Abstract
Purpose
Weight-bearing activities such as running have been shown to be osteogenic. However, investigations have also shown that running may lead to site-specific deficiencies in bone mineral density (BMD) as well as overall low BMD. The purpose of this investigation was to evaluate and compare the BMD of female and male collegiate cross-country runners with non-running controls. In addition, energy availability and disordered eating attitudes and behaviors were assessed.
Methods
BMD of 60 collegiate cross-country runners and 47 BMI and age-matched non-running controls were measured via DXA scans. Participants completed a Block 2014 Food Frequency Questionnaire and Eating Disorder Examination Questionnaire.
Results
Controlling for fat-free mass (FFM), male runners showed greater BMD at the femoral neck (0.934 ± 0.029 vs. 0.866 ± 0.028 g cm2, p < 0.05), total hip (1.119 ± 0.023 vs. 1.038 ± 0.021 g cm2, p < 0.05), and whole body (1.119 ± 0.023 vs. 1.038 ± 0.021 g cm2, p < 0.05) than male controls. The female runners had greater whole-body BMD than female controls (1.143 ± 0.018 vs. 1.087 ± 0.022 g cm2, p < 0.05). Runners scored significantly higher than controls in dietary restraint (1.134 ± 1.24 vs. 0.451 ± 0.75, p < 0.05), male runners were significantly higher than male controls in eating concern (1.344 ± 1.08 vs. 0.113 ± 0.27, p < 0.05) and female runners were significantly higher than male runners in shape concern (1.056 ± 1.27 vs. 0.242 ± 0.31, p < 0.05). Forty-two percent of the male runners and 29% of female runners had an energy availability of less than 30 kcals kg−1FFM.
Conclusion
It appears that distance running has beneficial effects on whole-body BMD and site-specific areas. Further research is warranted to further clarify the health effects of eating behaviors and EA of distance runners.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Regular participation in exercise is known to provide numerous physiological benefits including reducing the risk of some forms of cancer, improvements in body composition and mental health, and has been shown to reduce the risk of chronic diseases such as cardiovascular disease, diabetes mellitus, and osteoporosis (Garber et al. 2011). More specifically, weight-bearing activities have been shown to benefit bone health and, when performed during youth, help achieve an optimal peak bone mass (Baxter-Jones et al. 2003; Gunter et al. 2012; Kohrt et al. 2004). Optimizing development of peak bone mass during peripubertal and young-adult years may be a critical opportunity for lowering the risk of osteoporosis later in life (Baxter-Jones et al. 2003; Gunter et al. 2012).
Athletes performing weight-bearing exercise, such as running, appear to experience site-specific skeletal benefits and frequently exhibit greater bone mass than non-exercising controls (Kohrt et al. 2004). Interestingly, several investigations of distance runners have shown that bone mineral density (BMD) may be lower than expected (Barrack et al. 2017; Fredericson et al. 2007; Tenforde et al. 2015, 2018) and several investigations have shown site-specific deficiencies at the lumbar spine in distance runners (Barrack et al. 2008; Fredericson et al. 2007; Hind et al. 2006; Tam et al. 2018). However, few investigations have compared bone health of runners to non-running controls of similar age, height, and weight. Of those that have, runners seem to exhibit skeletal benefits due to training (Hind et al. 2006).
Some evidence suggests that distance runners may be prone to low energy availability (EA), which directly impacts bone accrual and risk for bone stress injuries (Barrack et al. 2017, 2008; Burke et al. 2018a, 2018b; Tenforde et al. 2016). EA represents the fuel available to the body for basic physiological needs including immune function, tissue growth and repair, and reproductive function, which remains after energy expended during exercise (Loucks 2004; Mountjoy et al. 2018). Brief periods of low EA have been shown to negatively influence bone metabolism in a manner which over time may lead to loss in bone density or failure to make progress toward peak bone mass (Ihle and Loucks 2004). EA has been identified as an underlying concept of the female athlete triad: a syndrome of inter-related disorders featuring menstrual disturbance, restrictive eating practices, and low bone density (Mountjoy et al. 2014; Nattiv et al. 2007). More recently, the International Olympic Committee has called attention to Relative Energy Deficiency in Sport (RED-S) with an underlying etiology of low EA. RED-S is a syndrome that expands upon the female athlete triad and includes physiological effects of energy deficiency on bone health and other bodily systems in both female and male athletes (Mountjoy et al. 2018, 2014).
Restrictive eating may be common among elite athletes, particularly those in thin-build sports such as cross-country running and is likely an underlying feature of the triad and RED-S (Barrack et al. 2014; Tenforde et al. 2017). Components of the female athlete triad have been studied frequently in female runners; however, there is a lack of research investigating EA and eating habits of male distance runners. Therefore, the purpose of this investigation was to evaluate and compare the BMD of female and male collegiate cross-country runners with like-sized, non-running controls. Secondarily, we aimed to estimate EA and assess disordered eating attitudes and behaviors in this population. It was hypothesized that runners with adequate EA would have greater BMD than non-running controls matched for age, height, and weight. It was anticipated that athletes would have lower EA than controls and that female athletes would have lower EA than male athletes.
Methods
Participants
Sixty cross-country runners, 27 males and 33 females, were recruited from an NCAA Division I team across several years. These 60 runners represent 97% of the available runners. Forty-seven non-running participants, 23 males and 24 females, were pulled from a larger study examining lifestyle choices, alcohol consumption, and bone health in first- and second-year college students to serve as a control group (LaBrie et al. 2018). The runners were training more than 100 km per week, across 9–10 running sessions, with a long run between 20 and 25 km week−1. In addition, the runners were performing two resistance training sessions and two workouts of aqua jogging each week. Non-running controls were recruited through announcements at Greek life events, in academic courses, via the study website, and through social media advertising. Inclusion criteria in the control group required physical activity and exercising energy expenditure of less than 500 kcals day−1, BMI ≤ 23 kg m−2, < 25 g day−1 of alcohol consumption, and < 1 binge drinking episode per month with no current smoking or previous tobacco use. In addition, volunteers with a history of participation in impact sports, such as gymnastics, soccer, basketball, and running were not included as controls in this analysis. The Loyola Marymount University Institutional Review Board approved the testing protocol and informed consent documentation was obtained from all participants prior to enrolling in the study.
Bone mineral density and body composition
Bone mineral density of runners and controls was measured using dual-energy x-ray absorptiometry (DXA; Hologic Discovery A, Waltham, MA). A single technician performed and analyzed all scans of the anterior–posterior (AP) spine (L1–L4), proximal left femur, and whole body. The DXA absorptiometer was calibrated daily during the testing period and previous assessment reliability for BMD at the hip and spine for this technician was demonstrated at greater than 99.0%. A less than 1% coefficient of variation was determined for this DXA technician via repeated measurements on 20 volunteers of similar age to the study population for the spine and hip regions after repositioning the participant before the second scan. The whole-body DXA scan also provided data for bone-free, fat-free mass (FFM) and percent body fat. FFM from the whole-body DXA scan, excluding bone mass, was used as a variable in calculating EA.
Diet analysis
The full-length block 2014 Food Frequency Questionnaire (FFQ) was used to assess energy, calcium, and vitamin D intake. The self-administered survey includes 127 food and beverage items, with additional questions to adjust for fat, protein, carbohydrate, sugar and whole grain content. The food list was developed from NHANES data which was based on the USDA Food and Nutrient Database. Frequency of consumption for each food and beverage item was recorded on a monthly, weekly, or daily basis. Previous research has established the Block FFQ as a valid assessment of dietary intake over the previous 12 months (Hartman et al. 1996). Photos were used to help participants more accurately record portion sizes. Supplemental and dietary sources for calcium and vitamin D were summed for analysis in this investigation.
All participants completed the Eating Disorder Examination Questionnaire (EDEQ), which assesses psychopathology of eating disorders (Fairburn et al. 2008). The EDEQ quantitatively examines four subscales of eating attitudes and behaviors including dietary restraint, eating concern, shape concern, and weight concern (Anderson et al. 2004). Scores range from 0 to 6, with the greater number indicating more extensive disorder. The validity and reliability of the EDEQ have been established (Luce et al. 2007) and the questionnaire has previously been used for investigations of bone health outcomes (Barrack et al. 2008).
Energy availability
EA was calculated as daily energy intake (EI) minus exercising energy expenditure (EEE) divided by FFM. EI was derived from the Block FFQ as total calorie intake. FFM was derived from the whole-body DXA scans. Training and physical activity logs were used to collect information about intensity and duration of regular physical activity and exercise over the previous 3 months to calculate EEE (Kohl et al. 1988; Pereira et al. 1997). Scoring of training data utilized metabolic equivalents (MET-h week−1) from the compendium of physical activity (Ainsworth et al. 2011) to account for energy expenditure at various intensities and duration of exercise. As in previous research (Guebels et al. 2014; Viner et al. 2015), to prevent overestimating energy expended during exercise, only activities with an intensity greater than 4.0 METs were included in the calculation. EEE was the sum of all exercise (> 4.0 METs) multiplied by the hours of activity and FFM. EEE was further adjusted to remove the number of calories contributed by resting metabolic rate (RMR) for the duration of exercise (Heikura et al. 2018; Koehler et al. 2013; Melin et al. 2015; Viner et al. 2015). Resting metabolic rate was estimated using the Cunningham equation of: RMR = 500 + (22 × FFM) (Cunningham 1991). Therefore, adjusted EEE was the raw EEE minus RMR per hour multiplied by hours of all reported activity at 4.0 METs or greater. To calculate EA, the adjusted EEE was subtracted from EI and divided by FFM. EA below 30 kcal kgFFM−1 was considered to be low EA (Loucks et al. 2011).
Statistical analysis
All measures are reported as mean ± standard deviation. A Kolgomorov–Smirnov test was used to check for normal distribution. Homogeneity of variance was investigated using Levine’s F test. For variables that were not normally distributed, a Mann–Whitney U test was utilized. Comparisons of the anthropometric and bone data were made using analysis of covariance (ANCOVA) with FFM serving as the covariant. The Bonferroni post hoc test was utilized when group differences were found following ANCOVA analysis. Pearson’s product correlations were used to determine the relationships between bone measurements and anthropometric and body composition measurements as well as to explore relationships between eating attitudes/behavior and bone health. The statistical package SPSS, version 22 (IBM Corp., Armonk, NY, USA) was used for all statistical analysis. The level of significance was set p < 0.05.
Results
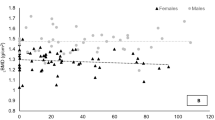
Demographic, anthropometric, body composition, dietary intake, and energy expenditure of the participants are presented in Table 1. There were no statistically significant differences (p > 0.05) in age, BMI, or EA between groups. Everyone meeting the RDA for vitamin D consumed a supplement. Results for BMD are displayed in Table 2. As noted in the table, the ANCOVA, controlling for FFM revealed several statistical differences in BMD between groups. Of note, there were significant differences between the male runners and controls at the femoral neck (p = 0.029), total hip (p = 0.001), and whole body (p = 0.001). For the females, the runners were significantly higher (p = 0.016) in whole body BMD versus the controls. Calcium intake was significantly (p < 0.05) correlated with BMD at the femoral neck (r = 0.243), total hip (r = 0.236), and whole body (r = 0.341) but not related to bone mass at the spine. Mean group z score results are presented in Table 3. Comparisons of z scores between groups were similar to BMD comparisons at two bone sites showing that male athletes have significantly higher z scores than controls at the total hip, while both male and female athletes have higher z scores of the whole body. Percent of participants (by group) with z scores < − 1.0 are presented in Fig. 1. Six runners (four male and two female) and three male controls had z scores at the AP spine ≤ − 2.0. Two women runners and three male controls had femoral neck z scores ≤ − 2.0. One female runner and 2 male controls had total hip z scores ≤ − 2.0. One female runner, five male controls and four female controls had whole body z scores ≤ − 2.0.
An examination of the medical history revealed eight (2 male, 6 female) of the runners (13.3%) had a previous history of stress fractures and one female runner (1.7%) had a history of a stress reaction. Analysis of the z scores for these runners at the AP Spine, femoral neck, total hip, and whole body revealed that three of the runners (1 male, 2 females) had a z score < -1.0 at the AP spine. All other z scores for these nine runners were > − 1.0. The majority of the z scores for these nine runners were > 0.0.
EDEQ results are presented in Table 4. The male runners had significantly higher scores for dietary restraint (p = 0.001) and eating concern (p = 0.008) than the male controls. The female runners were significantly higher (p = 0.047) in dietary restraint than the female controls. There were significant negative correlations between shape concern and BMD at the femoral neck (r = − 0.262, p < 0.01), total hip (r = − 0.367, p < 0.01), and whole body (r = − 0.228, p < 0.05). Additionally, weight concern was negatively correlated to BMD of the femoral neck (r = − 0.199, p < 0.05). The number of participants categorized as having low EA (< 30 kcals kgFFM−1) by sex and athletic status is presented in Table 5. Seven participants were excluded from the EA analysis: four (one male, three female runners) due to acute injury at the time of data collection and three females (one control and two runners) were excluded because application of the Goldberg and Black cutoffs indicated that their energy intake measurements were not likely valid (Black 2000). Comparisons of BMD between participants with low EA (< 30 kcals kgLBM−1) and those with EA ≥ 30 kcals kgLBM−1 demonstrate 1–3% greater bone mass in those with the higher EA, although this difference did not meet statistical significance (Fig. 2: p = 0.590 at AP Spine; p = 0.203 at femoral neck; p = 0.816 at total hip; p = 0.280 at whole body).
Discussion
The purpose of this investigation was to examine the BMD of male and female collegiate cross-country runners compared to age and size-matched, non-running controls. When controlling for FFM, several significant differences in BMD appeared. Female runners had significantly greater BMD at the spine than male runners: a relationship that was also seen in the control group and may be explained by timing of bone maturity. Even though the male and female athletes are the same chronological age, the later onset of pubertal growth experienced by males (~ 2 years) may explain why females at this age exhibit significantly greater BMD at the spine (Baxter-Jones et al. 2011) when controlling for FFM. Research has shown that skeletal tissue at the spine typically reaches peak BMD a few years after peak bone mass is achieved at the hip (Baxter-Jones et al. 2011). Because z scores compare BMD to norms for people of the same age and sex, the lack of significant differences in z scores (Table 3) at the AP spine supports the idea that timing of bone maturity explains the greater BMD exhibited by women in this study.
In runners, weight-bearing exercise may accelerate BMD accrual at the hip and whole body explaining why the BMD values for athletes in this study were higher at specific bone sites. In particular, the male runners demonstrated significantly greater (p < 0.05) BMD than male controls at the femoral neck (7.9%) and total hip (10.7%). The whole-body BMD of the runners was significantly greater (p < 0.05) than their sex-matched controls with male runners 7.8% greater than male controls and female runners 5.2% greater than female controls. In this cohort of runners and controls, it appears that running provided both site specific and general improvements in BMD and that maturity may help explain differences in spine BMD measurements between the sexes. These findings are similar to previous research in which runners were compared with control groups; however, in each one of these investigations the runner and control groups were at least 4 years older than the participants in the present investigation. Kemmler et al. (2006) found similar results in their investigation, with total body and pelvis BMD significantly greater than BMI-matched controls in an investigation of male distance runners (Kemmler et al. 2006). Tam et al. (2018) examined elite male Kenyan distance runners and found a significant difference in BMD at the proximal femur but not at the lumbar spine, femoral neck, or whole body when compared with a control group (Tam et al. 2018). However, the control group in the Tam et al. investigation was not age or size matched. In an investigation of male runners, gymnasts, swimmers and age-matched controls, Hind et al. (2012) concluded that athletes who competed in weight-bearing activities (running and gymnastics) had superior hip geometry and resistance to axial loading than those not engaged in regular weight-bearing activity (swimmers and controls).
In contrast to the findings at the hip which seem to support loading from weight-bearing activity, running does not seem to offer an advantage in bone health at the spine (Fredericson et al. 2007; Hind et al. 2006). Hind et al. (2006) concluded that runners who performed at least two resistance training sessions per week had greater lumber spine BMD due to the nature of forces applied to the skeleton while lifting weights. However, the twice weekly resistance training of the runners in the present investigation did not appear to promote increases in AP spine BMD. Sex differences in timing of peak bone mass and the loading patterns of running may explain why women displayed greater bone mass at the spine while men displayed greater bone mass at the hip in our cohort. The relatively high percentage of non-running controls with low z scores, especially for the males, suggests promotion of weight-bearing exercise among people of lower BMI could be important for long-term bone health.
One of the important factors that may influence bone accrual is EA. Previous research reported that an EA of less than 30 kcals kgFFM−1 impacts bone formation (Loucks et al. 2011) and reproductive function in women (Loucks 2004). Among the female participants in this study, there was no difference in the percentage of runners or controls who had EA of less than 30 kcals kgFFM−1. Although group differences were not observed, EA estimates for nearly 30% of the study participants fell short of healthy ranges (Burke et al. 2018a, 2018b). There have been very few studies which have examined EA and bone health in men. Our investigation reveals that a high percentage of the male runners (42%) did not achieve an EA of 30 kcals kgFFM−1 which was significantly greater than the male controls (14%). Zanker and Swaine (2000) reported that restricted energy intake in male runners impacted serum IGF-1 and may cause a decrease in bone collagen formation. Decreased bone formation and increased bone resorption among females with low EA (15 kcals kgFFM−1) have been shown but a similar result was not seen in males (Papageorgiou et al. 2017). The authors noted that the percentage decrease in bone turnover markers was nearly the same in the male (− 15%) and female (− 14%) participants in their study; however, changes for the males did not reach significance. Our estimation of EA is derived with habitual calorie intake and physical activity or training patterns over the previous 3 months; therefore, this assessment of EA reflects energy status in the 3 months directly prior to the DXA scan.
Operationally, EA may appear to be a simple equation; however, researchers agree and point to many factors which contribute to the difficulty in accurately estimating EA (Burke et al. 2018a, 2018b; Mountjoy et al. 2014 #574). The calculations presented in this paper benefit from the use of DXA, the reference standard in measurement of FFM (Buckinx et al. 2018). Our estimation of EA incorporated calorie intake derived from the Block 2014 FFQ which previous research has established as a valid and reliable measure of dietary intake (Block et al. 1990, 1992). Well-accepted methods of measuring calorie intake also include random 3-day diet recalls, yet, no method of dietary assessment is perfect and while the Block FFQ is designed to measure habitual intake, it is possible that some highly selective eaters are consuming uncommon foods that are not included in the questionnaire. Even though photos of portion sizes were provided to help improve estimates, it is possible that the participants underreported their dietary intake, which would impact the EA calculations. Our methods are among the most accurate currently available until a Best Practice Protocol is developed (Burke et al. 2018a, 2018b).
When comparing the EDEQ results of this cohort of runners to normative data, the male runners in this investigation scored in the 85–90th percentile for male competitive athletes (Darcy et al. 2013) in eating concern and shape concern and between the 60–65th percentile in dietary restraint and weight concern. The female athletes in this investigation scored below the 45th percentile in all categories except eating concern, where they were at the 60th percentile. The controls in this investigation were below the 50th percentile for American college students (Quick and Byrd-Bredbenner 2013) in all EDEQ categories. The high percentile ranking of the male runners in conjunction with the large percentage of male runners with an EA of less than 30 kcals kgFFM−1 may be indicative of a sport where leanness is viewed as necessary for elite level competitors. However, the tradeoff could be an impact on physiologic systems beyond the skeletal system such as hormonal changes, resting metabolic changes, increases in catabolic markers, as well as an impact on sports performance. Interpretation of these findings should acknowledge limitations in the self-report nature of the EDEQ. Future research, especially into male collegiate distance runners, is warranted and further examination is needed of the reasons underlying the EDEQ results in which the male runners scored in the 85–90th percentile in eating concern and shape concern, and were significantly higher in dietary restraint than the female runners and controls.
A major strength of this investigation is use of a control group that was closely matched for age and weight, but was not involved in large quantities of physical activity or sports. Many other investigations of bone health among runners have not utilized a comparison group which is similar in body size to the athletes (Smock et al. 2009; Stewart and Hannan 2000; Tam et al. 2018). Perhaps one of the greatest strengths of this research is that it adds to the scientific literature examining bone health and RED-S in males. The female athlete triad has been examined for decades; however, the consequences and contributing factors of low EA in male athletes need further exploration (Burke et al. 2018a, 2018b; Mountjoy et al. 2018).
This research could be improved by including assessment of indicators of health beyond the skeletal system such as resting metabolic rate, cardiovascular health, hormone levels, and immune health (Burke et al. 2018a, 2018b; Mountjoy et al. 2014). For example, it would be helpful to know blood levels for some nutrients and bone metabolism. In our population, less than 12% of participants achieved the DRI for vitamin D; however, with our location in southern California, it is possible that serum levels of vitamin D are adequate due to hormone production via skin exposure to the sun, especially in the runners, who train outdoors in the sun throughout the week. This research can be further improved by conducting longitudinal analysis of the health consequences of RED-S in male and female collegiate runners (Mountjoy et al. 2018; Tenforde and Fredericson 2011).
Conclusion
There are a number of interesting findings from this investigation, especially in male runners. It appears that in male collegiate runners, the weight-bearing activity provides a beneficial effect in BMD at the hip, femoral neck, and whole body. In female collegiate runners, it appears that running provides a beneficial effect in whole-body BMD. In this group of male runners, a high percentage (42%) were not achieving 30 kcals kgFFM−1 and were in the 85th to 90th percentile in Eating Concern and Shape Concern from their EDEQ results. Clinically, a majority of the z scores for the nine runners with bone injuries were above the expected norm for their age, suggesting that the injuries were due to training rather than low BMD. From a practical standpoint, education for elite endurance athletes, especially young elite endurance athletes, discussing the importance of EA for physiologic function and athletic performance, should be included in their training regimen. As Mountjoy et al. (2018) point out, it is imperative to detect low EA and/or RED-S early to avoid long-term health consequences and decreased athletic performance.
Abbreviations
- ANCOVA:
-
Analysis of covariance
- AP:
-
Anterior–posterior
- BMD:
-
Bone mineral density
- BMI:
-
Body mass index
- DXA:
-
Dual-energy X-ray absorptiometry
- EA:
-
Energy availability
- EDEQ:
-
Eating Disorder Examination Questionnaire
- EEE:
-
Exercise energy expenditure
- EI:
-
Energy intake
- FFM:
-
Fat-free mass
- FFQ:
-
Food Frequency Questionnaire
- MET:
-
Metabolic equivalent
- RED-S:
-
Relative energy deficiency in sport
- RMR:
-
Resting metabolic rate
References
Ainsworth BE, Haskell WL, Herrmann SD, Meckes N, Bassett DR Jr, Tudor-Locke C, Leon AS (2011) 2011 compendium of physical activities: a second update of codes and MET values. Med Sci Sports Exerc 43(8):1575–1581. https://doi.org/10.1249/MSS.0b013e31821ece12
Anderson DA, Lundgren JD, Shapiro JR, Paulosky CA (2004) Assessment of eating disorders: review and recommendations for clinical use. Behav Modif 28(6):763–782. https://doi.org/10.1177/0145445503259851
Barrack MT, Rauh MJ, Barkai HS, Nichols JF (2008) Dietary restraint and low bone mass in female adolescent endurance runners. Am J Clin Nutr 87(1):36–43
Barrack MT, Gibbs JC, De Souza MJ, Williams NI, Nichols JF, Rauh MJ, Nattiv A (2014) Higher incidence of bone stress injuries with increasing female athlete triad-related risk factors: a prospective multisite study of exercising girls and women. Am J Sports Med 42(4):949–958. https://doi.org/10.1177/0363546513520295
Barrack MT, Fredericson M, Tenforde AS, Nattiv A (2017) Evidence of a cumulative effect for risk factors predicting low bone mass among male adolescent athletes. Br J Sports Med 51(3):200–205. https://doi.org/10.1136/bjsports-2016-096698
Baxter-Jones AD, Mirwald RL, McKay HA, Bailey DA (2003) A longitudinal analysis of sex differences in bone mineral accrual in healthy 8–19-year-old boys and girls. Ann Hum Biol 30(2):160–175
Baxter-Jones AD, Faulkner RA, Forwood MR, Mirwald RL, Bailey DA (2011) Bone mineral accrual from 8 to 30 years of age: an estimation of peak bone mass. J Bone Miner Res 26(8):1729–1739. https://doi.org/10.1002/jbmr.412
Black AE (2000) Critical evaluation of energy intake using the Goldberg cut-off for energy intake: basal metabolic rate. A practical guide to its calculation, use and limitations. Int J Obes Relat Metab Disord 24(9):1119–1130
Block G, Woods M, Potosky A, Clifford C (1990) Validation of a self-administered diet history questionnaire using multiple diet records. J Clin Epidemiol 43(12):1327–1335
Block G, Thompson FE, Hartman AM, Larkin FA, Guire KE (1992) Comparison of two dietary questionnaires validated against multiple dietary records collected during a 1-year period. J Am Diet Assoc 92(6):686–693
Buckinx F, Landi F, Cesari M et al (2018) Pitfalls in the measurement of muscle mass: a need for a reference standard. J Cachexia Sarcopenia Muscle 9(2):269–278
Burke LM, Close GL, Lundy B, Mooses M, Morton JP, Tenforde AS (2018a) Relative energy deficiency in sport in male athletes: a commentary on its presentation among selected groups of male athletes. Int J Sport Nutr Exerc Metab 28(4):364–374. https://doi.org/10.1123/ijsnem.2018-0182
Burke LM, Lundy B, Fahrenholtz IL, Melin AK (2018b) Pitfalls of conducting and interpreting estimates of energy availability in free-living athletes. Int J Sport Nutr Exerc Metab 28(4):350–363. https://doi.org/10.1123/ijsnem.2018-0142
Cunningham JJ (1991) Body composition as a determinant of energy expenditure: a synthetic review and a proposed general prediction equation. Am J Clin Nutr 54(6):963–969. https://doi.org/10.1093/ajcn/54.6.963
Darcy AM, Hardy KK, Lock J, Hill KB, Peebles R (2013) The Eating Disorder Examination Questionnaire (EDE-Q) among university men and women at different levels of athleticism. Eat Behav 14(3):378–381. https://doi.org/10.1016/j.eatbeh.2013.04.002
Fairburn CG, Cooper Z, O’Connor M (2008) Eating Disorder Examination. In: Fairburn CG (ed) Cognitive behavior therapy and eating disorders, 16.0th edn. Guilford Press, New York
Fredericson M, Chew K, Ngo J, Cleek T, Kiratli J, Cobb K (2007) Regional bone mineral density in male athletes: a comparison of soccer players, runners and controls. Br J Sports Med 41(10):664–668. https://doi.org/10.1136/bjsm.2006.030783 (discussion 668)
Garber CE, Blissmer B, Deschenes MR, Franklin BA, Lamonte MJ, Lee IM, American College of Sports M (2011) American College of Sports Medicine position stand. Quantity and quality of exercise for developing and maintaining cardiorespiratory, musculoskeletal, and neuromotor fitness in apparently healthy adults: guidance for prescribing exercise. Med Sci Sports Exerc 43(7):1334–1359. https://doi.org/10.1249/mss.0b013e318213fefb
Guebels CP, Kam LC, Maddalozzo GF, Manore MM (2014) Active women before/after an intervention designed to restore menstrual function: resting metabolic rate and comparison of four methods to quantify energy expenditure and energy availability. Int J Sport Nutr Exerc Metab 24(1):37–46. https://doi.org/10.1123/ijsnem.2012-0165
Gunter KB, Almstedt HC, Janz KF (2012) Physical activity in childhood may be the key to optimizing lifespan skeletal health. Exerc Sport Sci Rev 40(1):13–21. https://doi.org/10.1097/JES.0b013e318236e5ee
Hartman AM, Block G, Chan W, Williams J, McAdams M, Banks WL Jr, Robbins A (1996) Reproducibility of a self-administered diet history questionnaire administered three times over three different seasons. Nutr Cancer 25(3):305–315
Heikura IA, Uusitalo ALT, Stellingwerff T, Bergland D, Mero AA, Burke LM (2018) Low energy availability is difficult to assess but outcomes have large impact on bone injury rates in elite distance athletes. Int J Sport Nutr Exerc Metab 28(4):403–411. https://doi.org/10.1123/ijsnem.2017-0313
Hind K, Truscott JG, Evans JA (2006) Low lumbar spine bone mineral density in both male and female endurance runners. Bone 39(4):880–885. https://doi.org/10.1016/j.bone.2006.03.012
Hind K, Gannon L, Whatley E, Cooke C, Truscott J (2012) Bone cross-sectional geometry in male runners, gymnasts, swimmers and non-athletic controls: a hip-structural analysis study. Eur J Appl Physiol 112(2):535–541. https://doi.org/10.1007/s00421-011-2008-y
Ihle R, Loucks AB (2004) Dose-response relationships between energy availability and bone turnover in young exercising women. J Bone Miner Res 19(8):1231–1240. https://doi.org/10.1359/JBMR.040410
Kemmler W, Engelke K, Baumann H, Beeskow C, von Stengel S, Weineck J, Kalender WA (2006) Bone status in elite male runners. Eur J Appl Physiol 96(1):78–85. https://doi.org/10.1007/s00421-005-0060-1
Koehler K, Achtzehn S, Braun H, Mester J, Schaenzer W (2013) Comparison of self-reported energy availability and metabolic hormones to assess adequacy of dietary energy intake in young elite athletes. Appl Physiol Nutr Metab 38(7):725–733. https://doi.org/10.1139/apnm-2012-0373
Kohl HW, Blair SN, Paffenbarger RS Jr, Macera CA, Kronenfeld JJ (1988) A mail survey of physical activity habits as related to measured physical fitness. Am J Epidemiol 127(6):1228–1239
Kohrt WM, Bloomfield SA, Little KD, Nelson ME, Yingling VR (2004) American College of Sports Medicine Position Stand: physical activity and bone health. Med Sci Sports Exerc 36(11):1985–1996
LaBrie JW, Boyle S, Earle A, Almstedt HC (2018) Heavy episodic drinking is associated with poorer bone health in adolescent and young adult women. J Stud Alcohol Drugs 79(3):391–398
Loucks AB (2004) Energy balance and body composition in sports and exercise. J Sports Sci 22(1):1–14. https://doi.org/10.1080/0264041031000140518
Loucks AB, Kiens B, Wright HH (2011) Energy availability in athletes. J Sports Sci 29(Suppl 1):S7–15. https://doi.org/10.1080/02640414.2011.588958
Luce KH, Engler PA, Crowther JH (2007) Eating disorders and alcohol use: group differences in consumption rates and drinking motives. Eat Behav 8(2):177–184. https://doi.org/10.1016/j.eatbeh.2006.04.003
Melin A, Tornberg AB, Skouby S, Moller SS, Sundgot-Borgen J, Faber J, Sjodin A (2015) Energy availability and the female athlete triad in elite endurance athletes. Scand J Med Sci Sports 25(5):610–622. https://doi.org/10.1111/sms.12261
Mountjoy M, Sundgot-Borgen J, Burke L, Carter S, Constantini N, Lebrun C, Ljungqvist A (2014) The IOC consensus statement: beyond the Female Athlete Triad-Relative Energy Deficiency in Sport (RED-S). Br J Sports Med 48(7):491–497. https://doi.org/10.1136/bjsports-2014-093502
Mountjoy M, Sundgot-Borgen J, Burke L, Ackerman KE, Blauwet C, Constantini N, Budgett R (2018) International Olympic Committee (IOC) Consensus Statement on Relative Energy Deficiency in Sport (RED-S): 2018 update. Int J Sport Nutr Exerc Metab 28(4):316–331. https://doi.org/10.1123/ijsnem.2018-0136
Nattiv A, Loucks AB, Manore MM, Sanborn CF, Sundgot-Borgen J, Warren MP (2007) American College of Sports Medicine position stand. The female athlete triad. Med Sci Sports Exerc 39(10):1867–1882. https://doi.org/10.1249/mss.0b013e318149f11100005768-200710000-00026
Papageorgiou M, Elliott-Sale KJ, Parsons A, Tang JCY, Greeves JP, Fraser WD, Sale C (2017) Effects of reduced energy availability on bone metabolism in women and men. Bone 105:191–199. https://doi.org/10.1016/j.bone.2017.08.019
Pereira MA, FitzerGerald SJ, Gregg EW, Joswiak ML, Ryan WJ, Suminski RR, Zmuda JM (1997) A collection of Physical Activity Questionnaires for health-related research. Med Sci Sports Exerc 29(6 Suppl):S1–205
Quick VM, Byrd-Bredbenner C (2013) Eating Disorders Examination Questionnaire (EDE-Q): norms for US college students. Eat Weight Disord 18(1):29–35. https://doi.org/10.1007/s40519-013-0015-1
Smock AJ, Hughes JM, Popp KL, Wetzsteon RJ, Stovitz SD, Kaufman BC, Petit MA (2009) Bone volumetric density, geometry, and strength in female and male collegiate runners. Med Sci Sports Exerc 41(11):2026–2032. https://doi.org/10.1249/MSS.0b013e3181a7a5a2
Stewart AD, Hannan J (2000) Total and regional bone density in male runners, cyclists, and controls. Med Sci Sports Exerc 32(8):1373–1377
Tam N, Santos-Concejero J, Tucker R, Lamberts RP, Micklesfield LK (2018) Bone health in elite Kenyan runners. J Sports Sci 36(4):456–461. https://doi.org/10.1080/02640414.2017.1313998
Tenforde AS, Fredericson M (2011) Influence of sports participation on bone health in the young athlete: a review of the literature. PMR 3(9):861–867. https://doi.org/10.1016/j.pmrj.2011.05.019
Tenforde AS, Fredericson M, Sayres LC, Cutti P, Sainani KL (2015) Identifying sex-specific risk factors for low bone mineral density in adolescent runners. Am J Sports Med 43(6):1494–1504. https://doi.org/10.1177/0363546515572142
Tenforde AS, Kraus E, Fredericson M (2016) Bone stress injuries in runners. Phys Med Rehabil Clin N Am 27(1):139–149. https://doi.org/10.1016/j.pmr.2015.08.008
Tenforde AS, Carlson JL, Chang A, Sainani KL, Shultz R, Kim JH, Fredericson M (2017) Association of the female athlete triad risk assessment stratification to the development of bone stress injuries in collegiate athletes. Am J Sports Med 45(2):302–310. https://doi.org/10.1177/0363546516676262
Tenforde AS, Parziale AL, Popp KL, Ackerman KE (2018) Low bone mineral density in male athletes is associated with bone stress injuries at anatomic sites with greater trabecular composition. Am J Sports Med 46(1):30–36. https://doi.org/10.1177/0363546517730584
Viner RT, Harris M, Berning JR, Meyer NL (2015) Energy availability and dietary patterns of adult male and female competitive cyclists with lower than expected bone mineral density. Int J Sport Nutr Exerc Metab 25(6):594–602. https://doi.org/10.1123/ijsnem.2015-0073
Zanker CL, Swaine IL (2000) Responses of bone turnover markers to repeated endurance running in humans under conditions of energy balance or energy restriction. Eur J Appl Physiol 83(4–5):434–440. https://doi.org/10.1007/s004210000293
Acknowledgements
We are very grateful to the research participants, Scott Guerrero, Caitlin Jennings, and Liam Shorrock for their role in the success of this research project. We also acknowledge contributions from student research assistants involved with the data collection and data management including Lily Bramble, Makenzie Cook, and Rowena Feng.
Author information
Authors and Affiliations
Contributions
WPM developed the original research inquiry, recruited participants, collected data, analyzed data, and collaborated in writing of manuscript. TCS assisted in data analysis and writing of the manuscript. JWL assisted in recruiting control participants and provided guidance on the composition of the paper. HCA collected data, analyzed data, and collaborated in writing of manuscript. The control group for this investigation was funded by a National Institute on Alcohol Abuse and Alcoholism Grant 5R21AA022942-02.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Communicated by Olivier Seynnes.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
McCormack, W.P., Shoepe, T.C., LaBrie, J. et al. Bone mineral density, energy availability, and dietary restraint in collegiate cross-country runners and non-running controls. Eur J Appl Physiol 119, 1747–1756 (2019). https://doi.org/10.1007/s00421-019-04164-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00421-019-04164-z