Abstract
Purpose
This study examined and compared select Triad–RED–S components/risk factors in high-level Kenyan male and female distance runners to corresponding control groups; focusing on examining energy intake (EI), bone indices, and hormonal markers.
Methods
A cross-sectional, observational design was used in which Kenyan male and female (n = 30 and n = 26, respectively) middle- and long-distance runners and corresponding male and female control groups (n = 29 and n = 29, respectively) were examined.
The participant’s bone mineral density (BMD) at the lumbar spine, right femur, and total body were measured using a dual-energy X-ray absorptiometry analysis. Complete blood counts (CBC) were done on the whole blood specimens and hormonal measurements were performed on plasma specimens. In addition, athletes completed metabolic testing to determine maximal oxygen uptakes and 7-day dietary diaries.
Results
Overall daily EI across runners and controls within each sex were low, but not significantly different (p > 0.05). Prevalence of low BMD values (Z score < − 2.0) was comparable across groups in each sex (p > 0.05). CBC measures suggested that both runners and controls were healthy. Finally, slight hormonal differences between runners and their respective controls existed (p < 0.05), but were not clinically meaningful or observed in typical Triad–RED–S-related parameters.
Conclusion
High-level Kenyan male and female runners had low daily EI, but no tendency toward a higher prevalence of low BMD, or Triad–RED–S–related hormonal abnormalities. The occurrence of low EI was not a major risk factor in our athletes; this calls into question whether the current criteria for Triad–RED–S are entirely applicable for athletes of African ethnicity.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Excessive physical activity without a concomitant increase in appropriate dietary intake can be associated with energy deficiency, which can disrupt the reproductive cycle and result in amenorrhea in females (Sangenis et al. 2006; Loucks et al. 1989, 1992). Furthermore, an inadequate caloric intake and the resulting consequence of decreases in endogenous estrogen may eventually result in an imbalance in bone remodeling leading to low bone mineral density (Sangenis et al. 2006; Drinkwater et al. 1984; Ilhe and Loucks 2004). In women, the consequences of these events are referred to as the Female Athlete Triad (Triad) condition. This model is defined as the existence of three specific components: (1) low energy availability (with or without disordered eating); (2) menstrual dysfunction; and (3) low bone mineral density, which can occur in isolation or all three concurrently. Over a period of time, an athlete may move along on a continuous spectrum ranging from optimal health with appropriate energy availability (EA), regular menses, and healthy bones to the opposite end of the spectrum characterized by amenorrhea, low EA, and osteoporosis (Nattiv et al. 2007; Mountjoy et al. 2014). According to De Souza et al. (2014) for female athletes who present with one or more of the Triad components, an early intervention is essential to prevent the progression of the condition to the potentially serious clinical endpoints.
As a new dimension to the Triad–related area of study, Mountjoy et al. (2014) in the IOC consensus statement introduced a more comprehensive, broader terminology for the overall syndrome—Relative Energy Deficiency in Sport (RED-S), which encompasses a range of negative health consequences from exercise training beyond that traditionally associated with the Triad, such as: cardiovascular, gastrointestinal, immunological, endocrine, metabolic, hematological, growth and development, and psychological health. Additionally, these investigators argued that a relative energy deficiency is also affecting male athletes (Mountjoy et al. 2014). Interestingly, there is growing evidence that males may experience low energy availability (LEA) in situations when there is a mismatch between energy intake and the exercise energy expenditure of training or competition. Populations of male athletes at increased risk for LEA and the resulting health consequences of RED-S include cyclists, rowers, runners, jockeys, and athletes in weight class combat sports (Burke et al. 2018). The need to further investigate potential effects of poor energetic status to male athletes was also acknowledged in the first publication of the Female Athlete Triad in 1992. In 2019, Male Athlete Triad was formally recognized as a syndrome which reflects that similar energetic, reproductive, and bone health problems occur in men (Nattiv et al. 2021). The underlying mechanism of RED-S/Triad is an inadequacy of energy to support the full range of body functions involved for optimal health and performance in female as well as male athletes. Low energy availability, which occurs with a reduction in energy intake and/or increased exercise load, causes adjustments to body systems to reduce/conserve energy expenditure, leading to disruption of an array of hormonal, metabolic, and functional characteristics (Mountjoy et al. 2014; Loucks 2004; Hackney 2008).
The success of east African middle- and long-distance runners, especially from Kenya and Ethiopia, is well known as they have dominated international running events for decades (Burke et al. 2018). To this end, Kenyan male and female runners have collectively won astonishing 63 Olympic medals in middle- and long-distance running since the 1996 Atlanta Olympic Games. However, a recent study (Muia et al. 2015) showed that elite-level adolescent Kenyan female runners had LEA, with 17.9% reaching clinical criteria levels and 76% at subclinical levels. Furthermore, it has been shown previously that Kenyan elite runners have low bone mineral density (BMD). For example, Tam and colleagues reported that 40% of their male study participants had a Z score less than − 2 at lumbar spine; however, they did not collect dietary intake data from their study cohort (Tam et al. 2017).
To date, published research on the Triad and/or RED-S status is greatly lacking in athletes of diverse racial backgrounds (i.e., African, African-American, Hispanic, and Asian athletes), especially relative to bone health indices. Thus, there is a need to include more diverse athlete populations into such research and to integrate race/ethnicity to determine appropriate prevention and treatment strategies (Mountjoy et al. 2018). Therefore, the purpose of this study is to examine and compare the prevalence of select Triad–RED–S components/risk factors in Kenyan male and female distance runners to corresponding control groups with special interest on examining and comparing energy intake, bone indices, and hormonal markers commonly associated with Triad–RED–S. Our primary hypothesis was that Kenyan male and female distance runners would present lower bone mineral indices compared to ethnically similar control groups.
Methods
Participants and study design
For this study, a cross-sectional, observational design approach was used in which high-level Kenyan male and female (n = 30 and n = 26, respectively) middle- and long-distance runners and corresponding male and female control groups (n = 29 and n = 29, respectively) were examined. However, due to technical reasons, the number of participants varies across the different measurements. Study participants were recruited from their training camps in the Eldoret area and from the Moi University campus area in Kenya. The control group consisted of students from Moi University of similar age to the runners. All participants received written and oral information about the study before signing the informed consent statement.
The seasonal best running performance of each runner was assessed using the International Association of Athletics Federations (IAAF) Scoring Tables (Spiriev 2017) which enables comparison between events and participants (Legaz and Eston 2005; Mooses et al. 2015). All runners were required to be actively engaged in training and competing at the time of the study for middle- to long-distance athletics events, had done so for a number of years, and were viewed by their coaches as high-level performers. For the study, each participant (runner and control) was required to complete three visits in our laboratory facilities in Kenya.
First visit
Anthropometry and body composition
During the first visit, body composition measurements were obtained for both runners and control group and a familiarization session for the runners with the equipment that was used during the metabolic testing to determine maximal oxygen uptake (i.e., VO2max [see later description]).
Height (Power Tape; Lidu Hardware, Zhejiang, China) and body mass (Seca robusta 813; Seca GmbH and Co., Hamburg, Germany) of the participants was measured to the nearest 0.01 m and 0.1 kg, respectively. Body mass index (BMI, kg/m2) was derived as body mass (kg) divided by body height squared (m2). The participant’s bone mineral density (BMD) at the lumbar spine (LS-BMD), right femur (RF-BMD), and total body (TB-BMD) were measured using a dual-energy X-ray absorptiometry (DXA) analysis (Hologic Discovery QDR Series, Waltham, MA, USA). All the DXA procedures were performed in the morning between 8 and 11 am and all the procedures were carried out by the hospital, and the DXA system was calibrated by the same technician in accordance with the manufacturer’s guidelines. DXA procedure was carried out by the same qualified person from the hospital. During the measurements, participants wore shorts and T-shirt. Low BMD was defined as Z score between − 1.0 and − 2.0, while an osteoporosis condition was considered if < − 2.0 (Nattiv et al. 2007). Body composition was evaluated from whole-body DXA scan as described by Shepherd and colleagues (2017).
Second visit
Blood analyses
During this visit, blood samples were obtained from all participants from a vein in the antecubital region of their preferred arm after completing an overnight fast between 7:30 and 9:30 am, and before the exercise testing was performed by the runners. Complete blood counts (CBC) (Analyser Coulter AC T 5 diff AL, Beckman Coulter, Brea, California, United States) were done immediately on the whole blood specimens at the University Moi Hospital Laboratory (Eldoret, Kenya). These blood specimens were then centrifuged, and the plasma separated and stored frozen at − 20 °C until later analysis. Hormonal measurements were performed on plasma specimens using a Cobas 6000 e601 module (Roche Holding AG, Basel, Switzerland) and consisted of luteinizing hormone, follicle-stimulating hormone, prolactin, estradiol, free thyroxine, thyroid-stimulating hormone, testosterone, triiodothyronine, insulin, and cortisol. An IDS-iSYS-automated immunoassay analyzer (Immunodiagnostic Systems Holdings PLC, Boldon, United Kingdom) was used for determining growth hormone and insulin-like growth factor. All hormonal analytical procedures were performed according to the manufacturer’s instructions at the University of Tartu, United Laboratories (Tartu, Estonia). All appropriate clinical analytical procedures and steps were utilized to assure the samples were viable and accurate results (Hackney and Viru 2008).
Exercise testing protocol
Also, during this second visit, the runners completed exercise testing. They were instructed to abstain from high-intensity training and/or competition for at least 24 h before exercise testing, and to maintain their usual dietary habits as well as to refrain from caffeine and alcohol consumption prior to returning for their second visit.
Following the warm-up period (10 min, freely chosen speeds) with the treadmill, participants performed an incremental running test on a motorized treadmill (Cardionics Type 3113, Sweden) until voluntary exhaustion. Before commencement of the test, each participant remained stationary on the treadmill for 3 min and resting cardio-respiratory data were collected. The initial running speed was set at 8 km·h−1 for female and 10 km·h−1 for male participants with a gradient of 1% (Mooses et al. 2015; Jones and Doust 1996). The running speed was increased 2 km·h−1 every 3 min until 16 km·h−1 for females and 18 km·h−1 for male participants. Following the 3 min stage at 16 km·h−1 for female and 18 km·h−1 for male participants, respectively, the speed remained constant until the end of the test; however, the elevation was increased 1% after each minute until voluntary exhaustion (MacDougall et al. 1982). Heart rate and expired gases were measured continuously using MetaMax 3B (Cortex Biophysic GmbH, Leipzig, Germany), which was calibrated before each test according to instructions by the manufacturer. The highest average oxygen uptake (VO2) during a 30 s period as well as a failure to further increase VO2 consumption despite an increase in the work rate was defined as the VO2max (Wasserman et al. 2004).
Finally, participants were given 7-day dietary intake diaries to record food consumption to all participants and additionally 7-day training diaries to the runners. All participants were instructed on how to record and approximate food amounts—quantities by members of the research team before being excused from our laboratory. This included going through the food diary step-by-step with a description of one example day, which was given to the participant with the diary. To ensure accurate completion of these diaries, verbal explanations were given by a member of the research team and written instructions were sent home with each participant. After returning the filled out food diary, researcher went through the diary and, if necessary, asked additional information/clarification from the participant.
Third visit
Energy intake
During the third and final visit, food diaries were collected from the participants, checked in their presence, and any discrepancies in entries were clarified by a member of the research team. The subsequent dietary analysis was conducted with NutriSurvey (Germany, 2007) software. Based on prior reports with similar populations, it was expected that there would be the potential for substantial underreporting of dietary intake (Mertz et al. 1991; Fudge et al. 2006; Scagliusi et al. 2006; Pfrimer et al. 2015; Orcholski et al 2015). To that end, to be conservative in our assessment of dietary status, we included three highest energy intakes (EI) days from the 7-day dietary intake diary and a correction factor was devised from this literature and applied (~ 46% underreport). Herein, we report the nutritional findings in both an uncorrected and corrected fashion.
Statistical analysis
Statistically, the data are presented descriptively as means and standard deviations (± SD); additionally, range values are reported. Between-groups comparisons (runners vs. controls) within each sex for most variables were performed with a one-way ANOVA with statistical significance set at p ≤ 0.05, and for comparison of prevalence of low bone mineral density (Tables 2 and 3), a Chi-square analysis with Fisher’s exact test was utilized. All statistical computations were performed with SPSS version 20.
Ethical statement
All ethical approaches and procedures were carried out according to the World Medical Association Declaration of Helsinki. The study procedures and protocols were approved by the Institutional Review Board of School of Medicine, Moi University, Kenya and Research Ethics Committee of the University of Tartu, Estonia.
Results
The participant’s physical characteristics and IAAF performance scores and VO2max values (runners only) are reported in Table 1. Male athletes were slightly older (p = 0.001), and had lower mass (p = 0.030) and a lower BMI (p = 0.024) compared to the male controls. Female athletes had lower body mass (p = 0.001) and BMI (p = 0.001) compared to the female controls. BMI was in the normal range for all study groups (i.e., importantly, no controls were obese). The mean IAAF performance scores of the runners confirmed them as being high-level performers (i.e., 10,000 M [< 28:30, < 34:00] or marathon [< 2:14:00, < 2:40:00] equivalences, for the men and women, respectively).
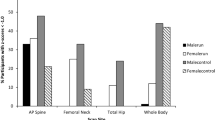
Table 2 for males and Table 3 for females present the prevalence of Z score values in three categories of bone measurements (LS-, RF-, and TB-BMD) and the p values for Fisher’s exact test from the Chi-square analysis. There were no significant differences in the prevalence of low LS-, RF-, or TB-BMD values between male runners and male controls nor between the female runners and female controls.
Tables 4 and 5 present the blood work findings for each of the study groups, specifically, the complete blood count (CBC, select values) and hormonal values and corresponding reference ranges. All the hormonal and CBC values measured were in the expected range for the specific study group. Male athletes did have higher cortisol (p = 0.005) and lower red blood cells (p = 0.003), hematocrit (p = 0.014), and estradiol (p = 0.049) values compared to male controls. For females, the athletes had higher neutrophils (p = 0.015), but lower lymphocytes (p = 0.036) and insulin (p = 0.001) levels compared to female controls.
Dietary intake values, absolute and relative to body mass (uncorrected and corrected) are presented in Table 6. We found that male athletes had higher calcium (p = 0.05) intake compared to the control group. Female athletes had higher carbohydrates (p = 0.035) and calcium (p = 0.039) intake compared to the control group. No other runner vs. control differences were noted for assessed measures.
Discussion
The purpose of this study was to examine and compare the prevalence of select Triad–RED–S components/risk factors in Kenyan male and female distance runners to corresponding control groups with special interest on bone indices, hormonal markers, and energy intake commonly associated with RED-S. We hypothesized that our key finding would be Kenyan male and female distance runners would present lower bone mineral indices compared to ethnically similar control groups. The data from the present study did not support this hypothesis.
BMD
We found that there was a remarkably similar prevalence of low BMD (Z score < − 1) in the male and female Kenyans, but no difference in prevalence between male runners and male controls and female runners and female controls. It has been shown previously that Kenyan elite runners have low BMD (Tam et al. 2017). However, similarly, to our study, Goodwin et al. (2014) found that there were no differences in bone mineral density by region in their study with females between athletes and controls.
In our study, there were some participants (runners and controls) with very low BMD (Z score < 2), but most did not have excessively low BMD values across our various measurement sites. It is known that long periods of inadequate energy consumption (low energy availability) can result in a low BMD status (Mountjoy et al. 2014). We speculate that our lack of substantial numbers of participants having low BMD could be the resultant of the combination of factors: (1) osteogenic effects of running (2) adequate intake of carbohydrates, which has been shown to acutely effects bone turnover (Hammond et al. 2019; Langan-Evans et al. 2021). That said, the overall EI of our participants (runners vs. controls) seems relatively low (see subsequent discussion). These findings suggest that further research is warranted on this topic with our unique sample population of runners. Finally, statistic testing for differences in prevalence of bone health indices between the sexes as that comparison was not conducted as it was not a focus of our work.
CBC and hormones
Our intent in examining the complete blood counts (CBC) was to assure ourselves that our male and female samples (athlete and control) were healthy and not suffering any illness; and, or displaying signs of dehydration. Furthermore, some evidence points to athletes with Triad–RED–S displaying immune systems dysfunctions as a consequence of their energy availability imbalance (Sangenis et al. 2006). While we did see some significant differences between athletes vs. controls within each sex, these were slight in magnitude and within normal clinical ranges (Kratz et al 2004; Feingold et al. 2000). Overall, these data suggest that our samples were normal in immune function and not dehydrated at the time of the study.
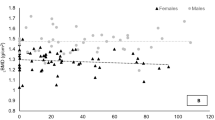
The hormonal values (see Table 5) we observed were variable, but relatively unremarkable as all values for athletes and controls were within clinical norms (Feingold et al. 2000). We did find that cortisol (p = 0.005) levels were somewhat higher, and estradiol (p = 0.049) levels lower in male athletes compared to male controls; and, insulin (p = 0.001) levels were lower in female athletes compared to female controls, but as noted, all within the normal expected range for healthy men and women within each of the respective hormonal measures (Feingold et al. 2000). This was a somewhat surprising finding as we had surmised that based on prior literature (specifically in women), there would be anomalies in hormones levels. Why none were seen is unclear, but as our BMD values were relatively normal, it could be expected that our hormones values would follow suit. Alternative possible explanations could also relate to: (1) Ethnic-race considerations—that is, could hormonal norms for African’s differ somewhat from those based on Caucasian populations? (2) Temporal factors—while low EI occurred in our participants, perhaps, it was not manifested long enough to result in hormonal disturbances. Further work is warranted to pursue this speculation on our part and determine the underlying factor(s) for these findings.
Dietary energy intake
There were no differences in overall EI between runners and controls in our study; although, we found extremely low EI values across all study groups in examining either the uncorrected or corrected results. We had anticipated potential errors in the dietary data based on previous reports (Mertz et al. 1991; Fudge et al. 2006; Scagliusi et al. 2006; Pfrimer et al. 2015; Orcholski et al 2015) and as such applied a correction factor to adjust; nonetheless, we were surprised by the relatively low caloric consumption by all our participants. That said, Christensen et al. (2002) found somewhat similar findings in male adolescent Kenyan runners; although, other studies have reported higher EI than we found (Fudge et al. 2006). There are multiple factors that could potentially contribute to this difference in study findings. First, in our study, the participants had a free living diet, whereas in some studies, dietary food was provided by the schools where participants were enrolled and as such could be consumed ad libitum. Second, Christensen and colleagues followed up with a 24 h recall interview which could have ensured a more accurate and detailed food consumption record. We too attempted to improve the accuracy of our dietary data by insuring explanations of diet records procedures were given by native Kenyan language(s) speakers.
Macronutrients
We found that the protein intake was elevated and composed 16.7% of male runners, 15.2% of female runners, 17.3% of male controls, and 16.7% of female controls’ dietary intake. This is in accordance with the studies done with Kenyan male runners by Fudge et al. (2006) and Christensen et al. (2002) where protein composed 15.3% and 13% of dietary intake, respectively. In addition, the carbohydrate intake in our study was also in accordance with these previous studies—64.4% for male runners, 65.6% for female runners, 62.0% for male control, and 60.8% for female controls from the total EI as compared to 71% in Christensen (2002) and colleagues study and 67.3% in Fudge et al.’s study (2006). Our findings do not shed light on this issue, but do show macro- and micro-nutrient intake among Kenyan athletes is remarkably similar to their non-athletic country men-women.
Limitations and strengths
As with any study, there were short comings within our research. A key limitation of our study was the self-reported aspects of our dietary records which could have affected the accuracy of the information. Due to the observational nature of this study, we cannot demonstrate a causative linkage between EI and metabolic or endocrine alterations. Also, we did not collect hormonal contraception nor menstrual cycle data. Finally, we had a varying number of subjects across several of our outcome measures, due to technical issues, causing incomplete data sets and as such affecting the robustness of our findings.
One of the strengths of our study is the overall high athletic level of the participants. To give perspective as to the level of our participants, we translated the average IAAF scores into marathon running times, which yielded a 2:39:57 for women and 2:13:41 for men in this study according to the IAAF Scoring Tables of Athletics. To add even more perspective, these marathon times would have given 8th and 13th places, respectively, at the 2019 World Athletics championship. Furthermore, our participants represent not just high-level athletes, but a racial group that has been understudied in the sport science community. Another strength of our study was the usage of DXA for BMD measurements, especially the segmental data of lumbar spine and right femur in addition to total body BMD as the latter may underestimate the prevalence and presence of low BMD (Tam et al. 2017).
Conclusions
Our current findings do not show a statistical linkage between EI and high prevalence of low BMD in male and female Kenyan runners. We did find that high-level Kenyan male and female runners do have, in addition to low overall EI, some occurrences of low BMD, which if were to be of a chronic nature could increase their risk for developing low EA. Nevertheless, even with the low EI observed, there were no major Triad—RED-S risk factors detected in our athletes, which might suggest that the current criteria for Triad–RED–S diagnosis may not be entirely applicable for athletes of African ethnicity. This latter point is in need of future investigation.
Availability of data and materials
The data that support the findings of this study are available from the corresponding author, upon reasonable request.
Code availability
Not applicable.
Change history
24 October 2021
A Correction to this paper has been published: https://doi.org/10.1007/s00421-021-04836-9
Abbreviations
- BMD:
-
Bone mineral density
- BMI:
-
Body mass index
- CBC:
-
Complete blood count
- Cort:
-
Cortisol
- DXA:
-
Dual-energy X-ray absorptiometry
- E2 :
-
Estradiol
- EA:
-
Energy availability
- EI:
-
Energy Intake
- FSH:
-
Follicle-stimulating hormone
- GH:
-
Growth hormone
- HCT:
-
Hematocrit
- HGB:
-
Hemoglobin
- IAAF:
-
International Association of Athletics Federations (world athletics since October 2019)
- IGF-1:
-
Insulin like growth factor-1
- Ins:
-
Insulin
- IOC:
-
International Olympic Committee
- LBM:
-
Lean body mass
- LEA:
-
Low energy availability
- LH:
-
Luteinizing hormone
- LS-BMD:
-
Lumbar spine bone mineral density
- Prol:
-
Prolactin
- RBC:
-
Red blood cells
- RED-S:
-
Relative energy deficiency in sport
- RF-BMD:
-
Right femur bone mineral density
- T3 :
-
Triiodothyronine
- T4 :
-
Thyroxine
- TB-BMD:
-
Total body bone mineral density.
- Testo:
-
Testosterone
- Triad:
-
Female athlete triad
- TSH:
-
Thyroid-stimulating hormone
- VO2 :
-
Oxygen consumption
- VO2max :
-
Maximal oxygen consumption
- WBC:
-
White blood cells
References
Burke LM, Close GL, Lundy B, Mooses M, Morton JP, Tenforde AS (2018) Relative energy deficiency in sport in male athletes: a commentary on its presentation among selected groups of male athletes. Int J Sport Nutr Exer Metab 28(4):364–374
Christensen DL, van Hall G, Hambraeus L (2002) Food and macronutrient intake of male adolescent Kalenjin runners in Kenya. Br J Nutr 88:711–717. https://doi.org/10.1079/BJN2002728
De Souza MJ, Nattiv A, Joy E, Misra M, Williams N, Mallinson R, Gibbs JC, Olmsted M, Goolsby M, Matheson G (2014) 2014 female athlete triad coalition consensus statement on treatment and return to play of the female athlete triad: 1st international conference held in San Francisco, California, May 2012 and 2nd international conference held in Indianapolis, Indiana, May 2013. Clin J Sport Med 24(2):96–119
Drinkwater BL, Nilson K, Chesnut CH 3rd, Bremner WJ, Shainholtz S, Southworth M (1984) Bone mineral content of amenorrheic and eumenorrheic athletes. N Eng J Med 311(5):277–281
Feingold KR, Anawalt B, Boyce A, et al., editors. Endotext. South Dartmouth (MA): MDText.com, Inc. 2000. http://www.ncbi.nlm.nih.gov/books/NBK278943/. Accessed 8 May 2020
Fudge BW, Westerterp KR, Kiplamai FK et al (2006) Evidence of negative energy balance using doubly labelled water in elite Kenyan endurance runners prior to competition. Br J Nutr 95:59–66
Goodwin Y, Monyeki MA, Boit MK et al (2014x) Profile of the female athlete triad among elite Kenyan endurance athletes and non-athletes. Afr J Phys Health Educ Recreat Dance 20:610–625
Hackney AC (2008) Effects of endurance exercise on the reproductive system of men: the “exercise-hypogonadal male condition.” J Endocrinol Invest 31(10):932–938
Hackney AC, Viru A (2008) Research methodology: endocrinologic measurements in exercise science and sports medicine. J Athl Train 43(6):631–639. https://doi.org/10.4085/1062-6050-43.6.631
Hammond KM, Sale C, Fraser W, Tang J, Shepherd SO, Strauss JA, Close GL, Cocks M, Louis J, Pugh J, Stewart C, Sharples AP, Morton JP (2019) Post-exercise carbohydrate and energy availability induce independent effects on skeletal muscle cell signalling and bone turnover: implications for training adaptation. J Physiol 597(18):4779–4796. https://doi.org/10.1113/JP278209
Ilhe R, Loucks AB (2004) Dose-response relationships between energy availability and bone turnover in young exercising women. J Bone Miner Res 19(8):1231–1240
Jones AM, Doust JH (1996) A 1% treadmill grade most accurately reflects the energetic cost of outdoor running. J Sports Sci 14(4):321–327. https://doi.org/10.1080/02640419608727717
Kratz A, Ferraro M, Sluss PM, Lewandrowski KB (2004) Normal reference laboratory values. N Engl J Med 351:1548–1563
Langan-Evans C, Germaine M, Artukovic M, Oxborough DL, Areta JL, Close GL, Morton JP (2021) The psychological and physiological consequences of low energy availability in a male combat sport athlete. Med Sci Sports Exerc 53(4):673–683. https://doi.org/10.1249/MSS.0000000000002519
Legaz A, Eston R (2005) Changes in performance, skinfold thicknesses, and fat patterning after three years of intense athletic conditioning in high level runners. Br J Sports Med 39(11):851–856. https://doi.org/10.1136/bjsm.2005.018960
Loucks AB, Heath EM (1994) Induction of low-T3 syndrome in exercising women occurs at a threshold of energy availability. Am J Physiol 266(3 Pt 2):R817–R823. https://doi.org/10.1152/ajpregu.1994.266.3.R817
Loucks AB, Thuma JR (2003) Luteinizing hormone pulsatility is disrupted at a threshold of energy availability in regularly menstruating women. J Clin Endocrinol Metab 88(1):297–311. https://doi.org/10.1210/jc.2002-020369
Loucks AB, Mortola JF, Girton L, Yen SS (1989) Alterations in the hypothalamicpituitary-ovarian and the hypothalamic-pituitary-adrenal axes in athletic women. J Clin Endocrinol Metab 68(2):402–411
Loucks AB, Laughlin GA, Mortola JF, Girton L, Nelson JC, Yen SS (1992) Hypothalamic-pituitary-thyroidal function in eumenorrheic and amenorrheic athletes. J Clin Endocrinol Metab 75(2):514–518
Macdougall JD, Wenger HA, Green HJ (1982) Physiological testing of the elite athlete. Canadian Association of Sports Sciences, Canada
Mertz W, Tsui JC, Judd JT et al (1991) What are people really eating? The relation between energy intake derived from estimated diet records and intake determined to maintain body weight. Am J Clin Nutr 54:291–295. https://doi.org/10.1093/ajcn/54.2.291
Mooses M, Mooses K, Haile DW, Durussel J, Kaasik P, Pitsiladis YP (2015) Dissociation between running economy and running performance in elite Kenyan distance runners. J Sports Sci 33(2):136–144. https://doi.org/10.1080/02640414.2014.926384
Mountjoy M, Sundgot-Borgen J, Burke L, Carter S, Constantini N, Lebrun C, Meyer N, Sherman R, Steffen K, Budgett R, Ljungqvist A (2014) The IOC consensus statement: beyond the female athlete triad—relative energy deficiency in sports (RED-S). Br J Sports Med 48(7):491–497
Mountjoy M et al (2018) IOC consensus statement on relative energy deficiency in sport (RED-S): 2018 update. Br J Sports Med 52:687–697. https://doi.org/10.1136/bjsports-2018-099193
Muia EN, Wright HH, Onywera VO, Kuria EN (2015) Adolescent elite Kenyan runners are at risk for energy deficiency, menstrual dysfunction and disordered eating. J Sports Sci. https://doi.org/10.1080/02640414.2015.1065340
Nattiv A, Loucks AB, Manore MM, Sanborn CF, Sundgot-Borgen J, Warren MP (2007x) American college of sports medicine position stand. The female athlete triad. Med Sci Sports Exerc 39(10):1867–1882
Nattiv A, De Souza MJ, Koltun KJ, Misra M, Kussman A, Williams NI, Barrack MT, Kraus E, Joy E, Fredericson M (2021) The male athlete triad-a consensus statement from the female and male athlete triad coalition part 1: definition and scientific basis. Clin J Sport Med 31(4):335–348. https://doi.org/10.1097/JSM.0000000000000946
Orcholski L, Luke A, Plange-Rhule J et al (2015) Under-reporting of dietary energy intake in five populations of the African diaspora. Br J Nutr 113:464–472. https://doi.org/10.1017/S000711451400405X
Papageorgiou M, Dolan E, Elliott-Sale KJ et al (2018) Reduced energy availability: implications for bone health in physically active populations. Eur J Nutr 57:847–859. https://doi.org/10.1007/s00394-017-1498-8
Pfrimer K, Vilela M, Resende CM et al (2015) Under-reporting of food intake and body fatness in independent older people: a doubly labelled water study. Age Ageing 44:103–108. https://doi.org/10.1093/ageing/afu142
Sangenis P, Drinkwater BL, Loucks A, Sherman RT, Sundgot-Borgen J, Thompson RA (2006x) IOC medical commission position stand on the female athlete triad. IOC, Lausanne
Scagliusi FB, Ferriolli E, Lancha AH (2006) Underreporting of energy intake in developing nations. Nutr Rev 64:319–330. https://doi.org/10.1111/j.1753-4887.2006.tb00216.x
Shepherd JA, Ng BK, Sommer MJ, Heymsfield SB (2017) Body composition by DXA. Bone 104:101–105. https://doi.org/10.1016/j.bone.2017.06.010
Spiriev B (2017) IAAF scoring tables of athletics. Monaco, Multiprint
Tam N, Santos-Concejero J, Tucker R et al (2017x) Bone health in elite Kenyan runners. J Sports Sci. https://doi.org/10.1080/02640414.2017.1313998
Wasserman K, Hansen JE, Sue D, Whipp BJ, Casaburi R (2004) Principles of exercise testing and interpretation, 4th edn. Lippincott Williams and Wilkins, Philadelphia
Funding
This study was funded by International Association of Athletics Federations.
Author information
Authors and Affiliations
Contributions
MM and ACH conceived and designed research. MM, SS, DWH, RO, ACH and ARL conducted experiments. ACH and LÕ analyzed data and wrote the manuscript. All authors read and approved the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
Authors’ have no conflict of interests to declare.
Additional information
Communicated by Kirsty Elliott-Sale.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Õnnik, L., Mooses, M., Suvi, S. et al. Prevalence of Triad–RED–S symptoms in high-level Kenyan male and female distance runners and corresponding control groups. Eur J Appl Physiol 122, 199–208 (2022). https://doi.org/10.1007/s00421-021-04827-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00421-021-04827-w