Abstract
Although not all centres still agree, histological evaluation of liver graft quality is crucial for the short-term outcome of orthotopic liver transplantation (OLT). Pre-transplant and “time zero” biopsies are useful for the assessment of macrovesicular steatosis, i.e. the histological alteration universally accepted as the major cause of early graft failure. Donor biopsies also provide information on graft preservation, fibrosis, and the occurrence of disregarded hepatitis or focal lesions.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Primary Sclerosing Cholangitis
- Orthotopic Liver Transplantation
- Chronic Rejection
- Acute Cellular Rejection
- Recurrent Hepatitis
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
1 Pre-transplant Evaluation of the Donor Liver
Although not all centres still agree, histological evaluation of liver graft quality is crucial for the short-term outcome of orthotopic liver transplantation (OLT). Pre-transplant and “time zero” biopsies are useful for the assessment of macrovesicular steatosis, i.e. the histological alteration universally accepted as the major cause of early graft failure. Donor biopsies also provide information on graft preservation, fibrosis, and the occurrence of disregarded hepatitis or focal lesions.
The material sent to the pathologist should always include a wedge resection and a core biopsy. The final diagnosis should be made on the balance between them: the wedge resection includes more portal tracts, but the subcapsular localization increases the fibrosis and the inflammatory infiltrate [1]. Frozen-section analysis is considered the approach of choice for the evaluation of liver biopsies from cadaveric donors, both on economic and time-effective grounds [2]. Freezing the samples increases the risk of overestimating microvesicular steatosis due to the formation of intracellular droplets of frozen water. Nonetheless, the microsteatosis score is generally not used as a donor exclusion criterion, and this artifact can be ruled out by avoiding excessive soaking in saline solution or other media and transporting the biopsy in dry gauze or empty vials [1].
Suggested checklist for histopathological evaluation of the donor liver:
-
Architecture and fibrosis assessment
-
Portal inflammatory infiltrate: grade of inflammation and extension (little/most portal tract)
-
Bile duct status, including biliocyte regression and ductular proliferation
-
Artery status, in particular the presence and amount of myointimal hypertrophy
-
Lobules: occurrence and amount of lobular necrosis and/or inflammatory infiltrate
-
Cholestasis
-
Percentage of macrovesicular and microvesicular steatosis
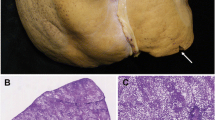
This checklist offers the transplant team a quick and complete overview of graft status. In any case, it should be kept in mind that only macrovesicular steatosis has a proven higher risk of graft failure, and macrovesicular steatosis >30 % is generally a contraindication to OLT [3, 4] (Fig. 11.1). Other findings such as lobular necrosis and diffuse fibrosis can influence the decision to proceed, although there are currently no standardized indications.
2 Graft Dysfunction
2.1 Preservation Injury
Also called “ischaemia/reperfusion injury”, preservation injury is the leading cause of early graft dysfunction and is defined as tissue damage occurring immediately after graft reperfusion, in the absence of other explicable causes of liver injury (e.g. vascular or biliary; see below).
The histopathological features of preservation injury depend on when the damage takes place: a warm ischaemia time of 120 min or more compromises both hepatocytes and endothelial cells, while a prolonged (usually more than 12 h) cold ischaemia is characterized by sinusoidal and endothelial damage, with a higher incidence of biliary complications [5, 6]. The reperfusion phase is the most crucial for the onset of liver preservation damage, since the reoxygenation after ischaemia causes activation of the Kupffer cells and complement factors, resulting in the production of reactive oxygen species and cytokines. The short-term consequences are granulocyte migration in the sinusoids and general vasoconstriction that can lead to graft circulatory failure [7].
Histopathology (Figs. 11.2 and 11.3)
(a) In some cases of ischaemia/reperfusion injury, architectural distortion, lobular haemorrhage with hepatocyte polymorphism and ballooning are visible. (b) Cholestasis and histiocytosis in zone 3 can be seen several weeks after OLT, associated with (c) portal tract distortion and bile duct regression. Magnification 20× (a); 40× (b, c)
Microscopically, mild preservation injuries are visible on biopsies taken 1 h after OLT as microvesicular steatosis with hepatocyte swelling, but these features are rapidly reversible. Conversely, the more severe forms of preservation injury are characterized microscopically by hepatocyte necrosis, especially in zone 3, with acidophilic bodies and/or zonal or confluent necrosis. The adjacent viable hepatocytes can show ballooning and mitotic activity. Bile duct degeneration is visible as a detachment of the biliocytes from the basement membrane and as a ductular reaction with ductular and lobular neutrophilic infiltrate [8, 9]. As a consequence, both intracellular and extracellular cholestases (with bile plugs) are common. Cholestasis, architectural lobular distortion, hepatocyte mitotic activity with nuclear polymorphism and ballooning, as well as histiocytosis in zone 3 can be seen several weeks after OLT.
Differential Diagnosis
In these early phases, the differential diagnosis includes acute infections, biliary complications, and antibody-mediated rejection. Cholangitis, bile duct obstruction, and reperfusion injury share predominant zone 3 damage, but cholangitis is commonly characterized by periductal oedema with neutrophilic granulocytes [9]. Interestingly, immunohistochemical positivity for C4d has been demonstrated in necrosis during reperfusion injury [10].
2.2 Hyperperfusion (“Small-for-Size” Syndrome)
This is a very early post-transplant complication (within 2 weeks), due to inadequate graft size (less than 0.8 % of the recipient’s weight) resulting in hyperdynamic portal flow. Allograft hyperperfusion is a critical condition that generally requires retransplantation.
Histopathology
The histopathological modifications of “small-for-size” syndrome are commonly sorted into early, intermediate and late changes [11]. Starting from a few minutes after reperfusion, denudation of portal veins and sinusoids can be seen, resulting in haemorrhages in the periportal zone (zone 1), all consequences of portal hyperperfusion. In the more severe cases, ischaemic bile duct damage with lobular infarcts can be seen [9]. Intermediate features include hypertrophic changes in the endothelia, oedema and fibrosis, while the late changes comprise thrombosis and obliteration of the portal veins with recanalization, together with nodular regenerative hyperplasia and biliary stenosis.
Differential Diagnosis
Arterial vascular complications (see below).
2.3 Vascular Complications
Post-OLT vascular complications include hepatic artery thrombosis, portal vein thrombosis and hepatic venous outflow obstruction (HVOO). Vascular complications represent a major technical problem after OLT and an important cause of liver damage and dysfunction in the first months after transplantation. Since liver biopsy is neither reliable nor safe in this setting, and the affected vascular structures are hilar and perihilar, histopathological examination is generally confined to the graft explant specimen [9].
Histopathology
At gross examination, the liver surface may be normal, while foci of cholestasis and/or necrosis can be seen in some cases. At histology, thrombosis of the hepatic artery leads to ischaemic necrosis of the bile ducts with epithelial denudation and bile leakage into the surrounding parenchyma. Lobular alterations, ranging from Councilman’s bodies to confluent hepatocytic necrosis, appear in the more severe and advanced cases. Prolonged arterial ischaemic injury can result in biliary strictures and fibrosis [12].
Histopathological examination of an allograft with portal vein thrombosis shows various degrees of parenchymal damage, from hepatocyte swelling and necrosis with haemorrhage and eventually thrombosis in the areas around the portal branches, to a pan-lobular necrosis of the allograft in cases with massive portal thrombosis. Chronic portal obstruction leads to nodular regenerative hyperplasia or similar changes [9].
HVOO can be due to problems at different levels, from congestive heart failure to Budd-Chiari syndrome and veno-occlusive disease affecting the portal venules or even sinusoids. The clinical presentations and the aetiologies of these conditions vary widely, but the histopathological picture is common. The first alterations affect the centrolobular zone (zone 3), with sinusoidal dilatation and centrolobular haemorrhage (in advanced cases), hepatocyte atrophy and loss of lobular architecture. Lobular or portal inflammation is lacking, and only the most severe cases show cholestasis. Chronicization (e.g. in Budd-Chiari syndrome, which is often subacute) leads to bridging fibrosis and the formation of hepatocellular nodules resembling focal nodular hyperplasia [12].
2.4 Large Bile Duct Obstruction
Strictures of the large bile ducts can be due to technical complications involving anastomoses, or other causes, including severe ischaemic and preservation injuries (see above) and chronic rejection with arteriopathy, among others.
Histopathology (Fig. 11.4)
A severe case of post-transplant biliary obstruction. (a) The lobule shows marked feathery degeneration of the hepatocytes and architectural distortion. (b, c) The portal tracts show inflammation with neutrophilic granulocytes, neoductulogenesis with bile plugs (black arrows) and intracellular cholestasis (red arrow). Magnification 10× (a); 20× (b); 40× (c)
Post-transplant biliary complications histologically resemble the biliary disease of the native liver. Portal oedema and inflammation with neutrophilic granulocytes, resembling cholangitis, are common findings: the predominance of the neutrophilic infiltrate is very important in the differential diagnosis between post-OLT biliary complications and acute rejection. Cholestasis can be associated, while portal fibrosis and biliary cirrhosis represent the chronic evolution of this condition [12, 13].
3 Rejection
3.1 Acute Cellular Rejection
Acute cellular rejection (ACR) is defined as a predominantly lymphocytic inflammation of the graft due to an antigen mismatch between donor and recipient. The liver structures most commonly affected are the bile ducts and the vascular endothelia. Due to the high variability in onset and clinical symptoms, liver biopsy is mandatory in cases of suspect ACR.
Histopathology (Fig. 11.5)
(a) Acute cellular rejection (ACR) is characterized by a portal inflammatory infiltrate with portal expansion. (b) The inflammatory infiltrate is prevalently lymphocytic, although macrophages and granulocytes can be present. There is aggression of the vascular (black arrow) and biliary (red arrow) structures. (c) Endothelialitis is defined as the presence of inflammatory cells in the subendothelial layer of the graft vascular structures (in this case, a centrolobular vein). Magnification 20× (a); 40× (b, c)
The main histological features of ACR are portal inflammatory infiltrate, endothelialitis and bile duct aggression. The inflammatory infiltrate characterizing ACR is mainly portal and with a prevalent lymphocytic component, although macrophages and both neutrophilic and eosinophilic granulocytes can be seen. The lymphocytes are CD8-positive and show the morphology of activated (or frankly blastic) T cells [14]. Lymphocyte-mediated bile duct injury is always present, with intraepithelial lymphocytes and associated bile duct regressive and reactive changes such as cytoplasmic eosinophilia and/or vacuolation, prominent nucleoli and occasional apoptosis [15]. Endothelialitis is defined as the presence of inflammatory cells in the subendothelial layer of the graft vascular (mainly venous) structures [16]. It is an important diagnostic feature in ACR, albeit not a specific finding since it can be detected in other pathological conditions (e.g. HCV recurrent hepatitis, infections) [17].
Differential Diagnosis
Recurrent and de novo viral hepatitis (HCV, HBV, CMV, EBV), drug hepatitis, lymphoproliferative disorders and other disorders with a predominant inflammatory component. Clinical onset, serological and clinical data are mandatory for the differential diagnosis. The presence of a plasma cell component requires the differential diagnosis from plasmacellular HCV recurrent hepatitis or post-OLT autoimmune hepatitis: the role of plasma cells in acute rejection is still debated [18] (Fig. 11.6).
A scarce (a) or more pronounced (b) plasma cell component, both in the portal tracts (a, b) and lobule (c) can be associated with rejection, recurrent viral hepatitis and de novo autoimmune hepatitis. The meaning of the plasma cell infiltrate after transplantation is controversial. Magnification 40×
Histopathological ACR is graded according to the 1997 Banff criteria as follows [14]:
Histopathological parameter | Description | Rejection activity index |
|---|---|---|
Portal inflammation | Mild/focal | 1 |
Diffuse, mixed with occasional blasts and granulocytes | 2 | |
Diffuse, mixed with numerous blasts and granulocytes, and interface activity | 3 | |
Bile duct involvement | Mild/focal inflammation | 1 |
Diffuse inflammation with epithelial injury to a few ducts | 2 | |
Diffuse inflammation of most bile ducts; biliary cells necrosis | 3 | |
Endothelialitis | Presence of subendothelial lymphocytes in a minority of portal vessels | 1 |
Presence of subendothelial lymphocytes in most portal and centrolobular vessels | 2 |
The absence of one or more criteria might lead to a diagnosis of indeterminate for rejection (Figs. 11.7 and 11.8), while according to these criteria a rejection activity index (RAI) of three to four represents mild ACR, a RAI of five to six moderate ACR, and a RAI greater than six severe ACR [14].
A histological picture with only one or two criteria for rejection poses the diagnosis of indeterminate for rejection according to Banff. In these cases there can be (a) a mild portal infiltrate possibly with bile duct aggression or (b) mild and focal endothelial aggression of centrolobular veins. Magnification 20×
3.2 Acute Antibody-Mediated Rejection
Antibody-mediated rejection (AMR), also called humoral rejection, is defined as liver injury mediated by pre-existing or de novo antibodies against graft antigens. The graft antigens most commonly involved are AB0 and other endothelial surface molecules or (more rarely) MHC class I on the graft lymphocytes. AMR can manifest after hours, especially in AB0-incompatible transplants with pre-existing recipient antibodies, or days after reperfusion: the terms hyperacute and acute AMR are used depending on the timing and severity of graft dysfunction onset. At gross examination, the graft is swollen, oedematous and flaccid, often with visible thrombosis of the major hepatic vessels.
Histopathology (Fig. 11.9)
AMR is primarily acute endothelial damage to virtually all liver vascular structures, which show fibrin deposition and granulocytic infiltrate. Accordingly, the main histopathological appearance is that of oedema and haemorrhage of the portal and periportal regions, with an inflammatory infiltrate predominantly composed of neutrophilic and eosinophilic granulocytes in the absence of lymphocytes. The most severe cases and all hyperacute cases present fibrin deposition in the sinusoids, portal and centrolobular veins, together with thrombosis. The lobular hepatocytes show necrosis and ballooning of variable degrees and extension [19].
As in the renal AMR, the antibody-antigen complexes activate the complement cascade, which mediates the endothelial damage. Accordingly, C4d positivity in the liver graft can also be detected by immunohistochemistry and immunofluorescence, while positivity in the portal tracts is considered of diagnostic help in cases of AMR. However, the usefulness of C4d is not as well established in the liver graft as it is in kidney transplantation [14, 20, 21] (Fig. 11.10).
(a) A case of antibody-mediated rejection (AMR) with specific immunohistochemical positivity for C4d in the endothelia (arrows): this kind of positivity is considered diagnostic for AMR (see arrows). (b) Another case with sinusoidal C4d positivity, considered not diagnostic for AMR. Magnification 40×
3.3 Chronic Rejection
Chronic rejection is defined as a progressive loss of graft bile ducts commonly resistant to immunosuppression. Although its incidence is low, chronic rejection represents a major cause of graft loss and retransplantation in the long term. It is characterized by a progressive loss of the bile ducts, without a significant inflammatory infiltrate, and by a post-transplant arteriopathy of the large arteries.
Histopathology (Fig. 11.11)
Although a lymphocytic bile duct infiltrate can be visible in the first phases of chronic rejection, the main histopathological feature is the loss of at least 50 % of the portal bile ducts, without direct inflammatory aggression and without ductular proliferation, possibly associated with “dysplastic-like” cell alterations [22]. Cholestasis is a common feature. Since bile duct loss is gradual, involving most of the portal tracts in the long term, it is even recommended that the graft biopsy contain no fewer than 20 portal tracts for a diagnosis of chronic rejection.
Graft arteriopathy represents another feature of chronic rejection: it begins with a subintimal infiltration by foam cells, activation of the endothelial cells and narrowing of the lumens. With time, a fibrotic obliteration of the hepatic arteries is visible, resulting in hepatocytic regression and necrosis of zone 3 [23].
Differential Diagnosis
“Vanishing bile duct syndrome” and recurrent primary sclerosing cholangitis. Portal tract expansion is normally present in cholangitis, together with regressive changes in the biliary epithelium. Primary sclerosing cholangitis as the primitive disease, immunosuppression status and the timing of onset help in the differential diagnosis [9, 24].
4 Recurrence of Primitive Disease
4.1 Recurrent Hepatitis C
End-stage liver disease due to chronic hepatitis C (HCV) is the principal indication for OLT in the Western world. Reinfection after OLT is universal, and up to 70 % of graft recipients experience recurrent HCV.
Histopathology (Figs. 11.12 and 11.13)
Recurrent HCV has recently been divided into three histopathological variants: the “usual” form (acute and chronic), fibrosing cholestatic HCV (FCH) and plasma cell-rich recurrent hepatitis [25, 26].
The “usual” acute recurrent HCV shows lobular damage, similar to acute HCV in the native liver, with lobular disarray, numerous Councilman bodies and spotty necrosis with Kupffer cell activation. When present, portal tract involvement is usually very mild. In the chronic phases, a portal lymphocytic infiltrate with interface activity becomes evident, and the lobular activity (Councilman bodies in particular) depends on direct viral replication in the chronic and acute phases. Recurrent HCV can sometimes overlap post-surgical damage or ACR hampering the diagnosis as some morphological features of recurrent HCV, such as endothelialitis and bile duct damage, mimic other graft diseases (Fig. 11.14). HCV RNA quantitation by means of RT-PCR should always be considered in HCV-positive recipients, as it has a good diagnostic and prognostic value in the early post-transplant phases [27–29].
FCH is a peculiar highly aggressive presentation of recurrent HCV, characterized by early onset (within 1 year), rapid fibrosis progression and a poor response to antiviral therapies. Overimmunosuppressed recipients are at higher risk. At liver biopsy, FCH shows hepatocyte swelling and ballooning, spotty necrosis with Councilman bodies, cholestasis with ductular reaction and a scarce mixed portal infiltrate. Periportal fibrosis and cirrhosis are common in the late stages.
Plasma cell-rich recurrent HCV and other variants such as granulomatous recurrent HCV are rare and not well studied. Nevertheless, they should be known so as not to miss this differential diagnosis. They probably represent variants related to a “hyperimmune” status of the recipients or the immunosuppressive regimen (e.g. pegylated-interferon). As stated above, the role of plasma cells in recurrent HCV and acute rejection, and their meaning in possible de novo autoimmune damage, is still debated, making any differential diagnosis difficult (especially in overlap ACR-HCV recurrent hepatitis) [18].
4.2 Recurrent Hepatitis B
Compared to the last decades, the risk of a hepatitis B virus (HBV) re-infection after OLT has diminished due to advances in antiviral therapies and the possibility to transplant HBV-positive recipients with no dosable viraemia.
Histopathology
Early HBV proliferation in the graft is visible with immunohistochemistry (anti-HBcAg antibody) also without appreciable histological modifications. Up to 6 months after OLT, lobular hepatitis is seen characterized by spotty necrosis, Kupffer cell activation and “ground-glass” appearance of the hepatocytes [30]. The overall picture resembles acute HBV in native livers, but antiviral therapy and immunosuppression can modify the histopathology [9]. Indeed, portal involvement is generally mild. In the late phases (>6 months), recurrent HBV becomes chronic, and a lymphocytic portal inflammation with interface activity (“piecemeal necrosis”) completes the picture. Although the response to antiviral therapy is generally good, fibrosis progression can lead to end-stage liver graft.
4.3 Recurrent Autoimmune Hepatitis and Biliary Diseases
Chronic conditions such as autoimmune hepatitis (AH), primary biliary cirrhosis (PBC) and primary sclerosing cholangitis (PSC) are minor causes of end-stage liver and OLT. However, the recurrence rates of these diseases in the graft are very high (up to 50 % according to some series). Generally, these diseases show the same histopathological features in primitive livers and recurrent forms.
Histopathology
Like primitive AH, recurrent AH shows a prominent lymphocyte- and plasma cell-rich portal inflammation, with interface activity (“piecemeal necrosis”) of moderate/severe degree [31]. The biliary structures are often spared. Mononuclear inflammatory cells, especially plasma cells, are often visible in the lobules and in the central veins, posing the differential diagnosis with rejection.
Recurrent PBC is characterized by a lymphocytic aggression of the bile ducts with occasional non-necrotizing epithelioid granulomas. These changes are diagnostic of recurrent PBC, but they are focal and often require a large biopsy with many portal tracts to be appreciable [32]. The lobules show spotty inflammation, intrahepatocellular cholestasis, Mallory bodies and sometimes copper deposition. Immunohistochemistry for Keratin 7 highlights ductular proliferation and hepatocytic ductular metaplasia. Serum autoantibody quantitation is required for a definite diagnosis.
The histopathological picture of recurrent PSC is characterized by bile duct loss and ductular proliferation, together with an infiltrate of neutrophilic granulocytes, with portal fibrosis and “onion-skin” fibrosis seen in the long term [24]. The lobules show the same changes as recurrent PBC. Biliary stenosis places the differential diagnosis with post-surgical bile duct obstruction: the timing of onset, the presence of “onion-skin” fibrosis and biliary non-anastomotic stenosis are useful for the diagnosis.
5 Allograft Infections
5.1 Cytomegalovirus
Cytomegalovirus (CMV) graft infection has an incidence from 17 to 29 % according to the different series, and overimmunosuppression is the principal risk factor.
Histopathology (Fig. 11.15)
(a) The overall histological picture of post-transplant CMV hepatitis is not specific, with lobular spotty necrosis, centrolobular haemorrhage, mixed portal and lobular inflammatory infiltrate; (b) nuclear eosinophilic inclusions with a clear halo are considered diagnostic. (c, d) Diagnostic confirmation comes from the immunohistochemical positivity for CMV in scattered cells. Magnification 10× (a); 20× (c); 40× (b, d)
The diagnostic hallmark of CMV infection is represented by nuclear inclusions, typically eosinophilic with a clear halo, seen in virtually all graft cells, albeit endothelial cells are the most commonly affected. Sometimes these nuclear inclusions are associated with smaller basophilic cytoplasmic inclusions. Lobular spotty necrosis, centrolobular haemorrhage, mixed portal tract inflammatory infiltrate and (more rarely) microgranulomas and lobular microabscesses define the picture of CMV hepatitis [9, 12]. Apart from the nuclear inclusions, CMV graft infection does not show specific histopathological features and can sometimes overlap conditions such as rejection and other infections. In these cases, the immunohistochemistry with anti-CMV antibodies can be of use in the differential diagnosis.
5.2 Epstein Barr Virus
The incidence of Epstein Barr virus (EBV) hepatitis after OLT is difficult to evaluate since it depends on donor and recipient EBV positivity before transplant, immunosuppression and viral replication in the graft [9]. Moreover, apart from post-transplant lymphoproliferative disease (PTLD), which represents the most severe EBV complication, the histological picture of the EBV graft damage is very variable and often masked by overlapping conditions.
Histopathology
In the milder form, post-OLT EBV infection shows a mild portal and sinusoidal infiltrate of small lymphocytes, sometimes with slight nuclear atypia. A typical feature of EBV infection is the tendency of lymphocytes to line up within the sinusoids. A more severe form of EBV hepatitis is characterized by lobular spotty necrosis with Councilman bodies and mixed portal inflammatory infiltrate, containing large irregular lymphocytes and immunoblasts, and bile duct damage [33]. In situ hybridization for EBER is needed to confirm the diagnosis.
References
Fiorentino M, Vasuri F, Ravaioli M, Ridolfi L, Grigioni WF, Pinna AD, D’Errico-Grigioni A. Predictive value of frozen-section analysis in the histological assessment of steatosis before liver transplantation. Liver Transpl. 2009;15:1821–5.
Lo IJ, Lefkowitch JH, Feirt N, Alkofer B, Kin C, Samstein B, et al. Utility of liver allograft biopsy obtained at procurement. Liver Transpl. 2008;14:639–46.
Selzner M, Clavien PA. Fatty liver in liver transplantation and surgery. Semin Liver Dis. 2001;21:105–13.
Verran D, Kusyk T, Painter D, Fisher J, Koorey D, Strasser S, et al. Clinical experience gained from the use of 120 steatotic donor livers for orthotopic liver transplantation. Liver Transpl. 2003;9:500–5.
Kootstra G, Kievit J, Nederstigt A. Organ donors: heartbeating and non-heartbeating. World J Surg. 2002;26:181–4.
Selzner N, Rudiger H, Graf R, Clavien PA. Protective strategies against ischemic injury of the liver. Gastroenterology. 2003;125:917–36.
Kukan M, Haddad PS. Role of hepatocytes and bile duct cells in preservation-reperfusion injury of the liver grafts. Liver Transpl. 2001;7:381–400.
Kakizoe S, Yanaga K, Starzl TE, Demetris AJ. Evaluation of protocol before transplantation and after reperfusion biopsies from human orthotopic liver allografts: considerations of preservation and early immunological injury. Hepatology. 1990;11:932–41.
Adeyi O, Fischer SE, Guindi M. Liver allograft pathology: approach to interpretation of needle biopsies with clinicopathological correlation. J Clin Pathol. 2010;63:47–74.
Silva MA, Mirza DF, Murphy N, Richards DA, Reynolds GM, Wigmore SJ, Neil DA. Intrahepatic complement activation, sinusoidal endothelial injury, and lactic acidosis are associated with initial poor function of the liver after transplantation. Transplantation. 2008;85:718–25.
Demetris AJ, Kelly DM, Eghtesad B, Fontes P, Wallis Marsh J, Tom K, Tan HP, Shaw-Stiffel T, Boig L, Novelli P, Planinsic R, Fung JJ, Marcos A. Pathophysiologic observations and histopathologic recognition of the portal hyperperfusion or small-for-size syndrome. Am J Surg Pathol. 2006;30:986–93.
Odze R, Goldblum JR. Surgical pathology of the G.I. tract, liver, biliary tract, and pancreas. 2nd ed. Philadelphia: Elsevier; 2009.
Sanchez-Urdazpal L, Gores GJ, Ward EM, Maus TP, Buckel EG, Steers JL, Wiesner RH, Krom RA. Diagnostic features and clinical outcome of ischemic-type biliary complications after liver transplantation. Hepatology. 1993;17:605–9.
Aguilera I, Sousa JM, Gavilan F, et al. Complement component 4d immunostaining in liver allografts of patients with de novo immune hepatitis. Liver Transplant. 2011;17:779–88. Banff schema for grading liver allograft rejection: an international consensus document. Hepatology. 1997;25:658–63.
Scholz M, Auth MK, Markus BH. The immunological role of biliary epithelial cells in human liver transplant rejection. Transpl Immunol. 1997;5:142–51.
Ludwig J, Batts KP, Ploch M, Rakela J, Perkins JD, Wiesner RH. Endothelialitis in hepatic allografts. Mayo Clin Proc. 1989;64:545–54.
Yeh MM, Larson AM, Tung BY, Swanson PE, Upton MP. Endotheliitis in chronic viral hepatitis: a comparison with acute cellular rejection and non-alcoholic steatohepatitis. Am J Surg Pathol. 2006;30:727–33.
Demetris AJ, Sebagh M. Plasma cell hepatitis in liver allografts: variant of rejection or autoimmune hepatitis? Liver Transpl. 2008;14:750–5.
Demetris AJ, Jaffe R, Tsakis A, Ramsey G, Todo S, Belle S, Esquivel C, Shapiro R, Markus B, Mroczeck E, Van Thiel DH, Sysyn G, Gordon R, Makowka L, Starzl T. Antibody-mediated rejection of human orthotopic liver allografts. A study of liver transplantation across AB0 blood group barriers. Am J Pathol. 1988;132:489–502.
Haga H, Egawa H, Fujimoto Y, et al. acute humoral rejection and C4d immunostaining in AB0 blood type-incompatible liver transplantation. Liver Transpl. 2006;12:457–64.
Kozlowski T, Rubinas T, Nickeleit V, et al. Liver allograft antibody-mediated rejection with demonstration of sinusoidal C4d staining and circulating donor specific antibodies. Liver Transpl. 2011;17:357–68.
Hubscher SG. Transplantation pathology. Semin Liver Dis. 2009;29:74–90.
Nakazawa Y, Jonsson JR, Walker NI, et al. Fibrous obliterative lesions of veins contribute to progressive fibrosis in chronic liver allograft rejection. Hepatology. 2000;32:1240–7.
Demetris AJ. Distinguishing between recurrent primary sclerosing cholangitis and chronic rejection. Liver Transpl. 2006;12:S68–72.
Demetris AJ. Evolution of hepatitis C virus in liver allografts. Liver Transpl. 2009;15:S35–41.
Vasuri F, Malvi D, Gruppioni E, Grigioni WF, D’Errico-Grigioni A. Histopathological evaluation of recurrent hepatitis C after liver transplantation: a review. World J Gastroenterol. 2014;20:2810–24.
Gottschlich MJ, Aardema KL, Burd EM, Nakhleh RE, Brown KA, Abouljoud MS, Hirst K, Moonka DK. The use of hepatitis C viral RNA levels in liver tissue to distinguish rejection from recurrent hepatitis C. Liver Transpl. 2001;7:436–41.
D’Errico-Grigioni A, Fiorentino M, Vasuri F, Gruppioni E, Fabbrizio B, Zucchini N, Ballardini G, Morelli C, Pinna AD, Grigioni WF. Tissue hepatitis C virus RNA quantification and protein expression help identify early hepatitis C virus recurrence after liver transplantation. Liver Transpl. 2008;14:313–20.
Vasuri F, Morelli MC, Gruppioni E, Fiorentino M, Ercolani G, Cescon M, Pinna AD, Grigioni WF, D’Errico-Grigioni A. The meaning of tissue and serum HCV RNA quantitation in hepatitis C recurrence after liver transplantation: a retrospective study. Dig Liver Dis. 2013;45:505–9.
Thung SN. Histologic findings in recurrent HBV. Liver Transpl. 2006;12:S50–3.
Duclos-Vallee JC, Sebagh M, Rifai K, Johanet C, Ballott E, Guettier C, Karam V, Hurtova M, Feray C, Reynes M, Bismuth H, Samuel D. A 10 year follow up study of patients transplanted for autoimmune hepatitis: histological recurrence precedes clinical and biochemical recurrence. Gut. 2003;52:893–7.
Khettry U, Anand N, Faul PN, Lewis WD, Pomfret EA, Pomposelli J, Jenkins RL, Gordon FD. Liver transplantation from primary biliary cirrhosis: a long-term pathologic study. Liver Transpl. 2003;9:87–96.
Randawa PS, Markin RS, Starzl TE, Demetris AJ. Epstein-Barr virus-associated syndromes in immunosuppressed liver transplant recipient. Am J Surg Pathol. 1990;14:538–47.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2015 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Vasuri, F., Malvi, D., D’Errico, A. (2015). Liver. In: Pinna, A., Ercolani, G. (eds) Abdominal Solid Organ Transplantation. Springer, Cham. https://doi.org/10.1007/978-3-319-16997-2_11
Download citation
DOI: https://doi.org/10.1007/978-3-319-16997-2_11
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-16996-5
Online ISBN: 978-3-319-16997-2
eBook Packages: MedicineMedicine (R0)