digital system [20]. The introduction of whole slide imaging scanners in retrieval hospitals would allow establishing a national or international network of on-call pathologists, reducing the costs for local on-call services. Some authors discuss if the pathologist’s assessment of donor liver steatosis could be replaced by automated software [3].
To avoid frozen section analysis outside normal working hours, some centers performed percutaneous pre-recovery liver biopsies to evaluate brain-dead donors with an increased risk for liver pathology. In the studies reported, the authors emphasize that the use of bedside percutaneous liver biopsies can help to avoid futile recovery and unnecessary surgical expenses but is reasonable only in cases where just liver donation is intended [21, 22]. However, in some cases of these studies, livers appeared histologically normal by bedside percutaneous liver biopsies but were deemed unsuitable when surgical recovery was begun (false negative). This may partly be due to sampling error because of the small size of the needle biopsy.
Quality of frozen section histology
An open wedge biopsy from deceased liver donors and frozen section evaluation is the usual method to provide information about the graft quality and has always been considered the gold standard. Studies that have compared frozen section analysis with corresponding slides of the formalin fixed, paraffin-embedded (FFPE) tissues have shown an excellent agreement for macrovesicular, microvesicular, and overall steatosis [23, 24, 2]. The concordance is especially high in cases with severe macrovesicular steatosis (>60 %). In livers with less fat, mild macrovesicular steatosis can sometimes (7.7 %) be overestimated [25]. The rate of overestimation for mild microvesicular steatosis in frozen sections is higher (8.2 %), possibly due to technical artifacts of water droplets trapped during the freezing procedure. However, this does not lead to exclusion of suitable organs from donation as microvesicular steatosis is generally not used as a criterion for exclusion.
Regarding inter-observer variability in quantitative and qualitative assessment of steatosis, some authors have demonstrated a high degree of congruency between different pathologists on frozen sections for pre-transplant diagnosis [25]. Other authors questioned the use of histopathological examinations for fat quantification due to poor agreement among pathologists and called for a computerized system [26]. It is noteworthy that this controversially debated study was not performed in the context of pre-transplant biopsies on frozen sections but on routine histology performed on any type of liver resection and included many cases with low rates of steatosis. Automated fat content calculated the surface area covered by fat, without distinguishing between macro- and microvesicular steatosis, which does not relate to the algorithms used for assessment of fat in pre-transplant livers.
Steatosis
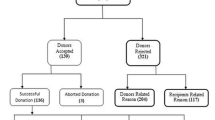
The presence of steatosis in pre-transplant liver biopsies is common and is detected with increasing frequency in up to ¾ of cases [27–30]. This is not surprising, as liver biopsies are commonly requested from higher risk donors (Fig. 2). The effects of steatosis after liver transplantation are not completely understood but are thought to be associated with greater susceptibility to ischemia and other transplant associated phenomena [31].
Typical biopsy from an extended criteria donor. a, b: Frozen section histology of wedge biopsy, H&E staining (a overview, b higher magnification) showing moderate macrovesicular steatosis (arrowhead), admixed with microvesicular steatosis (arrow). c, d: H&E (c) and modified Gomori’s staining (d) of FFPE tissue. Note centrilobular accumulation of fat droplets and portal fibrosis
Although hepatic steatosis is a widely accepted risk factor for early graft dysfunction and failure in deceased as well as in living donor liver transplantation [32, 33, 5], studies have been inconsistent regarding the relevant amount of fat or type of fat vacuoles necessary to be of harm. The information is of importance to safely define the donor pool.
The weakness of some of the studies is that only the amount of fat was evaluated, without subclassifying macro- and microvesicular steatosis and without evaluating the relation between macro- and microvesicular steatosis in the mixed steatosis livers.
The largest study that analyzed macrovesicular and microvesicular steatosis separately as a possible factor for increased liver graft loss within 1 year after transplantation used the data from the Scientific Registry of Transplant Recipients [34]. Of 21.777 adult liver transplants, 5.051 donor livers were biopsied. Biopsy itself introduced a bias as donors with biopsied livers had a higher Donor Risk Index, were older, and more obese. It could be shown that in this high risk donor group, microvesicular steatosis was not a risk factor for graft loss, whereas macrovesicular steatosis >30 % was an independent risk factor. Due to low number, severe macrovesicular steatosis (>60 %) could not be evaluated in this study. In another study, the impact of severe macrovesicular steatosis on allograft survival appeared greater than other donor factors, including the calculated Donor Risk Index [29].
Frequently extended criteria donors are accepted to expand the donor pool [35–37]. Some authors reported that without other additional risk factors, it was safe to use donor livers with >30 % but <60 % macrovesicular steatosis [38]. Therefore, in general, livers with >60 % macrovesicular steatosis are considered not safe to use [32, 39]. Studies that report the use of highly steatotic livers did not differentiate between macro- and microvesicular steatosis [40] or used mainly livers with high steatosis due to the high proportion of microvesicular steatosis [32]. Livers with severe microvesicular steatosis were reported to be safe to use [41]. Although associated with a delay in postoperative hepatic function, even high microvesicular steatosis does not affect outcome (Fig. 3). In the deceased, especially high-risk donor population, pure microvesicular steatosis is rare, but in living donor transplantation, pure microvesicular steatosis does not seem to impair post-transplant outcomes [42].
Histopathological report
Pre-transplant biopsies are not only performed to determine the degree of steatosis, but also to identify pathologic findings, which are considered as absolute or relative contraindications to transplantation, e.g., marked steatohepatitis, severe fibrosis, and severe necrosis or malignancy.
Steatohepatitis should be diagnosed if in addition to steatosis ballooning of hepatocytes and lobular inflammation, associated with necrosis of steatotic hepatocytes, are present. Surprisingly, in most of the studies reporting even high degrees of steatosis, the presence of steatohepatitis was not reported. So, it remains unclear, if the negative outcome in severely steatotic organs was due to the amount of fat or additional necro-inflammation.
Hepatitis should not be diagnosed in donor biopsies with mild portal inflammation as this may represent a frequent finding after prolonged intensive care unit treatment; it does not seem to have predictive value for the outcome after liver transplantation [23]. To rule out viral hepatitis in donors, serological tests have to be preferred.
If inflammation of the bile ducts is present, the quality and extent of cholangitis should be reported. This is not a frequent finding in donor biopsies and usually suspected according to laboratory results.
Fibrosis plays a role in long-term graft survival, especially in patients with hepatitis B or C, who receive organs from hepatitis B- or C-positive donors. The extent of fibrosis in the donor biopsy should be reported according to scoring systems established for chronic hepatitis [43–46] as portal fibrosis, portal fibrosis with rare septum formation, numerous septa without obvious cirrhosis, and cirrhosis. Livers with significant septal fibrosis are generally considered unsuitable for transplantation. Evaluation of fibrosis is a difficult task for the procurement surgeon and often results in underestimation of fibrosis, especially in the setting of multiple extended donor criteria and HCV-positive donor serology [2]. Also, pathologists may underestimate fibrosis on frozen sections compared to sections of FFPE tissue, as special stainings for fibrosis are not available for frozen sections [2]. However, higher grades of fibrosis, which will lead to organ exclusion, can be safely identified. It has to be pointed out that the extend of fibrosis directly (0.5 cm) subcapsular may not be representative for the whole organ, as portal tracts in this location are often more expanded; thus, small biopsy may also lead to overestimation of fibrosis.
Necrosis, especially centrilobular and subcapsular, may be evident in frozen sections if the liver has undergone ischemia prior to biopsy. Focal subcapsular necrosis is seen frequently and should be ignored if the deeper parts of the biopsy do not show necrosis. There is no consensus regarding the amount of necrosis that is acceptable; however, a cutoff of 10 % diffuse necrosis (ignoring focal subcapsular necrosis) has been suggested [3].
A pigment often found in pre-transplant biopsies, especially from older donors, is lipofuscin. This granular brown pigment, typically located in centrilobular hepatocytes, is a product of fatty acid oxidation and associated with aging and certain diseases. Lipofuscin storage has no predictive value for the outcome after liver transplantation [23].
Malignancies found in the liver are a contraindication for using the organ. On frozen sections, haematopoetic malignancies may be difficult to definitively diagnose, so in case such a malignancy is suspected, the pathologist should be informed. Suspicion should lead to discard.
Conclusion
Not only in cases of suspected malignancies, but in all cases where donor biopsies are performed, excellent communication between pathologists, surgeons, and all the other members of the transplant team is essential to optimize decision-making in pre-transplant livers. Several studies have shown the inaccuracy of macroscopic evaluation of donor livers even when performed by experienced surgeons. Frozen sections are of value in the selection of donor livers for transplantation, especially in case of extended donor criteria, and should be performed more frequently in order to avoid unnecessary loss of potentially suitable organs and to prevent transplantation of inappropriate organs.
Steatosis is a major factor in quality assessment, as steatosis is a widely accepted risk factor for postoperative complications in liver transplantation. There is no clear cutoff regarding the amount of fat that should lead to discarding a donor organ. In general, livers with >60 % macrovesicular steatosis are considered inappropriate for transplantation, while donor livers with >30 % but <60 % macrovesicular steatosis could be used in cases with no other additional risk factors. Livers with <30 % macrovesicular steatosis are in general considered safe to use. Microvesicular steatosis does not seem to impair post-transplant outcomes.
Further criteria to exclude an organ from transplantation are significant septal fibrosis, severe inflammation, diffuse necrosis (>10 %), or malignancies.
Pre-transplant biopsies are not mandatory in the transplantation process and until now, steatosis or other histologic changes are not included in the large transplantation database such as the European Liver Transplant Registry (ELTR) and the United Network for Organ Sharing (UNOS). Adoption and implementation of standardized pathologic reports for frozen section evaluation should be considered in order to provide conclusive data for future guidelines.
References
Adam R, Reynes M, Johann M, Morino M, Astarcioglu I, Kafetzis I, Castaing D, Bismuth H (1991) The outcome of steatotic grafts in liver transplantation. Transplant Proc 23(1 Pt 2):1538–1540
Lo IJ, Lefkowitch JH, Feirt N, Alkofer B, Kin C, Samstein B, Guarrera JV, Renz JF (2008) Utility of liver allograft biopsy obtained at procurement. Liver Transplant: Off Publ Am Assoc Stud Liver Dis Int Liver Transplant Soc 14(5):639–646. doi:10.1002/lt.21419
Marsman WA, Wiesner RH, Rodriguez L, Batts KP, Porayko MK, Hay JE, Gores GJ, Krom RA (1996) Use of fatty donor liver is associated with diminished early patient and graft survival. Transplantation 62(9):1246–1251
Ploeg RJ, D'Alessandro AM, Knechtle SJ, Stegall MD, Pirsch JD, Hoffmann RM, Sasaki T, Sollinger HW, Belzer FO, Kalayoglu M (1993) Risk factors for primary dysfunction after liver transplantation—a multivariate analysis. Transplantation 55(4):807–813
Strasberg SM, Howard TK, Molmenti EP, Hertl M (1994) Selecting the donor liver: risk factors for poor function after orthotopic liver transplantation. Hepatology 20(4 Pt 1):829–838
Yoong KF, Gunson BK, Neil DA, Mirza DF, Mayer AD, Buckels JA, McMaster P (1999) Impact of donor liver microvesicular steatosis on the outcome of liver retransplantation. Transplant Proc 31(1–2):550–551
Zamboni F, Franchello A, David E, Rocca G, Ricchiuti A, Lavezzo B, Rizzetto M, Salizzoni M (2001) Effect of macrovescicular steatosis and other donor and recipient characteristics on the outcome of liver transplantation. Clin Transplant 15(1):53–57
Markin RS, Wisecarver JL, Radio SJ, Stratta RJ, Langnas AN, Hirst K, Shaw BW Jr (1993) Frozen section evaluation of donor livers before transplantation. Transplantation 56(6):1403–1409
Dominguez Fernandez E, Schmid M, Bittinger F, Mauer D (2007) Intraoperative assessment of liver organ condition by the procurement surgeon. Transplant Proc 39(5):1485–1487. doi:10.1016/j.transproceed.2007.01.076
Baunsgaard P, Sanchez GC, Lundborg CJ (1979) The variation of pathological changes in the liver evaluated by double biopsies. Acta Pathol Microbiol Scand Section A, Pathol 87(1):51–57
Frankel WL, Tranovich JG, Salter L, Bumgardner G, Baker P (2002) The optimal number of donor biopsy sites to evaluate liver histology for transplantation. Liver Transplant: Off Publ Am Assoc Stud Liver Dis Int Liver Transpl Soc 8(11):1044–1050. doi:10.1053/jlts.2002.36492
Picciotto A, Ciravegna G, Lapertosa G, Celle G (1983) One or two liver biopsies during laparoscopy? Hepatogastroenterology 30(5):192–193
Reis H, Peterek PT, Wohlschlaeger J, Kaiser GM, Mathe Z, Juntermanns B, Sotiropoulos GC, Beckhove U, Canbay A, Wirges U, Scherag A, Treckmann JW, Paul A, Baba HA (2014) Oil Red O-assessed macrosteatosis in liver transplant donor biopsies predicts ischemia-reperfusion injury and clinical outcome. Virchows Arch: Int J Pathol 464(2):165–174. doi:10.1007/s00428-013-1512-3
Mogler C, Flechtenmacher C, Schirmacher P, Bergmann F (2012) Frozen section diagnostics in visceral surgery. Liver, bile ducts and pancreas. Der Pathologe 33(5):413–423. doi:10.1007/s00292-012-1602-z
Melin C, Miick R, Young NA, Ortiz J, Balasubramanian M (2013) Approach to intraoperative consultation for donor liver biopsies. Arch Pathol Lab Med 137(2):270–274. doi:10.5858/arpa.2011-0689-RA
Crowley H, Lewis WD, Gordon F, Jenkins R, Khettry U (2000) Steatosis in donor and transplant liver biopsies. Hum Pathol 31(10):1209–1213. doi:10.1053/hupa.2000.18473
Adani GL, Baccarani U, Sainz-Barriga M, Lorenzin D, Bresadola V, Risaliti A, Avellini C, Trevisan G, De Candia A, Bresadola F (2006) The role of hepatic biopsy to detect macrovacuolar steatosis during liver procurement. Transplant Proc 38(5):1404–1406. doi:10.1016/j.transproceed.2006.02.111
Yersiz H, Lee C, Kaldas FM, Hong JC, Rana A, Schnickel GT, Wertheim JA, Zarrinpar A, Agopian VG, Gornbein J, Naini BV, Lassman CR, Busuttil RW, Petrowsky H (2013) Assessment of hepatic steatosis by transplant surgeon and expert pathologist: a prospective, double-blind evaluation of 201 donor livers. Liver Transplant: Off Publ Am Assoc Stud Liver Dis Int Liver Transpl Soc 19(4):437–449. doi:10.1002/lt.23615
Rey JW, Wirges U, Dienes HP, Fries JW (2009) Hepatic steatosis in organ donors: disparity between surgery and histology? Transplant Proc 41(6):2557–2560. doi:10.1016/j.transproceed.2009.06.121
Neil DA, Roberts IS, Bellamy CO, Wigmore SJ, Neuberger JM (2014) Improved access to histopathology using a digital system could increase the organ donor pool and improve allocation. Transplant Int: Off J Eur Soc Organ Transplant 27(8):759–764. doi:10.1111/tri.12320
Ganz S, Abdullah K, Gedaly R, Henry S, Cravero L, Olson L, Kato T, Miller J, Tzakis A (2001) Use of percutaneous liver biopsies in marginal liver donors. Transplant Proc 33(1–2):1509–1511
Oliver JB, Peters S, Bongu A, Beidas AK, Dikdan G, Brown L, Koneru B (2014) Prerecovery liver biopsy in the brain-dead donor: a case-control study of logistics, safety, precision, and utility. Liver Transplant: Off Publ Am Assoc Study Liver Dis Int Liver Transplant Soc 20(2):237–244. doi:10.1002/lt.23786
Fiorentino M, Vasuri F, Ravaioli M, Ridolfi L, Grigioni WF, Pinna AD, D'Errico-Grigioni A (2009) Predictive value of frozen-section analysis in the histological assessment of steatosis before liver transplantation. Liver Transplant: Off Publ Am Assoc Stud Liver Dis Int Liver Transplant Soc 15(12):1821–1825. doi:10.1002/lt.21948
Heller B, Peters S (2011) Assessment of liver transplant donor biopsies for steatosis using frozen section: accuracy and possible impact on transplantation. J Clin Med Res 3(4):191–194. doi:10.4021/jocmr629w
D'Alessandro E, Calabrese F, Gringeri E, Valente M (2010) Frozen-section diagnosis in donor livers: error rate estimation of steatosis degree. Transplant Proc 42(6):2226–2228. doi:10.1016/j.transproceed.2010.05.033
El-Badry AM, Breitenstein S, Jochum W, Washington K, Paradis V, Rubbia-Brandt L, Puhan MA, Slankamenac K, Graf R, Clavien PA (2009) Assessment of hepatic steatosis by expert pathologists: the end of a gold standard. Ann Surg 250(5):691–697. doi:10.1097/SLA.0b013e3181bcd6dd
Busuttil RW, Tanaka K (2003) The utility of marginal donors in liver transplantation. Liver Transpl: Off Publ Am Assoc Stud Liver Dis Int Liver Transplant Soc 9(7):651–663. doi:10.1053/jlts.2003.50105
D'Alessandro AM, Kalayoglu M, Sollinger HW, Hoffmann RM, Reed A, Knechtle SJ, Pirsch JD, Hafez GR, Lorentzen D, Belzer FO (1991) The predictive value of donor liver biopsies on the development of primary nonfunction after orthotopic liver transplantation. Transplant Proc 23(1 Pt 2):1536–1537
de Graaf EL, Kench J, Dilworth P, Shackel NA, Strasser SI, Joseph D, Pleass H, Crawford M, McCaughan GW, Verran DJ (2012) Grade of deceased donor liver macrovesicular steatosis impacts graft and recipient outcomes more than the Donor Risk Index. J Gastroenterol Hepatol 27(3):540–546. doi:10.1111/j.1440-1746.2011.06844.x
Loinaz C, Gonzalez EM (2000) Marginal donors in liver transplantation. Hepatogastroenterology 47(31):256–263
Adam R, Sanchez C, Astarcioglu I, Bismuth H (1995) Deleterious effect of extended cold ischemia time on the posttransplant outcome of aged livers. Transplant Proc 27(1):1181–1183
McCormack L, Petrowsky H, Jochum W, Mullhaupt B, Weber M, Clavien PA (2007) Use of severely steatotic grafts in liver transplantation: a matched case-control study. Ann Surg 246(6):940–946. doi:10.1097/SLA.0b013e31815c2a3f, discussion 946-948
Soejima Y, Shimada M, Suehiro T, Kishikawa K, Yoshizumi T, Hashimoto K, Minagawa R, Hiroshige S, Terashi T, Ninomiya M, Shiotani S, Harada N, Sugimachi K (2003) Use of steatotic graft in living-donor liver transplantation. Transplantation 76(2):344–348. doi:10.1097/01.TP.0000071205.52835.A4
Spitzer AL, Lao OB, Dick AA, Bakthavatsalam R, Halldorson JB, Yeh MM, Upton MP, Reyes JD, Perkins JD (2010) The biopsied donor liver: incorporating macrosteatosis into high-risk donor assessment. Liver Transplant: Off Publ Am Assoc Stud Liver Dis Int Liver Transplant Soc 16(7):874–884. doi:10.1002/lt.22085
Schemmer P, Nickkholgh A, Hinz U, Gerling T, Mehrabi A, Sauer P, Encke J, Friess H, Weitz J, Buchler MW, Schmidt J (2007) Extended donor criteria have no negative impact on early outcome after liver transplantation: a single-center multivariate analysis. Transplant Proc 39(2):529–534. doi:10.1016/j.transproceed.2006.12.002
Sotiropoulos GC, Paul A, Gerling T, Molmenti EP, Nadalin S, Napieralski BP, Treckmann J, Lang H, Saner F, Frilling A, Broelsch CE, Malago M (2006) Liver transplantation with "rescue organ offers" within the eurotransplant area: a 2-year report from the University Hospital Essen. Transplantation 82(3):304–309. doi:10.1097/01.tp.0000229447.37333.ed
Schemmer P, Nickkholgh A, Gerling T, Weitz J, Buchler MW, Schmidt J (2009) Rescue allocation for liver transplantation within Eurotransplant: the Heidelberg experience. Clin Transplant 23(Suppl 21):42–48. doi:10.1111/j.1399-0012.2009.01109.x
Frongillo F, Avolio AW, Nure E, Mule A, Pepe G, Magalini SC, Agnes S (2009) Quantification of degree of steatosis in extended criteria donor grafts with standardized histologic techniques: implications for graft survival. Transplant Proc 41(4):1268–1272. doi:10.1016/j.transproceed.2009.03.096
Urena MA, Moreno Gonzalez E, Romero CJ, Ruiz-Delgado FC, Moreno Sanz C (1999) An approach to the rational use of steatotic donor livers in liver transplantation. Hepatogastroenterology 46(26):1164–1173
Chavin KD, Taber DJ, Norcross M, Pilch NA, Crego H, McGillicuddy JW, Bratton CF, Lin A, Baliga PK (2013) Safe use of highly steatotic livers by utilizing a donor/recipient clinical algorithm. Clin Transplant 27(5):732–741. doi:10.1111/ctr.12211
Fishbein TM, Fiel MI, Emre S, Cubukcu O, Guy SR, Schwartz ME, Miller CM, Sheiner PA (1997) Use of livers with microvesicular fat safely expands the donor pool. Transplantation 64(2):248–251
Han S, Ko JS, Kwon G, Park C, Lee S, Kim J, Kim G, Kwon CD, Gwak M, Ha S (2014) Effect of pure microsteatosis on transplant outcomes after living donor liver transplantation: a matched case-control study. Liver Transpl: Off Publ Am Assoc Stud Liver Dis Int Liver Transplant Soc 20(4):473–482. doi:10.1002/lt.23824
Batts KP, Ludwig J (1995) Chronic hepatitis. An update on terminology and reporting. Am J Surg Pathol 19(12):1409–1417
Bedossa P, Poynard T (1996) An algorithm for the grading of activity in chronic hepatitis C. The METAVIR Cooperative Study Group. Hepatology 24(2):289–293. doi:10.1002/hep.510240201
Desmet VJ, Gerber M, Hoofnagle JH, Manns M, Scheuer PJ (1994) Classification of chronic hepatitis: diagnosis, grading and staging. Hepatology 19(6):1513–1520
Ishak K, Baptista A, Bianchi L, Callea F, De Groote J, Gudat F, Denk H, Desmet V, Korb G, MacSween RN et al (1995) Histological grading and staging of chronic hepatitis. J Hepatol 22(6):696–699
Conflicts of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Flechtenmacher, C., Schirmacher, P. & Schemmer, P. Donor liver histology—a valuable tool in graft selection. Langenbecks Arch Surg 400, 551–557 (2015). https://doi.org/10.1007/s00423-015-1298-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00423-015-1298-7