Abstract
Background
Computer-assisted surgery is a wide field of technologies with the potential to enable the surgeon to improve efficiency and efficacy of diagnosis, treatment, and clinical management.
Purpose
This review provides an overview of the most important new technologies and their applications.
Methods
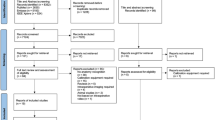
A MEDLINE database search was performed revealing a total of 1702 references. All references were considered for information on six main topics, namely image guidance and navigation, robot-assisted surgery, human-machine interface, surgical processes and clinical pathways, computer-assisted surgical training, and clinical decision support. Further references were obtained through cross-referencing the bibliography cited in each work. Based on their respective field of expertise, the authors chose 64 publications relevant for the purpose of this review.
Conclusion
Computer-assisted systems are increasingly used not only in experimental studies but also in clinical studies. Although computer-assisted abdominal surgery is still in its infancy, the number of studies is constantly increasing, and clinical studies start showing the benefits of computers used not only as tools of documentation and accounting but also for directly assisting surgeons during diagnosis and treatment of patients. Further developments in the field of clinical decision support even have the potential of causing a paradigm shift in how patients are diagnosed and treated.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Computer-assisted surgery (CAS) is a wide field of technologies with the potential to enable healthcare providers to improve efficiency and efficacy of diagnosis, treatment, and clinical management. Also, in abdominal surgery, CAS is widely adopted and increasingly used for different kinds of surgical assistance. This review will provide an overview of CAS especially in the field of laparoscopic minimally invasive surgery (MIS). It will present latest key technologies and exemplify whether and to what extent computer-assisted technologies impact present and future surgical therapy.
Literature search
A MEDLINE search based on the MeSH term in Table 1 was performed. Retrieved citations (n = 1702) were screened for inclusion. To limit retrieved publications, the search term was conducted for laparoscopic surgery assuming that a representative subset of publications would be retrieved. Further references were obtained through cross-referencing. Titles and abstracts of full papers were manually assessed for relevance by two authors (HK and FW) and presented to the other authors according to the topics image guidance and navigation in abdominal surgery, robot-assisted surgery, human-machine interface, surgical processes and clinical pathways, computer-assisted surgical training, surgical processes and clinical pathways, and clinical decision support. The authors then made decisions on presented publications upon their field of expertise based on abstract screening and full-text analysis. Based on this judgment, 64 articles were finally considered eligible to be included for the purposes of this review. To present an overview of the total number of publications on the topic, a statistical analysis on (the) annual and cumulative publication count with the term “computer-assisted surgery” was performed (see Fig. 1).
Definition of computer-assisted surgery
There is no single definition of CAS, and definitions vary widely since many different applications for computer assistance in surgery exist. Whereas CAS is often thought of as mere image guidance and navigation, the online encyclopedia Wikipedia refers to it more comprehensively as “computer-assisted […] to the use of a computer as an indispensable tool in a certain field, usually derived from more traditional fields of science and engineering” [1]. Thus, generally speaking, CAS entails a set of applications used at the surgical workplace preoperatively, intraoperatively, as well as postoperatively to improve surgical efficiency and efficacy.
Image guidance and navigation in abdominal surgery
Image guidance and navigation describe systems that assist the surgeon by means of radiologic imaging. They help exploring the patient’s anatomy in order to find targets faster and spare structures at risk.
Preoperative or intraoperative imaging is combined with a tracking system for the surgical instruments. Then, both are aligned in a registration step to the coordinate system of the patient. The system visualizes the points of interest in relation to the surgeon’s instruments and the patient’s anatomy in real time allowing insights and orientation for hidden anatomical features (see Fig. 2).
In neurosurgery, this technology has found its way into clinical routine and is also used in orthopedics and ear-nose-throat surgery [2]. However, navigation is of limited use in abdominal surgery, because tissue shift and organ deformation compromise the validity of the underlying preoperative imaging.
Possible clinical applications include various different procedures such as adrenalectomy [3], pediatricsplenectomy [4], pancreaticoduodenectomy [5], esophagectomy [6], and hepatectomy [7]. But, all of them remained limited to feasibility studies with case reports or small series, because they required a lot of effort in the setup, provided comparatively small advantages for the surgeon, and did not solve the problem of tissue shift. Only few navigation systems in these cases especially designed for liver surgery managed to bridge the translational gap and have been approved as a medical product [8, 9].
Recent developments are using novel intraoperative imaging modalities such as computed tomography (CT) to update preoperative imaging [10, 11] or combine preoperative MRI of the liver with intraoperative CT [12]. Although even with the use of intraoperative imaging, the tissue shift caused by respiration and intraoperative manipulation negatively affects accuracy of navigation systems considerably. Furthermore, intraoperative CT imaging generally results in increased radiation exposure for the patient and possibly even the surgical team. Intraoperative MRI could be a solution, and Tsutsumi et al. proved its feasibility visualizing the common bile duct in three patients undergoing laparoscopic cholecystectomy and the urinary bladder in two patients undergoing laparoscopic ventral hernia repair [13].
Despite navigation for abdominal surgery being “just around the corner” for more than a decade, limited progress has been made to overcome the well-known tissue-shift problems and complex setup [14, 15]. However, very promising commercial systems as the CAS-ONE Liver Surgery system (Cascination AG, Bern, Switzerland) have recently been developed [16], although it still remains to be proven whether benefits can be demonstrated in randomized controlled trials and if these outweigh the increased investments in the systems, the training, and the logistics.
Robot-assisted surgery
Robot-assisted surgery has been a fast growing field of surgery for nearly 15 years and is one of the most common technologies in CAS [17]. Today, the da Vinci® (da Vinci Surgical System®, Intuitive Surgical, Sunnyvale, CA, USA) is mostly used. The da Vinci® is entirely controlled by the surgeon, who uses hand controls and foot pedals to manipulate a camera arm and at least two instrument arms [18, 19]. It has mostly been used for laparoscopic prostatectomy easing troublesome conventional laparoscopy. Thus, compared to open surgery, robotic prostatectomy allowed to provide the well-known advantages of laparoscopic surgery such as less use of analgesics and shorter hospitalization for a cohort formerly treated with an open approach and improving the learning curve [20]. In two recent meta-analyses, there is evidence suggesting improved urinary function, lower intraoperative organ injuries, and improvements in positive surgical margins comparing robotic-assisted with laparoscopic radical prostatectomy; however, both authors state that these results should be interpreted with caution due to limited and heterogeneous evidence [21, 22]. Nonetheless, robot-assisted surgery in prostatectomy is even discussed as becoming gold standard in the near future [23]. Another field with similar characteristics is robotic hysterectomy which even surpasses case numbers of robotic prostatectomy [20]. In recent years, considerable effort has been put into robot-assisted colorectal surgery. A recent systematic review by Kim et al. for robot-assisted colorectal surgery analyzed 39 case series, 29 comparative studies, and one randomized controlled trial, 69 studies in total. They concluded that robot-assisted colorectal surgery is safe and feasible and shows at least comparable results to conventional laparoscopic or open surgery [24]. Nonetheless, this technology is encountering serious challenges in colorectal surgery. The system is currently incapable of performing multiquadrant surgery without readjusting the position of the system cart holding the instrument arms which results in time-consuming readjustments of the system during an operation causing longer operation times. Several surgeons solved this problem by a hybrid approach. They used the robot only for pelvic dissection and performing adhesiolysis and colonic mobilization by conventional laparoscopy [24]. Since the robot demands complex servicing and operation times tend to be longer, significantly higher costs have been reported in randomized control trials for robotic colorectal surgeries [25]. In addition, results from randomized controlled trials showed no significant differences in hospital stay, surgical complications, postoperative pain score, resection margin clearance, and number of lymph nodes harvested compared to conventional laparoscopic surgery [26]. For laparoscopic fundoplication, Müller-Stich et al. reported a lower operation time, but in a recent meta-analysis, total operating time was significantly higher, while perioperative complication rates and length of hospital stay were comparable between laparoscopic and robot-assisted fundoplication [27, 28].
Apart from complex telemanipulation systems for all instruments, there is increasing interest in stand-alone robotic camera holders. These systems aim to replace humans as a camera operator. Particularly in solo surgery or small medical centers with fewer personnel, this tool might be helpful. These systems have been reported to be much cheaper than the da Vinci® by a factor of 10–20 [25, 29]. Gumbs et al. proved safety and feasibility in a case series of 200 laparoscopic pancreatic procedures using a robotically controlled laparoscope holder (Viky EP®, EndoControl, Grenoble, France) [29]. Gillen et al. evaluated 123 patients using a similar system (Soloassist®; Actormed GmbH, Barbing, Germany) in a case-control study for laparoscopic cholecystectomy. They found a significantly increased total operating time (104 vs. 90 min; p = 0.001) but a lower overall surgical staff operation time (number of surgeons multiplied by operating time) (104 vs. 180 min; p = 0.001) [30]. However, it has to be mentioned that these robotic camera holders are lacking real autonomy as well as learning behavior and have to be constantly controlled by surgeons by voice or manual interaction during surgery which costs time and effort. A new generation of cognitive robots that are context-aware and help the surgeon based on individual view preferences may lead to a future with autonomous robots operating the camera mainly on their own [18].
Human-machine interface
The term human-machine interface (HMI) describes the junction between a human and a machine. It involves both human senses and actions on one side and input as well as output devices on the other side. The user interacts with the HMI to control the hardware or software and to receive helpful feedback.
In minimally invasive CAS, the interaction with the computer is a key factor for the success of an operation. The classical HMI combination of a personal computer, consisting of a monitor, keyboard, and mouse, needs direct human interaction and is thus very impractical intraoperatively requiring a nurse to operate the system [31]. In contrast, modern devices tend to be mobile and available at the point of care. Tablet- or smartphone-based products to access healthcare information systems or view radiological images are commercially available and in clinical use even in the sterile operation area [32–34]. Tablet-based augmented reality (see Fig. 3) and 3D visualization have been reported for hepatectomy and pulmonary segmentectomy [35, 36], neurosurgery [37], and percutaneous access to the renal pelvis for, i.e., nephrolithotomy by Rassweiler et al. [38, 39]. Recently, optical see-through head-mounted displays like google glass™ (Google Inc., Mountain View, USA) were used for intraoperative augmented reality and medical training [40]. More intuitive input methods like voice or gesture control are now in focus of research. Examples are electromyographic armbands like the Myo™ armband (Thalmic Labs Inc, Kitchener, Canada) or the Microsoft Kinect™ gesture detector (Microsoft Corp, Redmond, USA) for touchless interaction in a sterile environment [41, 42]. Another way is to use voice control and voice recognition for intraoperative control of computers [43].
Experimental and clinical research to validate these new methods demand time, effort, and thoroughly planned studies. Unresolved issues remain data privacy, patient safety, and compatibility in the current clinical environment [44]. New HMI are fascinating at first sight, but for a successful clinical implementation, abovementioned challenges have to be addressed.
Surgical processes and clinical pathways
Clinical pathways (CP) are defined descriptions of diagnostics and therapies to solve a specific problem of a patient and aim for standardization of patient care and optimization of the clinical outcome. There is strong evidence for CP decreasing length of stay and postoperative complications as well as to improving comprehensive documentation and decreasing inpatient costs [45]. In order to develop, discuss, and implement CP, there is need for an intuitive and clear depiction. For this purpose, the Business Process Model and Notation (BPMN) was developed in the organizational and business science to establish a standardized taxonomy of organizational processes and can also be used in CP modeling [46–48].
To establish the link between CP and CAS, the modeling and the implementing processes have to be considered. As most CP are comprehensive and complex, thorough implementation as well as optimization will need mathematical and computational support. Doebbeling et al. used the VirtECS Scheduling engine to run simulations in the perioperative management workflows of the operating room. These simulations balanced the workload which resulted in less overtime, fewer cancelled shifts, less resource strain, and increased staff satisfaction [49].
In conclusion, modeling CP reveals organizational responsibilities of patient care within the multidisciplinary network of modern medicine. It governs specific diagnostic and therapeutic tasks, which makes it liable to mathematical optimization. In the case of jurisdictional and insurance-related arguments, it is also able to document and report the whole process of patient care conclusively and automatically. In the future, simulators for CP optimization may help to develop and improve integrated CP.
Computer-assisted surgical training
Competent skill levels and procedural knowledge are vital for successful patient outcome in surgery. With the increasing public demand for patient safety and the rising complexity of modern operations, the necessity of operative training outside the operating room is not doubted anymore. Modern computer- and media-based training modalities enable surgeons to acquire the psychomotor skills and surgical knowledge necessary before operating on real patients [50–54]. Due to additional technical challenges and psychomotor demands, training is especially relevant for MIS [55–58].
The psychomotor aspects of minimally invasive surgery can be trained with virtual reality (VR) trainers that use computer simulation with haptic feedback to train psychomotor skills and procedural aspects of operations (see Fig. 4). The trainees receive automated instructions and feedback. Their performance can be continuously recorded, and training progress can be monitored [59, 60]. VR trainers have proven advantages for patient safety. In a study by Zendejas et al., a group of surgical residents with an extensive simulator training program had faster operative times and improved patient outcome with significantly less complications in endoscopic hernia repair compared to the group with the shorter VR training program [61].
Online learning platforms provide videos of operations, explanations and teaching of surgical techniques, the relevant anatomy, and perioperative management [62, 63]. The efficacy of online learning modules has been studied with positive results for online learning both alone and combined with other training modalities [64].
As liver anatomy is very complex, 3D visualization techniques have been developed to improve learning performance of medical students. In a cohort of 156 medical students, it has been shown that students exposed to 3D liver anatomy visualization performed significantly better to a set of anatomical and evaluative questions [65].
Surgical training courses have been established worldwide to ensure adequate skill learning before performing operations on patients. Different training modalities and their combinations have been compared regarding training effects. Multimodality training combines the available training modalities for optimal training outcome [66–68]. Future developments of training modalities for surgical interventions include the use of automated detection of the movements of the surgeon and the instrument to collect their movement patterns reflecting experience and creating continuous individualized feedback for trainees to enhance their learning curves. The emerging technologies open up new possibilities of accumulating experience and surgical knowledge to assist surgeons in their learning curves and decision-making processes in multidimensional ways.
Clinical decision support and cognition-guided surgery
Nowadays, surgeons often face a large and complex amount of information, i.e., increasing amounts of individual patient data and prognostic factors, rising number of diagnostic guidelines, and treatment concepts. Because of this information overload, clinical decision support (CDS) systems have gained interest in the field of medicine over the past few years [69]. The general definition of CDS by Musen et al. is “a computer which is able to solve clinical problems together with a clinician” [70]. They defined three types of these decision-making support systems: systems which store information or patient data, systems which analyze information, and systems which assist in a patient-specific problem. Eberhardt et al. defined CDS as follows: a computer which provides intelligent information for a problem. To solve a problem intelligently, the computer requires information that pertain to the current problem. Furthermore, the computer needs a database containing relevant information on a certain topic (domain knowledge) as well as an interacting algorithm which integrates the current information of the specific situation with the general information of the database. Finally, a CDS needs an HMI which allows interaction between the users and the computer [69].
There is a variety of technical approaches which can be applied to CDS, the easiest of which is the rule-based approach which solves problems by applying determined rules in the “if-then” manner. The first clinical decision support called MYCIN used a rule-based approach (“if-then”) to diagnose infectious diseases and was named after the antibiotics it was designed to recommend [71]. As this approach is rigid and only able to solve problems, which were previously defined in the database, machine-learning algorithms have gained importance over the past few years. The advantage is based on the ability to learn from previous cases and hence to continuously extend knowledge of the medical problem. Machine-learning algorithms used for these systems are artificial neural networks, random forest models, and Bayesian belief network models.
Stojadinovic et al. developed a CDS to predict the individual survival rate of patients diagnosed with colon cancer [72]. This CDS was built with a database containing knowledge about colonic cancer (e.g., clinical or pathological findings), a machine-learned Bayesian belief network algorithm, and a user interface to insert the necessary data and to depict the results respectively. To construct the database and validate the CDS, a total of 146,248 records from the Surveillance Epidemiology and End Results Database (SEER) were used. Among these records, the overall survival was analyzed, and a subgroup analysis was made for the use of the CDS. They found a positive predictive for their CDS value for mortality rates at 1, 2, 3, and 5 years at 74, 80, 82, and 84 %, respectively. Moreover, key variables and their importance for the patient’s outcome that changed over the years have been discovered. They can be applied to an individualized CDS. Although this program was tested on retrospective data, the potential of CDS is shown.
Currently, further CDS models have been constructed to facilitate a surgeon’s work, for example, prediction of the survival rates of patients after liver transplantation and treatment for acute appendicitis [73–75]. Recently, the Transregional Collaborative Research Centre “Cognition-Guided Surgery” has been funded at the University of Heidelberg, the Karlsruhe Institute of Technology, and German Cancer Research Center by the German Research Foundation (DFG). The aim is to create a technical, knowledge-based cognitive system to support the surgeon. It will act in a similar way to a human assistant while permanently retaining vital knowledge to be transferred, accumulated, and reused for future operations. One of the main objectives includes knowledge-based intraoperative surgical assistance.
However, CDS is not yet ready to be implemented in daily practice, and a paradigm shift is on the rise, in which computers develop from mere data capturing and storing systems to teachable, context-aware assistants.
Discussion
CAS is a heterogeneous field of advanced computer sciences. A steadily increasing yearly number of publications on this topic can be observed suggesting an exponential growth interest on the field (Table 1). The use-cases presented in this review do not represent the entirety of CAS but serve as examples of present and future directions in surgery. Based on the judgment of the authors, a technology rating table was drawn and can be seen in Table 2. Especially, further developments in the field of clinical decision support have the potential of causing a paradigm shift in how patients are diagnosed and treated on a broader scale. Medical device manufacturers are keen on these new techniques. Unfortunately, recent legislative medical device acts and regulations signify high regulatory and thus financial challenges for academic researchers as they often render it impossible to evaluate self-developed technologies in the clinical setting. On the other hand, financially potent companies such as Karl Storz (endoscopy equipment and integrated operating rooms), Intuitive Surgical (da Vinci®), SAP (Medical Research Insights), IBM (Watson Oncology), and Apple (Healthkit) are either already global medical device manufacturers or are investing in healthcare applications to a great degree. These financially potent companies will be adopting promising technologies fast and at the same time they will provide approved, integrated, and workflow-oriented tools. Clinical reality may thus change faster than expected due to company-driven innovation before academic researchers will be able to evaluate the changes in time. However, thoroughly planned clinical studies that evaluate these new products are a bare necessity and will be the foundation of broad acceptance in the surgical community. As a consequence, research-oriented surgeons should thus partner with potent manufacturers and implement evaluation processes early in the development to finally perform well-designed clinical studies alongside new products entering the market.
Conclusion
As presented, CAS is increasingly used not only in experimental but also in clinical studies. Although CAS is at an early stage of development, clinical studies start showing the benefits of computers not only as tools of documentation and accounting but also for diagnosis and treatment. In the upcoming years, those tools and technologies will be increasingly used by early adopters and innovators on the verge to cross the chasm of the technology adoption lifecycle onto wide-spread use.
References
Foundation W (2015) Computer-assisted. http://en.wikipedia.org/wiki/Computer-aided. Accessed 25 Jan 2015
Mezger U, Jendrewski C, Bartels M (2013) Navigation in surgery. Langenbeck’s Arch Surg 398(4):501–514. doi:10.1007/s00423-013-1059-4
Marescaux J, Rubino F, Arenas M, Mutter D, Soler L (2004) Augmented-reality-assisted laparoscopic adrenalectomy. JAMA 292(18):2214–2215. doi:10.1001/jama.292.18.2214-c
Ieiri S, Uemura M, Konishi K, Souzaki R, Nagao Y, Tsutsumi N, Akahoshi T, Ohuchida K, Ohdaira T, Tomikawa M, Tanoue K, Hashizume M, Taguchi T (2012) Augmented reality navigation system for laparoscopic splenectomy in children based on preoperative CT image using optical tracking device. Pediatr Surg Int 28(4):341–346. doi:10.1007/s00383-011-3034-x
Onda S, Okamoto T, Kanehira M, Suzuki F, Ito R, Fujioka S, Suzuki N, Hattori A, Yanaga K (2014) Identification of inferior pancreaticoduodenal artery during pancreaticoduodenectomy using augmented reality-based navigation system. J Hepato-Biliary-Pancreat Sci 21(4):281–287. doi:10.1002/jhbp.25
Kenngott HG, Neuhaus J, Muller-Stich BP, Wolf I, Vetter M, Meinzer HP, Koninger J, Buchler MW, Gutt CN (2008) Development of a navigation system for minimally invasive esophagectomy. Surg Endosc 22(8):1858–1865. doi:10.1007/s00464-007-9723-9
Okamoto T, Onda S, Matsumoto M, Gocho T, Futagawa Y, Fujioka S, Yanaga K, Suzuki N, Hattori A (2013) Utility of augmented reality system in hepatobiliary surgery. J Hepato-Biliary-Pancreat Sci 20(2):249–253. doi:10.1007/s00534-012-0504-z
Kingham TP, Scherer MA, Neese BW, Clements LW, Stefansic JD, Jarnagin WR (2012) Image-guided liver surgery: intraoperative projection of computed tomography images utilizing tracked ultrasound. HPB (Oxford) 14(9):594–603. doi:10.1111/j.1477-2574.2012.00487.x
Peterhans M, vom Berg A, Dagon B, Inderbitzin D, Baur C, Candinas D, Weber S (2011) A navigation system for open liver surgery: design, workflow and first clinical applications. Int J Med Robot 7(1):7–16. doi:10.1002/rcs.360
Nozaki T, Fujiuchi Y, Komiya A, Fuse H (2013) Efficacy of DynaCT for surgical navigation during complex laparoscopic surgery: an initial experience. Surg Endosc 27(3):903–909. doi:10.1007/s00464-012-2531-x
Nozaki T, Iida Y, Morii A, Fujiuchi Y, Fuse H (2012) Laparoscopic radical nephrectomy under near real-time three-dimensional surgical navigation with C-arm cone beam computed tomography. Surg Innov 19(3):263–267. doi:10.1177/1553350611429690
Kenngott HG, Wagner M, Gondan M, Nickel F, Nolden M, Fetzer A, Weitz J, Fischer L, Speidel S, Meinzer HP, Bockler D, Buchler MW, Muller-Stich BP (2014) Real-time image guidance in laparoscopic liver surgery: first clinical experience with a guidance system based on intraoperative CT imaging. Surg Endosc 28(3):933–940. doi:10.1007/s00464-013-3249-0
Tsutsumi N, Tomikawa M, Uemura M, Akahoshi T, Nagao Y, Konishi K, Ieiri S, Hong J, Maehara Y, Hashizume M (2013) Image-guided laparoscopic surgery in an open MRI operating theater. Surg Endosc 27(6):2178–2184. doi:10.1007/s00464-012-2737-y
Lamade W, Vetter M, Hassenpflug P, Thorn M, Meinzer HP, Herfarth C (2002) Navigation and image-guided HBP surgery: a review and preview. J Hepato-Biliary-Pancreat Sci 9(5):592–599. doi:10.1007/s005340200079
Beller S, Hunerbein M, Eulenstein S, Lange T, Schlag PM (2007) Feasibility of navigated resection of liver tumors using multiplanar visualization of intraoperative 3-dimensional ultrasound data. Ann Surg 246(2):288–294. doi:10.1097/01.sla.0000264233.48306.99
Buchs NC, Volonte F, Pugin F, Toso C, Fusaglia M, Gavaghan K, Majno PE, Peterhans M, Weber S, Morel P (2013) Augmented environments for the targeting of hepatic lesions during image-guided robotic liver surgery. J Surg Res 184(2):825–831. doi:10.1016/j.jss.2013.04.032
Kranzfelder M, Staub C, Fiolka A, Schneider A, Gillen S, Wilhelm D, Friess H, Knoll A, Feussner H (2013) Toward increased autonomy in the surgical OR: needs, requests, and expectations. Surg Endosc 27(5):1681–1688. doi:10.1007/s00464-012-2656-y
Pandya A, Reisner L, King B, Lucas N, Composto A, Klein M, Ellis R (2014) A review of camera viewpoint automation in robotic and laparoscopic surgery. Robotics 3(3):310–329. doi:10.3390/robotics3030310
Gutt CN, Oniu T, Mehrabi A, Kashfi A, Schemmer P, Buchler MW (2004) Robot-assisted abdominal surgery. Br J Surg 91(11):1390–1397. doi:10.1002/bjs.4700
Kenngott HG, Fischer L, Nickel F, Rom J, Rassweiler J, Muller-Stich BP (2012) Status of robotic assistance—a less traumatic and more accurate minimally invasive surgery? Langenbeck’s Arch Surg 397(3):333–341. doi:10.1007/s00423-011-0859-7
Robertson C, Close A, Fraser C, Gurung T, Jia X, Sharma P, Vale L, Ramsay C, Pickard R (2013) Relative effectiveness of robot-assisted and standard laparoscopic prostatectomy as alternatives to open radical prostatectomy for treatment of localised prostate cancer: a systematic review and mixed treatment comparison meta-analysis. BJU Int 112(6):798–812. doi:10.1111/bju.12247
Moran PS, O’Neill M, Teljeur C, Flattery M, Murphy LA, Smyth G, Ryan M (2013) Robot-assisted radical prostatectomy compared with open and laparoscopic approaches: a systematic review and meta-analysis. Int J Urol Off J Jpn Urol Assoc 20(3):312–321. doi:10.1111/iju.12070
Sood A, Jeong W, Peabody JO, Hemal AK, Menon M (2014) Robot-assisted radical prostatectomy: inching toward gold standard. Urol Clin N Am 41(4):473–484. doi:10.1016/j.ucl.2014.07.002
Kim CW, Kim CH, Baik SH (2014) Outcomes of robotic-assisted colorectal surgery compared with laparoscopic and open surgery: a systematic review. J Gastrointest Surg 18(4):816–830. doi:10.1007/s11605-014-2469-5
Turchetti G, Palla I, Pierotti F, Cuschieri A (2012) Economic evaluation of da Vinci-assisted robotic surgery: a systematic review. Surg Endosc 26(3):598–606. doi:10.1007/s00464-011-1936-2
Park JS, Choi GS, Park SY, Kim HJ, Ryuk JP (2012) Randomized clinical trial of robot-assisted versus standard laparoscopic right colectomy. Br J Surg 99(9):1219–1226. doi:10.1002/bjs.8841
Muller-Stich BP, Reiter MA, Wente MN, Bintintan VV, Koninger J, Buchler MW, Gutt CN (2007) Robot-assisted versus conventional laparoscopic fundoplication: short-term outcome of a pilot randomized controlled trial. Surg Endosc 21(10):1800–1805. doi:10.1007/s00464-007-9268-y
Mi J, Kang Y, Chen X, Wang B, Wang Z (2010) Whether robot-assisted laparoscopic fundoplication is better for gastroesophageal reflux disease in adults: a systematic review and meta-analysis. Surg Endosc 24(8):1803–1814. doi:10.1007/s00464-009-0873-9
Gumbs AA, Croner R, Rodriguez A, Zuker N, Perrakis A, Gayet B (2013) 200 consecutive laparoscopic pancreatic resections performed with a robotically controlled laparoscope holder. Surg Endosc 27(10):3781–3791. doi:10.1007/s00464-013-2969-5
Gillen S, Pletzer B, Heiligensetzer A, Wolf P, Kleeff J, Feussner H, Furst A (2014) Solo-surgical laparoscopic cholecystectomy with a joystick-guided camera device: a case-control study. Surg Endosc 28(1):164–170. doi:10.1007/s00464-013-3142-x
Nicolau S, Soler L, Mutter D, Marescaux J (2011) Augmented reality in laparoscopic surgical oncology. Surg Oncol 20(3):189–201. doi:10.1016/j.suronc.2011.07.002
John S, Poh AC, Lim TC, Chan EH (2012) The iPad tablet computer for mobile on-call radiology diagnosis? Auditing discrepancy in CT and MRI reporting. J Digit Imaging 25(5):628–634
Berger E (2010) The iPad: gadget or medical godsend? Ann Emerg Med 56(1):A21–A22
Robinson JD (2012) The skeptical technophile: iPad review. J Digit Imaging 25(3):365–368. doi:10.1007/s10278-012-9467-5
Volonte F, Robert JH, Ratib O, Triponez F (2011) A lung segmentectomy performed with 3D reconstruction images available on the operating table with an iPad. Interact Cardiovasc Thorac Surg 12(6):1066–1068. doi:10.1510/icvts.2010.261073
MEVIS DF-IfBM Mobile Liver Explorer. Accessed 04 May 2014
Deng W, Li F, Wang M, Song Z (2013) Easy-to-use augmented reality neuronavigation using a wireless tablet PC. Stereotact Funct Neurosurg 92(1):17–24. doi:10.1159/000354816
Rassweiler JJ, Muller M, Fangerau M, Klein J, Goezen AS, Pereira P, Meinzer HP, Teber D (2012) iPad-assisted percutaneous access to the kidney using marker-based navigation: initial clinical experience. Eur Urol 61(3):628–631. doi:10.1016/j.eururo.2011.12.024
Muller M, Rassweiler MC, Klein J, Seitel A, Gondan M, Baumhauer M, Teber D, Rassweiler JJ, Meinzer HP, Maier-Hein L (2013) Mobile augmented reality for computer-assisted percutaneous nephrolithotomy. Int J Comput Assist Radiol Surg 8(4):663–675. doi:10.1007/s11548-013-0828-4
Peregrin T (2014) Surgeons see future applications for Google Glass. Bull Am Coll Surg 99(7):9–16
Inc TL (2015) Touch Free: The Myo Armband in Surgery. Thalmic Labs Inc. https://www.thalmic.com/blog/myo-armband-surgery/. Accessed 26 Jan 2015
Iannessi A, Marcy PY, Clatz O, Ayache N, Fillard P (2014) Touchless user interface for intraoperative image control: almost there. Radiographics 34(4):1142–1144. doi:10.1148/rg.344135158
Perrakis A, Hohenberger W, Horbach T (2013) Integrated operation systems and voice recognition in minimally invasive surgery: comparison of two systems. Surg Endosc 27(2):575–579. doi:10.1007/s00464-012-2488-9
Schreinemacher MH, Graafland M, Schijven MP (2014) Google glass in surgery. Surg Innov 21(6):651–652. doi:10.1177/1553350614546006
Rotter T, Kinsman L, James E, Machotta A, Gothe H, Willis J, Snow P, Kugler J (2010) Clinical pathways: effects on professional practice, patient outcomes, length of stay and hospital costs. Cochrane Database Syst Rev 3, CD006632. doi:10.1002/14651858.CD006632.pub2
Strasser M, Pfeifer F, Helm E, Schuler A, Altmann J (2011) Defining and reconstructing clinical processes based on IHE and BPMN 2.0. Stud Health Technol Inform 169:482–486
Scheuerlein H, Rauchfuss F, Dittmar Y, Molle R, Lehmann T, Pienkos N, Settmacher U (2012) New methods for clinical pathways-Business Process Modeling Notation (BPMN) and Tangible Business Process Modeling (t.BPM). Langenbeck’s Arch Surg 397(5):755–761. doi:10.1007/s00423-012-0914-z
Poulymenopoulou M, Papakonstantinou D, Malamateniou F, Vassilacopoulos G (2014) Adaptive healthcare processes for personalized emergency clinical pathways. Stud Health Technol Inform 205:423–427
Doebbeling BN, Burton MM, Wiebke EA, Miller S, Baxter L, Miller D, Alvarez J, Pekny J (2012) Optimizing perioperative decision making: improved information for clinical workflow planning. AMIA Annu Symp Proc 2012:154–163
Aggarwal R, Balasundaram I, Darzi A (2008) Training opportunities and the role of virtual reality simulation in acquisition of basic laparoscopic skills. J Surg Res 145(1):80–86. doi:10.1016/j.jss.2007.04.027
Aggarwal R, Darzi A (2005) Training in laparoscopy—which model to use? Indian J Gastroenterol 24(3):95–96
Aggarwal R, Moorthy K, Darzi A (2004) Laparoscopic skills training and assessment. Br J Surg 91(12):1549–1558. doi:10.1002/bjs.4816
Korndorffer JR Jr, Stefanidis D, Scott DJ (2006) Laparoscopic skills laboratories: current assessment and a call for resident training standards. Am J Surg 191(1):17–22. doi:10.1016/j.amjsurg.2005.05.048
Undre S, Darzi A (2007) Laparoscopy simulators. J Endourol 21(3):274–279. doi:10.1089/end.2007.9980
EU Hernia Trialists Collaboration (2000) Laparoscopic compared with open methods of groin hernia repair: systematic review of randomized controlled trials. Br J Surg 87(7):860–867. doi:10.1046/j.1365-2168.2000.01540.x
Clinical Outcomes of Surgical Therapy Study Group (2004) A comparison of laparoscopically assisted and open colectomy for colon cancer. N Engl J Med 350(20):2050–2059. doi:10.1056/NEJMoa032651
Hamad GG, Curet M (2010) Minimally invasive surgery. Am J Surg 199(2):263–265. doi:10.1016/j.amjsurg.2009.05.008
Harrell AG, Heniford BT (2005) Minimally invasive abdominal surgery: lux et veritas past, present, and future. Am J Surg 190(2):239–243
Ayodeji ID, Schijven M, Jakimowicz J, Greve JW (2007) Face validation of the Simbionix LAP Mentor virtual reality training module and its applicability in the surgical curriculum. Surg Endosc 21(9):1641–1649. doi:10.1007/s00464-007-9219-7
Schijven MP, Jakimowicz JJ, Broeders IA, Tseng LN (2005) The Eindhoven laparoscopic cholecystectomy training course—improving operating room performance using virtual reality training: results from the first E.A.E.S. accredited virtual reality trainings curriculum. Surg Endosc 19(9):1220–1226. doi:10.1007/s00464-004-2240-1
Zendejas B, Cook DA, Bingener J, Huebner M, Dunn WF, Sarr MG, Farley DR (2011) Simulation-based mastery learning improves patient outcomes in laparoscopic inguinal hernia repair: a randomized controlled trial. Ann Surg 254(3):502–509. doi:10.1097/SLA.0b013e31822c6994, discussion 509–511
Pape-Koehler C, Chmelik C, Aslund AM, Heiss MM (2010) An interactive and multimedia-based manual of surgical procedures: Webop—an approach to improve surgical education. Zentralbl Chir 135(5):467–471. doi:10.1055/s-0030-1262538
Mutter D, Vix M, Dallemagne B, Perretta S, Leroy J, Marescaux J (2011) WeBSurg: an innovative educational Web site in minimally invasive surgery—principles and results. Surg Innov 18(1):8–14. doi:10.1177/1553350611398880
Pape-Koehler C, Immenroth M, Sauerland S, Lefering R, Lindlohr C, Toaspern J, Heiss M (2013) Multimedia-based training on Internet platforms improves surgical performance: a randomized controlled trial. Surg Endosc 27(5):1737–1747. doi:10.1007/s00464-012-2672-y
Muller-Stich BP, Lob N, Wald D, Bruckner T, Meinzer HP, Kadmon M, Buchler MW, Fischer L (2013) Regular three-dimensional presentations improve in the identification of surgical liver anatomy—a randomized study. BMC Med Educ 13:131. doi:10.1186/1472-6920-13-131
Nickel F, Bintintan VV, Gehrig T, Kenngott HG, Fischer L, Gutt CN, Muller-Stich BP (2013) Virtual reality does not meet expectations in a pilot study on multimodal laparoscopic surgery training. World J Surg 37(5):965–973. doi:10.1007/s00268-013-1963-3
Brinkman WM, Havermans SY, Buzink SN, Botden SM, Jakimowicz JJ, Schoot BC (2012) Single versus multimodality training basic laparoscopic skills. Surg Endosc 26(8):2172–2178. doi:10.1007/s00464-012-2184-9
Zimmerman H, Latifi R, Dehdashti B, Ong E, Jie T, Galvani C, Waer A, Wynne J, Biffar D, Gruessner R (2011) Intensive laparoscopic training course for surgical residents: program description, initial results, and requirements. Surg Endosc 25(11):3636–3641. doi:10.1007/s00464-011-1770-6
Eberhardt J, Bilchik A, Stojadinovic A (2012) Clinical decision support systems: potential with pitfalls. J Surg Oncol 105(5):502–510. doi:10.1002/jso.23053
Musen MA, Middleton B, Greenes RA (2014) Clinical decision-support systems. In: Biomedical informatics. Springer, pp 643–674
Shortliffe EH (1976) Computer-based medical consultations, MYCIN. Artificial intelligence series, vol 2. Elsevier, New York
Stojadinovic A, Bilchik A, Smith D, Eberhardt JS, Ward EB, Nissan A, Johnson EK, Protic M, Peoples GE, Avital I, Steele SR (2013) Clinical decision support and individualized prediction of survival in colon cancer: bayesian belief network model. Ann Surg Oncol 20(1):161–174. doi:10.1245/s10434-012-2555-4
Cucchetti A, Vivarelli M, Heaton ND, Phillips S, Piscaglia F, Bolondi L, La Barba G, Foxton MR, Rela M, O’Grady J, Pinna AD (2007) Artificial neural network is superior to MELD in predicting mortality of patients with end-stage liver disease. Gut 56(2):253–258. doi:10.1136/Gut.2005.084434
Hsieh CH, Lu RH, Lee NH, Chiu WT, Hsu MH, Li YC (2011) Novel solutions for an old disease: diagnosis of acute appendicitis with random forest, support vector machines, and artificial neural networks. Surgery 149(1):87–93. doi:10.1016/J.Surg.2010.03.023
Prabhudesai SG, Gould S, Rekhraj S, Tekkis PP, Glazer G, Ziprin P (2008) Artificial neural networks: useful aid in diagnosing acute appendicitis. World J Surg 32(2):305–309. doi:10.1007/s00268-007-9298-6, discussion 310-301
Acknowledgments
This study has been conducted within the Transregional Collaborative Research Center (TCRC) 125 “Cognition-Guided Surgery”, funded by the German Research Foundation (DFG).
We thank Ms. Béivin-Vanessa Pyne for reviewing the manuscript as a native speaker.
Compliance with Ethical Standards
This study has been conducted in compliance with ethical standards as set in the declaration of Helsinki. The figures supplied were conducted within our working group; detailed results are yet to be published. Informed consent was obtained for the tablet-based augmented reality experiment (Fig. 3). An animal trial (Fig. 2) has been approved by the local authorities.
Conflicts of interest
None.
Author’s contributions
Kenngott HG: study conception and design, acquisition of data, analysis and interpretation of data, and drafting of manuscript.
Wagner M: acquisition of data, analysis and interpretation of data, and drafting of manuscript.
Nickel F: acquisition of data, analysis and interpretation of data, and drafting of manuscript.
Wekerle AL: acquisition of data, analysis and interpretation of data, and drafting of manuscript.
Preukschas A: acquisition of data, analysis and interpretation of data, and drafting of manuscript.
Apitz M: acquisition of data, analysis and interpretation of data, and drafting of manuscript.
Schulte T: acquisition of data, analysis and interpretation of data, and drafting of manuscript.
Rempel R: acquisition of data, analysis and interpretation of data, and drafting of manuscript
Mietkowski P: acquisition of data, analysis and interpretation of data, and drafting of manuscript
Wagner F: acquisition of data, analysis and interpretation of data, and drafting of manuscript
Termer A: acquisition of data, analysis and interpretation of data, and drafting of manuscript
Beat P. Müller-Stich: study conception and design, analysis and interpretation of data, and drafting of manuscript
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kenngott, H.G., Wagner, M., Nickel, F. et al. Computer-assisted abdominal surgery: new technologies. Langenbecks Arch Surg 400, 273–281 (2015). https://doi.org/10.1007/s00423-015-1289-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00423-015-1289-8