Abstract
Purpose
This study measured the influence of acute hypoxic exercise on Interleukin-6 (IL-6), hepcidin, and iron biomarkers in athletes.
Methods
In a repeated measures design, 13 moderately trained endurance athletes performed 5 × 4 min intervals at 90 % of their peak oxygen consumption velocity (vVO2peak) in both normoxic [NORM, fraction of inspired oxygen (F IO2) = 0.2093, 15.3 ± 1.7 km h−1] and simulated hypoxic (HYP, F IO2 = 0.1450, 13.2 ± 1.5 km h−1) conditions. Venous blood samples were obtained pre-, post-, and 3 h post-exercise, and analysed for serum hepcidin, IL-6, ferritin, iron, soluble transferrin receptor (sTfR), and transferrin saturation.
Results
Peak heart rate was significantly lower in HYP compared with NORM (p = 0.01); however, the rating of perceived exertion was similar between trials (p = 0.24). Ferritin (p = 0.02), transferrin (p = 0.03), and IL-6 (p = 0.01) significantly increased immediately post-exercise in both conditions, but returned to baseline 3 h later. Hepcidin levels significantly increased in both conditions 3 h post-exercise (p = 0.05), with no significant differences between trials. A significant treatment effect was observed between trials for sTfR (p = 0.01), but not iron and transferrin saturation.
Conclusion
Acute exercise in hypoxia did not influence post-exercise IL-6 production, hepcidin activity or iron metabolism compared with exercise at the same relative intensity in normoxia. Hence, acute exercise performed at the same relative intensity in hypoxia poses no further risk to an athlete’s iron status, as compared with exercise in normoxia.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Athletes undertake acute (minutes to hours) and/or chronic (weeks) hypoxic exposure to enhance normoxic exercise performance. Chronic hypoxic exposure improves tissue oxygen transport and extraction by augmenting several haematological and non-haematological biomarkers (Gore et al. 2007), which may translate into improved sea-level aerobic performance. Maintaining a healthy iron status (serum ferritin >30 µg L−1) during hypoxic exposure ensures that bone marrow iron delivery sufficiently supports accelerated erythropoiesis, because iron is involved in haemoglobin and myoglobin synthesis whilst also playing a key role in energy metabolism and cellular respiration (Ackrell et al. 1984; McLane et al. 1981). However, low iron stores have been shown to compromise the haematological adaptations associated with chronic hypoxic exposure; thus low iron stores may potentially reduce the effectiveness of this training method (Stray-Gundersen et al. 1992).
Exercise transiently compromises iron metabolism in athletes via an Interleukin-6 (IL-6) mediated increase in the iron regulatory hormone, known as hepcidin, 3–6 h after exercise (Newlin et al. 2012; Peeling et al. 2009a, b, c; Sim et al. 2012, 2013). Elevated hepcidin levels reduce iron recycling from senescent erythrocytes and intestinal iron absorption by degrading ferroportin iron export channels, consequently blocking the efflux of iron contained on iron storage cells (Nemeth et al. 2004) into the blood, which in turn decreases iron delivery to the bone marrow for erythropoiesis. As such, increased hepcidin activity during post-exercise recovery, coupled with a transient reduction in dietary iron absorption, may contribute to the high incidence of deficient erythropoiesis reported in athletes (Peeling et al. 2008).
In addition to IL-6, serum iron concentration, hypoxia, and erythropoietic activity also regulate hepcidin synthesis (Nicolas et al. 2002). However, because IL-6 and hypoxia have opposite effects on hepcidin synthesis, the influence of exercise performed in hypoxia on post-exercise iron metabolism is currently unclear. Indeed, although acute hypoxic-exercise augments IL-6 production (Lundby and Steensberg 2004), continuous hypoxic exposure and an increase in erythropoietic activity have been shown to suppress hepcidin levels within 24–48 h (Ashby et al. 2010; Robach et al. 2009; Talbot et al. 2012). However, several other mechanisms, including an enhanced transcription of hypoxic inducible factor (HIF) 1α and 2α (Kapitsinou et al. 2010; Mastrogiannaki et al. 2012), an increase in serum erythropoietin (EPO) concentration (Robach et al. 2009, 2013), and an up-regulation of growth differentiation factor-15 (GDF-15) (Tanno et al. 2007) or TWSG1 (Tanno et al. 2009) may also suppress hepcidin expression.
Reduced hepcidin activity in hypoxic conditions enhances intestinal iron absorption and iron mobilisation from storage sites in the liver, macrophages, intestine, and skeletal muscle (Chaston et al. 2011; Hintze and McClung 2011). Increased mobilisation improves iron delivery to the bone marrow for erythropoiesis during times of limited iron bioavailability, such as during hypoxic exposure, or when an individual is iron deficient. With this in mind, it is possible that a hypoxic-mediated reduction in hepcidin expression during hypoxic training could improve the dietary iron absorption post-exercise, despite an exercise-induced increase in IL-6 levels immediately post-exercise, thus enabling athletes to maintain a positive iron balance. Hence, for athletes undertaking chronic altitude training, reduced hepcidin levels during recovery from exercise may benefit haematological adaptations by improving dietary iron absorption and bone marrow iron delivery, regardless of an exercise-induced increase in IL-6 production immediately post-exercise. However, whether hepcidin suppression occurs following an acute exercise session performed in hypoxic conditions is currently unknown.
Therefore, this study compared the influence of high-intensity exercise performed in normoxic and hypoxic conditions on post-exercise IL-6, hepcidin and iron responses. It was hypothesised that exercise in hypoxia would attenuate hepcidin activity post-exercise, independent of elevated IL-6 levels.
Methods
Participants
Thirteen (7 males, 6 females) moderately trained endurance runners (n = 9) or triathletes (n = 4) participated in this study (mean ± standard deviation for age: 28.8 ± 5.3 year, body mass: 63.9 ± 11.8 kg, peak oxygen consumption (VO2peak): males: 61.0 ± 6.3 mL kg−1 min−1, females: 55.0 ± 5.9 mL kg−1 min−1). Prior to testing, written informed consent was obtained from each participant. The Human Research Ethics Committee at Edith Cowan University granted approval for this study in accordance with the Declaration of Helsinki.
Experimental overview
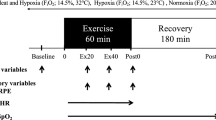
Participants performed four exercise sessions, scheduled at least 7 days apart. The first two sessions were graded exercise tests (GXT), which were performed in a counterbalanced order in either a normoxic (NORM, fraction of inspired oxygen (F IO2) = 0.2093) or hypoxic (HYP, F IO2 = 0.1450) environment. This test determined the athlete’s VO2peak, running velocity attained at VO2peak (vVO2peak), and peak heart rate (HRpeak) within each environment. The remaining two experimental sessions included a high-intensity interval run (INT) on a motorised treadmill in either NORM or HYP, in a counterbalanced order. These two interval sessions consisted of 5 × 4 min efforts at 90 % vVO2peak separated by 1.5 min of passive recovery. Before each INT session, a standardised 5 min treadmill-based running warm-up was conducted at 65 % vVO2peak, making the total session duration 31 min. During each INT session, venous blood samples were collected from a forearm antecubital vein before, immediately after exercise, and after 3 h of passive recovery in normoxic conditions.
Experimental procedures
Graded exercise test (GXT)
The GXT commenced at a speed of 10 km h−1 (females) or 12 km h−1 (males) in NORM, and at 8 km h−1 (females) or 10 km h−1 (males) in HYP at a 1 % gradient throughout (Jones and Doust 1996). In a similar design to Blondel et al. (2001), the treadmill speed increased by 1 km h−1 every 4 min, separated by 1 min of passive recovery until participants reached volitional exhaustion. During the GXT, VO2 for each workload was calculated based upon expired gas fractions and volumes. Briefly, expired gas volumes were collected during the final 30 s of each workload into a Collins 120 L chain-compensated gasometer (Warren E Collins, Braintree, MA, USA) as per (Hart and Withers 1996). Thereafter, expired gas fractions were determined by drawing an expirate sample out the gasometer into a 2-L Douglas bag, which were analysed using a calibrated electrochemical oxygen analyser and an infrared carbon dioxide analyser (Ametek Gas Analyzers, Applied Electrochemistry, SOV S-3A/1 and COV CD-3A, Pittsburgh, PA). The vVO2peak was calculated as the peak running velocity attained during the last successfully completed 4 min workload, which was multiplied by 0.9 to calculate the velocity for the NORM and HYP sessions.
High-intensity interval running session (INT)
Participants attended the laboratory having refrained from exercise and caffeine for 12 h. After collection of the pre-exercise venous blood sample, participants performed a standardised warm-up of 5 min treadmill running at 65 % vVO2peak followed by 5 min of dynamic stretching. Thereafter, participants ran 5 × 4 min intervals on a motorised treadmill in either NORM or HYP conditions. Additionally, during each session, heart rate (HR) was recorded continuously and a rating of perceived exertion (RPE) was obtained at the conclusion of each interval.
Simulated altitude environment
Altitude was simulated through nitrogen injection (flow rate 270 L min−1, VPSA S325 V16, van Amerongen, Tiel, The Netherlands) in a 40 m3 environmental chamber (b-Cat BV S879, Tiel, The Netherlands). To simulate 3,000 m altitude, the chamber was set at a F IO2 of 0.1450 because the chamber was located at sea-level (The University of Western Australia; Perth, Australia).
Venous blood collection
After 10 min of supine rest to control for postural shifts in plasma volume (Ahlgrim et al. 2010), a venous blood sample was collected from a forearm antecuitbal vein using a 22-gauge needle, filling two 8.5 mL blood collection tubes (SST II Gel; BD Vacutainer™, New Jersey, USA). These tubes then clotted at room temperature for 30 min before centrifugation at 10 °C and a speed of 1.1 r.c.f (3,000g) for 10 min. After centrifugation, the serum supernatant was transferred into 4 × 1 mL aliquots and stored at −80 °C for batch analysis.
Serum hepcidin
Serum hepcidin measurements were performed (Testing lab: Hepcidinanalysis.com, Nijmegen, The Netherlands) by a combination of weak cation exchange chromatography and time-of-flight mass spectrometry (WCX-TOF MS) using an internal standard (synthetic hepcidin-24; custom made Peptide International Inc.) for quantification (Kroot et al. 2010; Swinkels et al. 2008). Peptide spectra were generated on a Microflex LT matrix-assisted laser desorption/ionisation TOF-MS platform (Bruker Daltonics). Serum hepcidin-25 concentrations were expressed as nmol L−1 [nM]. The lower limit of detection of this method was 0.5 nM. The median reference level of serum hepcidin-25 (Dutch population) is 4.5 nM for men, 2.0 nM for premenopausal women, and 4.9 nM for postmenopausal women. The reference levels for the WCX-TOF MS method are derived from those of our enzyme method (Galesloot et al. 2011), based on the regression line between the Enzyme Linked Immunosorbent Assay (ELISA) and WCX-TOF MS results obtained for the same samples from patients without hepcidin isoforms. Details on the WCX-TOF MS reference values for hepcidin in adults can be found at www.hepcidinanalysis.com.
Serum interleukin-6
Serum IL-6 was analysed using a commercially available ELISA kit (Quantikine, HS, R & D Systems, Minneapolis, USA) with an assay range of 0.447–9.96 ng L−1.
Iron studies
Serum ferritin concentration was analysed on an Architect i4000SR analyser (Abbott Diagnostics, Abbott Laboratories, Abbott Park, IL 60064 USA) using Chemoluminesence Microparticle Immunoassay (CMIA) technology. Serum iron and transferrin were measured on the Architect analyser (c1600210, Abbott Diagnostics, Abbott Laboratories, Abbott Park, IL 60064, USA). Serum iron levels were measured using spectrophotometry after adding an iron reagent (Sentinel Diagnostics, Milan, Italy). Serum transferrin levels were measured using immunoturbidimetry after adding transferrin antibodies (Abbott Diagnostics, Abbott Laboratories, Abbott Park, IL 60064 USA). Additionally, transferrin saturation was calculated by dividing serum iron by the total iron binding capacity. Soluble transferrin receptor (sTfR) was measured by latex enhanced immunoturbidimetry via Tina Quant® (Roche Diagnostics GmbH, Mannheim, Germany) on a Cobas Integra 800 modular system (Roche Diagnostics GmbH) with absorbance measured at 583 nM.
Statistical analysis
A 2 × 2 repeated measures ANOVA analysed the effects of Time (2 levels: pre-exercise, post-exercise) and Treatment (2 levels: NORM, HYP) on IL-6 and hepcidin levels. Additionally, a 3 × 2 ANOVA analysed the effects of Time (3 levels: pre-exercise, post-exercise, 3 h post-exercise), Treatment (2 levels: NORM, HYP), and their interaction on the abovementioned iron parameters. In the event of a significant main or interaction effects, a post hoc Bonferroni correction for multiple comparisons was applied to adjust the family-wise error rate. Significance was set at an alpha level of p ≤ 0.05.
Data were also analysed via a contemporary statistical approach using standardised mean differences as a Cohen’s d effect size (Hopkins et al. 2009). To calculate the standardised mean difference, the mean difference between pre-exercise and post-exercise values was divided by the standard deviation of the pre-exercise value. Effect sizes were then interpreted using Cohen’s Scale for Effect Sizes (Cohen 1988) with the following qualitative descriptors: “small” (0.2–0.6), “moderate” (0.6–1.2), “large” (1.2–2.0), “very large” (2.0–4.0). Additionally, 90 % confidence intervals were used to ascertain the certainty with which the effects occurred. Situations where the confidence limits simultaneously overlapped positive and the negative thresholds were deemed unclear.
Results
Interval running session
There was no significant difference in ambient temperature (NORM: 22.2 ± 1.4 °C, HYP: 22.9 ± 1.3 °C, p = 0.12) or barometric pressure (NORM: 759.5 ± 9.41 mmHg, HYP: 758.1 ± 3.1 mmHg, p = 0.52) between the NORM or HYP sessions. The F IO2 was 0.2081 ± 0.0026 and 0.1450 ± 0.0005 for NORM and HYP, respectively. The relative running velocity (90 % vVO2peak) and HRpeak were significantly lower during HYP as compared to NORM (vVO2peak: p = 0.01, HRpeak: p = 0.01); however, there was no significant difference (p = 0.24) in RPE between trials (Table 1).
Serum interleukin-6
Baseline IL-6 rose significantly post-exercise in both conditions (p = 0.01); however, there were no significant treatment (p = 0.60) or interaction effects (p = 0.48) (Table 2). The significant time effect was supported by moderate (d = 0.60) and large (d = 1.49) effect sizes for NORM and HYP, respectively (Table 2).
Hepcidin
A significant time effect indicated that serum hepcidin increased from baseline levels at 3 h post-exercise in both conditions (p = 0.05). This time effect was supported by moderate effect sizes in both the NORM and HYP (Table 2). No treatment (p = 0.66) or interaction effect (p = 0.39) was present in NORM and HYP, respectively (Table 2).
Iron parameters
Iron parameters are presented in Table 3. No significant time (p = 0.27), treatment (p = 0.49) or interaction effect (p = 0.62) was present for serum iron concentration. However, a significant time effect existed for serum ferritin (p = 0.03) and transferrin concentrations (p = 0.01) in both conditions. These parameters significantly increased from baseline levels immediately post-exercise (serum ferritin: p = 0.04, serum transferrin: p = 0.08) but returned to baseline levels by 3 h post-exercise (serum ferritin: p = 0.05, serum transferrin: p = 0.02). Serum sTfR showed a significant treatment effect (p = 0.01), corresponding to a trivial effect in the NORM (d = 0.08) and HYP conditions (d = 0.14) (Table 3), but no significant time (p = 0.07) or interaction effect (p = 0.06) existed. No significant time (serum iron: p = 0.27, transferrin saturation: p = 0.65), treatment (serum iron: p = 0.49, transferrin saturation: p = 0.62) or interaction effect (serum iron: p = 0.62, transferrin saturation: p = 0.72) existed for serum iron or transferrin saturation.
Discussion
To our knowledge, this is the first study to examine the relationship between IL-6, hepcidin, and iron metabolism after acute exercise conducted in hypoxia. We hypothesised that acute exercise in hypoxia, performed at the same relative intensity as exercise in normoxia, would attenuate hepcidin activity 3 h after passive recovery in normoxia. Our findings did not support this hypothesis, with similar responses in IL-6 and hepcidin reported following interval exercise in both conditions. Furthermore, no differences in post-exercise iron biomarkers were observed between the two conditions, indicating a similar iron response between trials.
Exercise intensity and duration are key moderators of the IL-6 response, which has recently been proposed as the main mechanism responsible for elevated hepcidin levels 3–6 h after exercise in normoxic conditions (Newlin et al. 2012; Peeling et al. 2009a, b, c; Sim et al. 2012, 2013). As such, the matched relative exercise intensity may account for the similarity of the post-exercise biomarker responses between the two conditions observed in the present study, since the overall mechanical work performed during the hypoxic session was less than that performed during the normoxic session. Indeed, exercise intensity, rather than hypoxic exposure itself, may be a greater mediator of IL-6 production after an acute exercise session in hypoxia. Similar to our findings, comparable IL-6 production immediately after 60 min of cycling in both a normoxic (F IO2 = 0.2093) and hypoxic environment (F IO2 = 0.1240) at a matched relative intensity of ~45 % VO2max has previously been reported (Lundby and Steensberg 2004), although cycling at the same absolute intensity (154 W, ~54 % VO2max) in hypoxia augmented IL-6 production post-exercise. Thus, performing an acute exercise session at the same absolute exercise intensity may augment rather than suppress hepcidin production after exercise, owing to an augmented IL-6 response immediately after exercise, which may impair post-exercise iron absorption. However, further research is required to compare the hepcidin and iron biomarker response between hypoxic and normoxic exercise performed at a matched absolute, rather than a relative intensity.
The length of hypoxic exposure in this study (~31 min) may have been insufficient to up-regulate EPO, suppress hepcidin synthesis or affect iron uptake kinetics. Hepcidin suppression occurs within 24–48 h when induced by low- and high-dose EPO administration (Ashby et al. 2010; Lainé et al. 2011; Robach et al. 2009, 2013), blood removal (Ashby et al. 2010), or chronic hypoxic exposure (Piperno et al. 2010; Talbot et al. 2012). Although EPO concentration was not measured in the current study, Rodríguez et al. (1999) previously observed a significant increase in EPO after 90 min of hypoxic exposure at a simulated altitude of 5,500 m (540 hPa). Furthermore, Mackenzie et al. (2008) reported a significant increase in EPO levels associated with 90 min of hypoxic exposure (F IO2 = 0.1480) preceding a 30-min treadmill run at 50 % VO2max, although Knaupp et al. (1992) observed no increase in EPO after ~120 min of hypoxic exposure (F IO2 = 0.1050). Considering that the duration of the hypoxic exposure used in these studies was 3–4 times greater than ours, but no clear or consistent increase in EPO levels were present, the length of hypoxic exposure in the current study was likely insufficient to up-regulate EPO synthesis, thus having little influence on hepcidin synthesis and iron metabolism. However, these protocols were performed at a lower intensity than that used in the current study and do not represent a typical intermittent hypoxic interval training session utilised by endurance athletes. From a practical perspective, further research may be required to elucidate the response of these biomarkers during exercise recovery when manipulating the frequency, duration or intensity of hypoxic exercise.
Whilst athletes do not typically recover in hypoxic conditions after intermittent hypoxic training (IHT) protocols (Humberstone-Gough et al. 2013), it is unlikely that recovering in normoxia affected the iron biomarker responses seen here. Recovering in hypoxia would have extended the total hypoxic dose to ~3.5 h, which is still considerably less than the 24–48 h reportedly required to suppress hepcidin activity (Ashby et al. 2010; Piperno et al. 2010; Robach et al. 2013; Talbot et al. 2012) and lower than that experienced by athletes during chronic altitude training (21 days, 14 h day−1). Nevertheless, Badenhorst et al. (2014) showed that hepcidin levels were ~27 and ~34 % lower, respectively, 3 h and 24 h after interval exercise (8 × 3 min at 85 % vVO2peak) when athletes recovered for 3 h in hypoxia (F IO2 = 0.1508) as compared to normoxia (F IO2 = 0.2093). However, a similar iron biomarker response existed 3 and 24 h post-exercise between these two recovery conditions. Hence, athletes who recover in hypoxic conditions may experience lower hepcidin levels, which may promote iron mobilisation from storage sites and improve dietary iron absorption. Conceivably, recovering in hypoxic conditions could assist athletes to maintain a healthy iron status throughout competitive season by transiently improving iron absorption from post-exercise meals.
Athletes may recover in hypoxic conditions after exercise during simulated and natural live high, train low (LHTL) and live high, train high (LHTH) protocols; thus hepcidin expression after exercise in these protocols is likely to be lower than compared to acute IHT, owing to a larger total time spent in hypoxia. Indeed, from a practical perspective, Saunders (2009) recommended that athletes avoid interval exercise upon initial exposure to moderate (2,000–3,000 m) and high altitudes (>3,500 m) to reduce the effect of an exercise-induced increase in haemolysis or inflammatory cytokines, and an impairment in EPO production resulting from the acidic nature of high-intensity exercise. Thus, given that high-intensity exercise is unlikely to be performed in the first 24–48 h of hypoxic exposure during LHTL and LHTH protocols, it is probable that hepcidin levels would already be suppressed if exercise were performed on the third or fourth day of chronic hypoxic exposure. If so, then recovering in hypoxic conditions may lower hepcidin activity during recovery from exercise, thus improving iron absorption from post-exercise meals and its mobilisation from storage sites, consequently providing the bone marrow with sufficient iron to cope with accelerated erythropoiesis in hypoxia. However, whether the cumulative hypoxic exposure experienced during chronic intermittent hypoxic training protocols similarly influences post-exercise hepcidin expression or not is currently unknown.
Iron parameters
In the current investigation, serum ferritin and transferrin concentrations significantly increased from baseline levels post-exercise in both trials, but returned to baseline levels within 3 h of recovery. However, serum iron, transferrin saturation, and soluble transferrin receptor concentrations did not significantly increase above baseline levels at any time point in either exercise trial. The rise in IL-6 and serum ferritin immediately post-exercise in the current study are analogous to an acute-phase response (Fallon et al. 2001), which serves to reduce the availability of iron to invading pathogens in a process known as iron withholding. Serum iron, ferritin and transferrin saturation are positive acute-phase reactants (Epstein et al. 1999), although an increase in serum iron and transferrin saturation may also result from exercise-related haemolysis. Our findings are comparable to Sim et al. (2012) who also reported an increase in serum ferritin and transferrin in male endurance athletes immediately after a 90-min treadmill run at 75 % vVO2peak. Whilst transferrin is considered a negative acute-phase protein (Epstein et al. 1999), a small increase (Sim et al. 2012) or no change in this biomarker (Schumacher 2002) has been reported after acute exercise. Indeed, as Gimenez et al. (1988) proposed, haemoconcentration could explain the elevation in transferrin after high-intensity exercise seen in the current study. In contrast to Sim et al. (2012), sTfR was not significantly elevated post-exercise in either condition. An elevation in sTfR reflects increased bone marrow iron requirements associated with accelerated erythropoiesis (Beguin 2003), which is known to occur during prolonged hypoxic exposure (Clark et al. 2009; Garvican et al. 2010). However, in this study, an exercise-induced increase in inflammatory cytokines and/or haemolysis rather than hypoxic exposure was likely the key driver of changes in these markers of iron status after exercise.
Conclusion
Acute (~31 min) interval exercise performed in hypoxic conditions did not attenuate post-exercise hepcidin activity or alter iron metabolism as compared to exercise performed at the same relativity intensity in normoxic conditions. Such an outcome was most likely a result of the short-duration hypoxic exposure. Thus, from a practical perspective, whilst exercising in acute hypoxic conditions may not negatively affect dietary iron absorption after exercise, it may not improve it either. However, future research is required to determine the effect of manipulating the frequency, duration, and intensity of exercise performed in hypoxic conditions on the hepcidin and iron biomarker responses after exercise. Additionally, investigating the influence of chronic (several weeks), intermittent, and continuous hypoxic training on hepcidin activity and iron metabolism could also improve our understanding of how iron balance is maintained during training that utilises such environmental manipulation, which may enable sports scientists to maximise the haematological adaptations associated with hypoxic exposure.
Declarations
Dorine Swinkels is medical director of the “hepcidinanalysis.com” initiative, which aims to serve the scientific and medical communities with high quality hepcidin measurements (www.hepcidinanalysis.com).
Abbreviations
- ANOVA:
-
Analysis of variance
- CL:
-
Confidence limit
- CV:
-
Coefficient of variation
- EPO:
-
Erythropoietin
- F IO2 :
-
Fraction of expired oxygen
- GDF-15:
-
Growth differentiation factor-15
- GXT:
-
Graded exercise test
- HIF:
-
Hypoxic inducible factor
- HR:
-
Heart rate
- HYP:
-
Hypoxic exercise trial
- IL-6:
-
Interleukin-6
- INT:
-
High-intensity interval running session
- NORM:
-
Normoxic exercise trial
- RPE:
-
Rating of perceived exertion
- SD:
-
Standard deviation
- sTfR:
-
Soluble transferrin receptor
- VO2peak :
-
Peak oxygen consumption
- vVO2peak :
-
Velocity attained at peak oxygen consumption
- V E(ATPS):
-
Volume of expired gas (Atmospheric temperature and pressure saturated)
- V E(STPD):
-
Volume of expired gas (Standard temperature and pressure dry)
- WCX-TOF MS:
-
Weak cation-exchange time-of-flight mass spectroscopy
References
Ackrell BA, Maguire JJ, Dallman PR, Kearney EB (1984) Effect of iron deficiency on succinate- and NADH-ubiquinone oxidoreductases in skeletal muscle mitochondria. J Biol Chem 259:10053–10059
Ahlgrim C, Pottgiesser T, Robinson N, Sottas P, Ruecker G, Schumacher Y (2010) Are 10 min of seating enough to guarantee stable haemoglobin and haematocrit readings for the athlete’s biological passport? Int J Lab Hematol 32:506–511
Ashby DR, Gale DP, Busbridge M, Murphy KG, Duncan ND, Cairns TD, Taube DH, Bloom SR, Tam FWK, Chapman R, Maxwell PH, Choi P (2010) Erythropoietin administration in humans causes a marked and prolonged reduction in circulating hepcidin. Haematologica 95:505–508
Badenhorst CE, Dawson B, Goodman C, Sim M, Cox GR, Gore CJ, Tjalsma H, Swinkels DW, Peeling P (2014) Influence of post-exercise hypoxic exposure on hepcidin response in athletes. Eur J Appl Physiol 114(5):1–9
Beguin Y (2003) Soluble transferrin receptor for the evaluation of erythropoiesis and iron status. Clin Chim Acta 329:9–22
Blondel N, Berthoin S, Billat V, Lensel G (2001) Relationship between run times to exhaustion at 90, 100, 120, and 140% of vVO2max and velocity expressed relatively to critical velocity and maximal velocity. Int J Sports Med 22:27–33
Chaston TB, Matak P, Pourvali K, Srai SK, McKie AT, Sharp PA (2011) Hypoxia inhibits hepcidin expression in HuH7 hepatoma cells via decreased SMAD4 signaling. Am J Physiol Cell Physiol 300:C888–C895
Clark SA, Quod MJ, Clark MA, Martin DT, Saunders PU, Gore CJ (2009) Time course of haemoglobin mass during 21 days live high: train low simulated altitude. Eur J Appl Physiol 106:399–406
Cohen J (1988) Statistical power analysis for the behavioural sciences. Lawrence Erlbaum, Mahwah (NJ)
Epstein FH, Gabay C, Kushner I (1999) Acute-phase proteins and other systemic responses to inflammation. N Engl J Med 340:448–454
Fallon KE, Fallon SK, Boston T (2001) The acute phase response and exercise: the ultramarathon as prototype exercise. Clin J Sport Med 11:38–43
Galesloot TE, Vermeulen SH, Geurts-Moespot AJ, Klaver SM, Kroot JJ, van Tienoven D, Wetzels JFM, Kiemeney LALM, Sweep FC, den Heijer M, Swinkels DW (2011) Serum hepcidin: reference ranges and biochemical correlates in the general population. Blood 117:e218–e225
Garvican LA, Martin DT, Quod MJ, Stephens B, Sassi A, Gore CJ (2010) Time course of the hemoglobin mass response to natural altitude training in elite endurance cyclists. Scand J Med Sci Sports 22:95–103
Gimenez M, Uffholtz H, Paysant P, Belleville F, Nabet P (1988) Serum iron and transferrin during an exhaustive session of interval training. Eur J Appl Physiol 57:154–158
Gore CJ, Clark SA, Saunders PU (2007) Non-hematological mechanisms of improved sea-level performance after hypoxic exposure. Med Sci Sports Exerc 39:1600–1609
Hart JD, Withers RT (1996) The calibration of gas volume measuring devices at continuous and pulsatile flows. Aust J Sci Med Sport 28:61–65
Hintze KJ, McClung JP (2011) Hepcidin: a critical regulator of iron metabolism during hypoxia. Adv Hematol
Hopkins WG, Marshall SW, Batterham AM, Hanin J (2009) Progressive statistics for studies in sports medicine and exercise science. Med Sci Sports Exerc 41:3–12
Humberstone-Gough CE, Saunders PU, Bonetti DL, Stephens S, Bullock N, Anson JM, Gore CJ (2013) Comparison of live high: train low altitude and intermittent hypoxic exposure. J Sports Sci Med 12:394–401
Jones AM, Doust JH (1996) A 1% treadmill grade most accurately reflects the energetic cost of outdoor running. J Sports Sci 14:321–327
Kapitsinou PP, Liu Q, Unger TL, Rha J, Davidoff O, Keith B, Epstein JA, Moores SL, Erickson-Miller CL, Haase VH (2010) Hepatic HIF-2 regulates erythropoietic responses to hypoxia in renal anemia. Blood 116:3039–3048
Knaupp W, Khilnani S, Sherwood J, Scharf S, Steinberg H (1992) Erythropoietin response to acute normobaric hypoxia in humans. J Appl Physiol 73:837–840
Kroot JJ, Laarakkers CM, Geurts-Moespot AJ, Grebenchtchikov N, Pickkers P, van Ede AE, Peters HP, van Dongen-Lases E, Wetzels JF, Sweep FC (2010) Immunochemical and mass-spectrometry-based serum hepcidin assays for iron metabolism disorders. Clin Chem 56:1570–1579
Lainé F, Laviolle B, Ropert M, Bouguen G, Morcet J, Hamon C, Massart C, Westermann M, Deugnier Y, Loréal O (2011) Early effects of erythropoietin on serum hepcidin and serum iron bioavailability in healthy volunteers. Eur J Appl Physiol 112:1391–1397
Lundby C, Steensberg A (2004) Interleukin-6 response to exercise during acute and chronic hypoxia. Eur J Appl Physiol 91:88–93
Mackenzie RWA, Watt PW, Maxwell NS (2008) Acute normobaric hypoxia stimulates erythropoietin release. High Alt Med Biol 9:28–37
Mastrogiannaki M, Matak P, Delga S, Deschemin J-C, Vaulont S, Peyssonnaux C (2012) Deletion of HIF-2α in the enterocytes decreases the severity of tissue iron loading in hepcidin knockout mice. Blood 119:587–590
McLane J, Fell R, McKay R, Winder W, Brown E, Holloszy J (1981) Physiological and biochemical effects of iron deficiency on rat skeletal muscle. Am J Physiol 241:C47–C54
Nemeth E, Tuttle MS, Powelson J, Vaughn MB, Donovan A, Ward DM, Ganz T, Kaplan J (2004) Hepcidin regulates cellular iron efflux by binding to ferroportin and inducing its internalization. Science 306:2090–2093
Newlin MK, Williams S, McNamara T, Tjalsma H, Swinkels DW, Haymes EM (2012) The effects of acute exercise bouts on hepcidin in women. Int J Sport Nutr Exerc Metab 22:79–88
Nicolas G, Chauvert C, Viatte L, Danan J, Bigard X, Devaux I, Beaumont C, Kahn A, Vaulont S (2002) The gene encoding the iron regulatory peptide hepcidin is regulated by anemia, hypoxia, and inflammation. J Clin Invest 110:1037–1044
Peeling P, Dawson B, Goodman C, Landers G, Trinder D (2008) Athletic induced iron deficiency: new insights into the role of inflammation, cytokines and hormones. Eur J Appl Physiol 103:381–391
Peeling P, Dawson B, Goodman C, Landers G, Wiegerinck E, Swinkels D, Trinder D (2009a) Cumulative effects of consecutive running sessions on hemolysis, inflammation and hepcidin activity. Eur J Appl Physiol 106:51–59
Peeling P, Dawson B, Goodman C, Landers G, Wiegerinck ET, Swinkels DW, Trinder D (2009b) Effects of exercise on hepcidin response and iron metabolism during recovery. Int J Sport Nutr Exerc Metab 19:583–597
Peeling P, Dawson B, Goodman C, Landers G, Wiegerinck ET, Swinkels DW, Trinder D (2009c) Training surface and intensity: inflammation, hemolysis, and hepcidin expression. Med Sci Sports Exerc 41:1138–1145
Piperno A, Galimberti S, Mariani R, Pelucchi S, Ravasi G, Lombardi C, Bilo G, Revera M, Giuliano A, Faini A, Mainini V, Westerman M, Ganz T, Valsecchi MG, Mancia G, Parati G (2010) Modulation of hepcidin production during hypoxia-induced erythropoiesis in humans in vivo: data from the HIGHCARE project. Blood 117:2953–2959
Robach P, Recalcati S, Girelli D, Gelfi C, Aachmann-Andersen NJ, Thomsen JJ, Norgaard AM, Alberghini A, Campostrini N, Castagna A, Vigano A, Santambrogio P, Kempf T, Wollert KC, Moutereau S, Lundby C, Cairo G (2009) Alterations of systemic and muscle iron metabolism in human subjects treated with low-dose recombinant erythropoietin. Blood 113:6707–6715
Robach P, Recalcati S, Girelli D, Campostrini N, Kempf T, Wollert KC, Corbella M, Santambrogio P, Perbellini L, Brasse-Lagnel C, Christensen B, Moutereau S, Lundby C, Cairo G (2013) Serum hepcidin levels and muscle iron proteins in humans injected with low- or high-dose erythropoietin. Eur J Haematol 91:74–84
Rodríguez FA, Casas H, Casas M, Pagés T, Rama R, Ricart A, Ventura JL, Ibanez J, Viscor G (1999) Intermittent hypobaric hypoxia stimulates erythropoiesis and improves aerobic capacity. Med Sci Sports Exerc 31:264–268
Saunders PU (2009) Endurance training at altitude. High Alt Med Biol 10:135–148
Schumacher YO (2002) Effects of exercise on soluble transferrin receptor and other variables of the iron status. Br J Sports Med 36:195–200
Sim M, Dawson B, Landers G, Wiegerinck ET, Swinkels DW, Townsend MA, Trinder D, Peeling P (2012) The effects of carbohydrate ingestion during endurance running on post-exercise inflammation and hepcidin levels. Eur J Appl Physiol 112:1889–1898
Sim M, Dawson B, Landers G, Swinkels DW, Tjalsma H, Trinder D, Peeling P (2013) Effect of exercise modality and intensity on post-exercise interleukin-6 and hepcidin Levels. Int J Sport Nutr Exerc Metab 23:178–186
Stray-Gundersen J, Alexander C, Hochstein A, deLemos D, Levine BD (1992) Failure of red cell volume to increase to altitude exposure in iron deficient runners. Med Sci Sports Exerc 24:S90
Swinkels DW, Girelli D, Laarakkers C, Kroot J, Campostrini N, Kemna EHJM, Tjalsma H (2008) Advances in quantitative hepcidin measurements by time-of-flight mass spectrometry. PLoS One 3:e2706
Talbot NP, Lakhal S, Smith TG, Privat C, Nickol AH, Rivera-Ch M, Leon-Velarde F, Dorrington KL, Mole DR, Robbins PA (2012) Regulation of hepcidin expression at high altitude. Blood 119:857–860
Tanno T, Bhanu NV, Oneal PA, Goh S-H, Staker P, Lee YT, Moroney JW, Reed CH, Luban NL, Wang R-H (2007) High levels of GDF15 in thalassemia suppress expression of the iron regulatory protein hepcidin. Nat Med 13:1096–1101
Tanno T, Porayette P, Sripichai O, Noh S-J, Byrnes C, Bhupatiraju A, Lee YT, Goodnough JB, Harandi O, Ganz T (2009) Identification of TWSG1 as a second novel erythroid regulator of hepcidin expression in murine and human cells. Blood 114:181–186
Acknowledgments
The Australian Sports Commission’s High Performance Sport Research Fund provided funding for this project. The authors would like to thank the athletes for their participation in this study and Mark Entwistle, Renate van Dordrecht, Hayley Paterson and Leslie Bishop who assisted with data collection.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Fabio Fischetti.
Rights and permissions
About this article
Cite this article
Govus, A.D., Abbiss, C.R., Garvican-Lewis, L.A. et al. Acute hypoxic exercise does not alter post-exercise iron metabolism in moderately trained endurance athletes. Eur J Appl Physiol 114, 2183–2191 (2014). https://doi.org/10.1007/s00421-014-2938-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00421-014-2938-2