Abstract
The effect of two running sessions completed within a 12-h period on hemolysis, inflammation, and hepcidin activity in endurance athletes was investigated. Ten males completed two experimental trials in a randomized, counterbalanced order. The two trials included (a) a one-running-session trial (T1) including 10 × 1 km interval repeats (90% peak \( \dot{V}{\text{O}}_{2} \) velocity), and (b) a two-running-session trial (T2), comprising a continuous 10-km run (70% peak \( \dot{V}{\text{O}}_{2} \) velocity), and a 10 × 1 km interval run (90% peak \( \dot{V}{\text{O}}_{2} \) velocity) completed 12 h later. Interleukin-6 (IL-6), free hemoglobin (Hb), haptoglobin (Hp), iron, ferritin, and hepcidin were assessed post-exercise. After the T1 and T2 interval runs, free Hb was significantly increased and Hp significantly decreased (p ≤ 0.05), with a cumulative effect shown in T2 after the second run (p ≤ 0.05). The IL-6, serum iron, ferritin, and hepcidin activity were increased after each running session (p ≤ 0.05), with no cumulative effect in T2. In conclusion, a cumulative effect of two running sessions on hemolysis was shown, but no similar effect with inflammation and hepcidin activity was evident.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Exercise-induced iron deficiency is a common nutritional disorder amongst endurance athletes (Beard and Tobin 2000; Zoller and Vogel 2004). Mechanisms such as hemolysis, hematuria, sweating, and gastrointestinal bleeding contribute to this disrupted metabolism of iron (Babić et al. 2001; DeRuisseau et al. 2002; McInnis et al. 1998; Zoller and Vogel 2004). Additionally, recent attention has focused on the post-exercise activity of the iron-regulatory hormone hepcidin, suggesting that up-regulation of this hormone may have a negative impact on the body’s ability to recycle and absorb dietary iron (Peeling et al. 2008a; Roecker et al. 2005). Recently, it has been suggested that post-exercise increases in the activity of hepcidin may result from exercise-induced inflammation and/or hemolysis (Peeling et al. 2008b).
Exercise-induced hemolysis has been shown to result after swimming, cycling, rowing, and weight training (Schobersberger et al. 1990; Selby and Eichner 1986; Telford et al. 2003). However, the impact stress of heel strike during running has been identified as the largest contributor to the destruction of red blood cells, due to a force-dependent relationship between heelstrike and the degree of hemolysis experienced (Miller et al. 1988; Telford et al. 2003). Although it is possible that the hemolysis induced by the impact stress of running may have adverse affects on the iron status of an athlete, it is questionable as to whether a single hemolytic episode will cause clinically significant iron loss (Poortmans and Haralambie 1979). Telford et al. (2003) suggested that daily, or twice-daily hemolytic episodes during hard training may have a cumulative effect on exercise-induced hemolysis and iron status that could potentially put athletes at significant risk of developing an iron deficiency. To date, however, no research has looked at the effect of consecutive running sessions on hemolysis, and the timing of appropriate recovery between sessions is unknown.
Hemolysis leads to the release of free Hb and iron into the circulation, which is scavenged and recycled via Ferroportin (Fpn) transport channels, housed on the cell surface of the macrophage. However, the levels of the iron-regulatory hormone hepcidin are also increased after exercise (Roecker et al. 2005; Peeling et al. 2008b). Hepcidin is a liver-produced peptide hormone, up-regulated in response to elevated iron levels and the inflammatory cytokine interleukin-6 (IL-6) (Kemna et al. 2005a; Nemeth et al. 2004a, b). Hepcidin acts by internalizing and degrading the Fpn transport channels on the macrophage surface, and at the intestinal duodenum. This ultimately blocks the release of iron from macrophages that have collected senescent erythrocytes from hemolysis, in addition to inhibiting the absorption of iron from the gut (Nemeth et al. 2004a, b).
As with hemolysis, it is also unknown as to the effect of twice-daily training sessions on the hepcidin response to exercise. Ronsen et al. (2002) showed that a second bout of high-intensity exercise on the same day is associated with a more pronounced increase in the levels of IL-6. Such an outcome may also translate to hepcidin activity. However, Ronsen et al. (2002) also showed that if the recovery period between bouts of exercise was sufficient enough, this cumulative effect was diminished. With this in mind, it was the aim of this investigation to assess the cumulative effects of two training sessions within a 12-h period on hemolysis, inflammation, and hepcidin production in well trained male athletes.
Methods
Subjects
Ten highly trained male triathletes and endurance runners were recruited for participation in this study (Table 1). Participants were completing an average of 6.7 (±1.5) training sessions per week at the time of the investigation, with approximately 70% of these sessions being running based. Due to the training status of these athletes, it is possible that more than one training session on any given day is customary to this population, and as such the results here are applicable to well-trained individuals only. All athletes were of healthy iron status (average resting serum ferritin, 82.8 µg L−1; range, 37.3–173.1 µg L−1), and none took any form of iron supplement during this investigation. Subjects were briefed on the purpose, requirements, and risks involved with participation, and signed a written informed consent prior to commencement. Ethical approval for this study was granted by the Human Ethics Committee of The University of Western Australia (Reference Number 0867).
Experimental overview
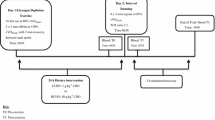
Participants were required to attend three laboratory-based testing sessions. Initially, a graded-exercise test (GXT) was performed to determine peak oxygen uptake (\( \dot{V}{\text{O}}_{{ 2 {\text{peak}}}} \)) and peak \( \dot{V}{\text{O}}_{ 2} \) running velocity (Table 1). Subsequently, a two-session (T2) and a one-session training condition (T1) were completed in a randomized, counterbalanced, cross over fashion. The T2 trial comprised evening and next morning running sessions (separated by 12 h), whereas the T1 trial comprised the morning running session only. An overnight recovery period was used in this study to allow complete rest between sessions, and to avoid any further coincidental activity that may occur throughout the day. Each condition was conducted over a period of 3 days, and was separated by a minimum of 7 days. All running training sessions were completed on a motorized treadmill (VR3000, NuryTech Inc., Germany), set at a given percentage of peak \( \dot{V}{\text{O}}_{2} \) running speed. In the 24 h prior to each trial, all participants were instructed not to perform any training sessions, structured exercise or manual labor.
Experimental procedures
Graded exercise test (GXT)
The GXT was conducted on the motorized treadmill using 4 min exercise and 1 min rest periods. The starting speed was 12 km h−1, with subsequent 1 km h−1 increments over each exercise period until volitional exhaustion. The treadmill was set to a 1% gradient in order to simulate conditions commonly encountered outdoors (Jones and Doust 1996). During the GXT, expired air was analysed for concentrations of O2 and CO2 via Ametek Gas Analysers (Applied Electrochemistry, SOV S-3A/1 and COV CD-3A, Pittsburgh, PA), which were calibrated pre-test and verified post-test with certified gravimetric gas mixtures (BOC Gases, Chatswood, Australia). Ventilation was recorded at 15-s intervals via a turbine ventilometer (Morgan, 225 A, Kent, England), which was calibrated before, and verified after exercise using a 1-L syringe in accordance with the manufacturer’s specifications. The \( \dot{V}{\text{O}}_{{2{\text{peak}}}} \) was determined by summing the four highest consecutive 15 s \( \dot{V}{\text{O}}_{ 2} \) values. The velocity at which subjects ran to achieve \( \dot{V}{\text{O}}_{{2{\text{peak}}}} \) was recorded and used to determine the velocities required for each of the running training sessions that followed.
Two running session trial (T2)
Day 1. On day 1, participants attended the laboratory at 1800 hour to provide a pre-run venous blood and urine sample. Subsequently, a 1-km warm up was performed at 60% of the individual peak \( \dot{V}{\text{O}}_{2} \) running velocity, followed by 5 min of static stretching. Next, they completed a continuous 10-km-long, slow distance run (T2LSD) at 70% of the peak \( \dot{V}{\text{O}}_{2} \) running velocity on the motorized treadmill. Heart rate (HR) data were collected every km using a polar heart rate monitor (620X, Finland), and blood lactate (BLa) was analyzed via a 35-μL−1 capillary blood sample collected from the earlobe during a 30-s rest period at 5 km and at the conclusion of 10 km, using a blood-gas analyser (ABL 625, Radiometer Medical A/S, Copenhagen, Denmark). A post-exercise venous blood sample was collected before the athlete went home at 2000 hour, taking with them a 75-mL sterilized collection cup to provide a urine sample at 3 h post-run, which was then stored in a refrigerator (4°C) and returned to the lab the next day.
Day 2. The athletes returned to the laboratory at 0600 hour. Pre-run venous blood and urine samples were then collected before they completed the same 1-km warm up and stretching routine as on the previous day. Subsequently, a 10 × 1 km interval running session (T2INT) at 90% of the individual peak \( \dot{V}{\text{O}}_{2} \) running velocity was completed (work to rest ratio of 2:1). As in the T2LSD, HR data were collected at 1-km intervals, and BLa was measured after the fifth and tenth km. A post-run venous blood sample was then collected, and the athletes were allowed to leave by 0800 hour, taking with them a 75-ml collection cup, to provide a urine sample at 3 h post-exercise. This sample was stored in a refrigerator (4°C) and brought back to the lab at the end of the day. Athletes were instructed to perform no further exercise or manual labor during the subsequent 24 h.
Day 3. Twenty-four hours later, subjects were asked to return at 0800 hour for collection of a final venous blood sample, marking the end of the T2 condition. The two different training intensities employed during the T2 condition (LSD and INT) were chosen to reflect the changes in intensity commonly scheduled between successive sessions within a distance running training program.
One-running-session trial (T1)
Day 1. Participants were required to attend the laboratory at 1800 hour to provide a resting venous blood and urine sample. Subsequently, they were allowed to go home with instructions to only eat and sleep as per their normal routine.
Days 2 and 3. On day 2, participants completed the 10 × 1 km interval running session (T1INT) at 90% of the individual peak \( \dot{V}{\text{O}}_{2} \) running velocity (work to rest ratio of 2:1). The remaining procedures and timing of days two and three in the T1 condition were an exact replication of those performed on days two and three of the T2 condition outlined above.
Sample collection and analysis
Urine
Urine samples were collected into a 75-ml, sterilized container and centrifuged at 10°C and 3,000 rpm for 10 min. The supernatant was divided into aliquots and stored at −80°C until analysis. Urinary hepcidin-25 was measured at the Department of Clinical Chemistry, Radboud University Nijmegen Medical Centre, The Netherlands, as described previously (Kemna et al. 2007; Swinkels et al. 2008). In brief, 5 μL (0.3 μM) of the solution of the lyophilized internal standard (synthetic hepcidin-24, Peptide Int., Louisville KY) in distilled water was added to 495 μL urine before hepcidin was extracted from the sample with the use of Macro-Prep CM support beads (Biorad Laboratories) (Park et al. 2001). Next, 1 μL of the extract of hepcidin was applied to a normal phase chip (NP20; Bio-Rad Laboratories, Hercules, CA) followed by the addition of energy-absorbing matrix (Kemna et al. 2007), all in a nitrogen atmosphere. Hepcidin quantification via surface-enhanced laser desorption/ionization time-of-flight mass spectrometry (SELDI-TOF-MS) has been described previously (Kemna et al. 2005b; Kemna et al. 2007). The standard curve of hepcidin-25 that was constructed by exploiting hepcidin-24 as the internal standard and by serially diluting hepcidin-25 in tubes with blank urine from a patient with juvenile hemochromatosis (Van Dijk et al. 2007), was linear (R 2 > 0.99). In addition, spiking of both hepcidin isoforms in different concentration combinations to blank urine revealed no influence of the internal standard hepcidin-24 to the peak height and position of the human hepcidin-25. Hepcidin results are expressed here relative to urinary creatinine levels. The lower limit of detection with this method is 0.05 nM with an intra-run variation of 3.0% at 3.3 and 9.9 nM, and an inter-run variation of 12.6% at 1.5 nM, and 10.2% at 9.1 nM.
Blood
Venous blood was collected from an antecubital vein in the forearm with the athlete lying down for a minimum of 5 min to control for postural shifts in plasma volume. The venous samples were collected in an identical fashion to that of Telford et al. (2003) using a 21-gage needle together with minimal stasis and a gentle, slow movement of the syringe plunger in order to avoid unnecessary hemolysis from the blood collection. Subsequently, blood was gently deposited down the side of one 4.5 ml EDTA and two 8.5 ml SST II Gel collection tubes (BD Vacutainer™, NJ, USA) with the vacuum seals removed, and allowed to clot for 60 min at room temperature. The samples were then centrifuged at 10°C and 3,000 rpm for 10 min. Serum supernatant was then divided into 1-ml aliquots and stored at −80°C until further analysis. These samples were analyzed for a full blood picture, Hematocrit (Hct), IL-6, serum Hp and free Hb at the pre- and post-exercise time points, and for serum iron and ferritin at the pre-, post- and 24 h post-exercise time points. Blood analysis was conducted at the Sir Charles Gairdiner Hospital Pathology Laboratory (Perth, Western Australia) and the Fremantle Hospital Pathology Laboratory (Fremantle, Western Australia).
A full blood picture including hemoglobin (Hb) analysis was conducted on a Coulter Counter (Beckman Coulter, LH750, Fullerton, CA, USA) Hematocrit were calculated from the Hb, red cell count, and mean corpuscular volume values derived from a red cell histogram. Serum IL-6 was measured using a commercially available ELISA (Quantikine HS, RandD Systems, Minneapolis, USA) with an assay range of 0.38–10 ng L−1. The coefficient of variation (CV) for IL-6 determination at 0.49 and 2.78 ng L−1 was 9.6 and 7.2%, respectively. Iron, transferrin and ferritin were measured on the Roche Modular System (Roche Diagnostics, Switzerland). Serum iron levels were determined using FerroZineÒ reagent and absorbance was measured at 552 nm. The CV for iron determination at 23.3 and 49.0 mmol L−1 was 2.5 and 1.0%, respectively. Ferritin levels were determined using a latex enhanced immunoturbidimetric assay with the absorbance measured at 552 nm. The CV for ferritin determination at 15.0 and 279.0 μg L−1 was 16.7, and 1.8%, respectively.
Free Hb was determined using a scanning spectrophotometer between wavelengths of 650–480 nm. The CV for free Hb determination at 161.0 and 82.0 μg L−1 was 2.2, and 3.4%, respectively. Quantitative serum Hp determination was achieved using rate nephelometry and an anti-human antibody (processed goat sera) reagent. The increase in light scatter from particles suspended in solution was measured resulting from the human antigen-human antibody reaction. The CV for serum Hp determination at 157.0 μg L−1 was 5%.
Statistical analysis
Results are expressed as mean and standard error (±SE). Repeated measures ANOVA were used to analyze time, trial and time*trial effects of the number of running sessions completed in one day on the hematological and urine variables measured. Post hoc, paired samples t tests were used to determine where specific trial differences existed. The alpha level was set at p ≤ 0.05.
Results
Running
The running velocity, average 1 km split times, average HR and the BLa at the 5- and 10-km time points during T1 and T2 are shown in Table 2. The running velocity, average 1 km split times, HR and BLa were significantly faster in the T2INT when compared to the T2LSD (p < 0.05). There were no differences between the T2INT and the T1INT sessions in any of these variables (p > 0.05).
Free hemoglobin
Free Hb levels are presented in Table 3. There was a significant time (p = 0.0001) but no trial effect (p = 0.507) between T1 and T2. The time effect showed that the free Hb levels were significantly increased at the completion of the T2INT and the T1INT trials (p = 0.013 and 0.002, respectively), and also at the conclusion of the T2LSD run (p = 0.003), compared to pre-exercise levels. The increased post-T2LSD free Hb levels had decreased significantly by the commencement of T2INT (p = 0.042), approximately 12 h later, but were still significantly higher than the pre-T2LSD values. Finally, the post-T2LSD free Hb levels were significantly lower than those recorded post-T2INT (p = 0.013).
Serum haptoglobin
Serum Hp levels are also shown in Table 3. A significant time effect (p = 0.003) and a significant time*trial effect (p = 0.001) were recorded. The time effect showed that the post-T1INT serum Hp levels were significantly lower than pre-T1INT (p = 0.037). Within the T2 trial, the serum Hp levels fell significantly at the conclusion of the T2LSD (p = 0.006), and remained at the same levels 12 h later (pre-T2INT), still significantly lower than pre-T2LSD (p = 0.011). The post-T2INT serum Hp levels then fell significantly further from pre-T2INT levels (p = 0.002), being also significantly lower than those recorded post-T2LSD (p = 0.001). The time*trial effect showed that both the pre-T2INT and post-T2INT serum Hp levels were significantly lower than those recorded at the pre-T1INT and post-T1INT time points (p = 0.034 and 0.027, respectively).
Interleukin-6
Interleukin-6 levels are shown in Fig. 1. There was a significant time effect (p = 0.0001) but no trial effect (p = 0.441). In the T1 condition, the post-T1INT IL-6 levels were significantly greater than those recorded pre-T1INT (p = 0.001). In the T2 condition, post-T2LSD levels of IL-6 were significantly greater than at pre-T2LSD (p = 0.0001). These elevated levels had returned to baseline 12 h later (pre-T2INT; p = 0.0001), but were again significantly increased at post-T2INT (p = 0.004). There were no differences between the IL-6 levels recorded post-T2LSD and post-T2INT (p = 0.379).
Hepcidin
Hepcidin levels are presented in Fig. 2. There was a significant time effect (p = 0.004) but no trial effect (p = 0.306) between the T1 and T2 conditions. The post-T1INT hepcidin levels were significantly greater than those recorded pre-T1INT (p = 0.030). In the T2 condition, the 3-h post-T2LSD levels of hepcidin were significantly greater than at pre-T2LSD (p = 0.049). These elevated levels had returned to baseline 12 h later (pre-T2INT), but were again significantly increased after the T2INT session (p = 0.028). There were no differences between the hepcidin levels recorded post-T2LSD and post-T2INT (p = 0.789).
Serum iron
Serum iron levels are shown in Table 4. A significant effect for time (p = 0.002), but no trial effect (p = 0.528) existed between the T1 and T2 conditions. The time effect within the T1 trial showed that serum iron levels were significantly elevated post-T1INT and 24 h later (p = 0.004 and 0.010, respectively). In the T2 condition, there were no differences between pre-T2LSD and post-T2LSD values (p = 0.247); however, the post-T2INT serum iron levels were significantly increased (p = 0.002), and remained elevated 24 h later (p = 0.019).
Serum ferritin
Serum ferritin levels are also shown in Table 4. There was a significant effect for time (p = 0.007), but no trial effect between the T1 and T2 conditions (p = 0.568). The post-T1INT serum ferritin levels were significantly greater than pre-T1INT (p = 0.028). Twenty-four hours later, these levels had returned to baseline (p = 0.039). In the T2 condition, the pre-T2LSD serum ferritin levels were not different to the post-T2LSD (p = 0.144). However, the post-T2INT serum ferritin levels were significantly greater than the pre-T2INT (p = 0.048), but again had fallen to baseline levels by 24 h (p = 0.018). The post-T2INT serum ferritin levels were significantly higher than those recorded post-T2LSD (p = 0.008).
Hemoglobin and hematocrit
The Hb levels recorded pre- and post-R1INT were 152 (±2) g L−1 and 151 (±2) g L−1, respectively. The Hct levels recorded pre- and post-R1INT were 0.44 (±0.01) and 0.43 (±0.01), respectively. The Hb levels recorded during the R2 condition were, pre-LSD: 151 (±3) g L−1, post-LSD: 153 (±2) g L−1, pre-INT: 152 (±3) g L−1, post-INT: 152 (±2) g L−1. The Hct recorded during the R2 condition were, pre-LSD: 0.44 (±0.01), post-LSD: 0.44 (±0.01), pre-INT: 0.44 (±0.01), post-INT: 0.44 (±0.01). There were no significant time or trial effects for changes in Hb (p = 0.974; p = 0.741, respectively) or Hct (p = 0.690; p = 0.546, respectively).
Discussion
The findings of this investigation showed that inflammation, hemolysis, serum iron, ferritin, and urinary hepcidin were elevated at the conclusion of a high intensity interval running session. Further, a cumulative effect of two running training sessions within 12 h on the amount of hemolysis incurred was evident, shown via the significantly greater decreases in serum Hp during T2INT. The increases in inflammation, iron status, and hepcidin levels were not affected by the addition of a second session. The implications of these results are discussed below.
Hemolysis
Hemolysis was evident here in both the T1 and the T2 conditions, shown via significant post-run increases in free Hb and decreases in serum Hp. In response to hemolysis, serum Hp forms a complex with free Hb in an attempt to restrict the rise in oxidative stress (Giblett 1968). The Hp–Hb complex is cleared from the circulation by binding to the CD163 hemoglobin scavenger receptors on the surface of macrophages (Kristiansen et al. 2001), and is taken up by endocytosis in the liver, stimulating rapid ferritin synthesis (Van Vlierberghe et al. 2004). Such a mechanism may in part explain the increased post-exercise ferritin levels seen here. In the T2 condition, the significant post-exercise changes to free Hb and serum Hp remained evident 12 h post-T2LSD, suggesting that the scavenging process of Hp and macrophages in response to exercise-induced hemolysis is not completely finished within 12 h of recovery, and that the body is still attempting to ‘clean up’ the damage created by running.
The INT session in the T2 trial resulted in significantly greater increases to free Hb and decreases to serum Hp than those seen after the T2LSD run. Furthermore, the T2INT decreases in serum Hp was also significantly lower than that seen after the T1INT trial. Telford et al. (2003) suggested that twice-daily hemolytic episodes could have a cumulative effect in athletes that may eventually begin to influence iron stores. This theory is supported by the serum Hp data of the current investigation. However, the lack of difference between the free Hb levels recorded post-INT between the T1 and T2 conditions may also be explained by the greater decreases in serum Hp after T2INT, since it is likely that this corresponds to more Hp–Hb complexes being created to deal with the cumulative stress of multiple running sessions.
Inflammation
Previously, it has been shown that a second bout of high-intensity exercise on the same day is associated with a more pronounced increase in IL-6 (Ronsen et al. 2002). Here, the inflammatory response to running showed a significant increase in IL-6 activity at the conclusion of the T2LSD run, which then decreased and returned to baseline levels within 12 h of recovery (pre-T2INT). The performance of the T2INT session then showed a further significant increase to post-exercise IL-6, which was also significantly greater than that of the T2LSD trial. However, the IL-6 responses were identical at the conclusion of the T1INT. These results agree with those of Ronsen et al. (2002), since the second bout of exercise performed here was associated with a more pronounced increase in IL-6. However, it is likely that this increase was the result of the greater intensity INT run when compared to the LSD run, since positive relationships between the intensity of exercise and increases in IL-6 have previously been reported (R 2 = 0.69 and 0.32, respectively) (Ostrowski et al. 1998, 2000). In addition, there were no differences in the increase to IL-6 levels between the T2INT and the T1INT trial.
The more pronounced increases in IL-6 activity as a result of a second exercise bout seen by Ronsen et al. (2002) would come as a result of the recovery time given between the two bouts of exercise. These greater IL-6 levels occurred when the second bout of exercise came after only 3 h of recovery. In fact, when the rest period between bouts of exercise was increased to 6 h, an attenuation of this cumulative IL-6 response was shown. Furthermore, previous investigations have shown that increases in plasma IL-6 begin to occur after 30 min of exercise (Ostrowski et al. 1998), with peak post-exercise activity occurring for up to 1.5 h (Bruunsgaard et al. 1997; Margeli et al. 2005). Thereafter, these heightened levels of IL-6 are followed by a rapid decline (Pedersen et al. 2001). The current investigation implemented a recovery period between exercise bouts that represents what is commonly employed by elite athletes (~12 h). Since no cumulative IL-6 effects were seen from the two consecutive sessions, it would seem that the current training methodologies used by endurance athletes provides a safe amount of recovery time to sufficiently return elevated IL-6 to resting levels prior to the subsequent session, when running training of the intensity and duration used here is performed.
Hepcidin
The increases in IL-6 activity recorded here may also provide explanation for the up-regulation of hepcidin activity seen 3 h post-exercise. Previously, it was shown that urinary hepcidin levels peaked 3 h after the peak in IL-6 activity in healthy subjects intravenously injected with a bolus of lipopolysaccharide (Kemna et al. 2005a). Furthermore, a significant rise in hepcidin levels 3 h after the completion of 10 × 1 km interval run session at 90–95% of the peak \( \dot{V}{\text{O}}_{2} \) running velocity has also been reported (Peeling et al. 2008b). Therefore, we considered that the 3-h post-exercise time point was the most appropriate to show any changes in hepcidin activity. Elevations in post-exercise hepcidin levels may impose a challenge to an athlete’s iron stores via attenuation of iron recycling from the macrophage, in addition to incurring a period of reduced iron absorption from the gut due to the degradation and internalization of Fpn transport channels situated on the intestinal enterocytes and on the macrophage cell surface (Nemeth et al. 2004a, b).
Although significant hepcidin increases 3 h post-T2LSD were evident in the current investigation, it would appear that there were no cumulative effects of two running sessions completed within 12 h, since the increases seen post-T2INT were not greater than recorded after the T2LSD, or after the T1INT. Such an outcome would suggest that 12 h of recovery provides a sufficient amount of time for an athlete to restore their hepcidin activity to resting levels when conducting the type of running training outlined here, since the hepcidin response showed a significant decline in the 12-h post-T2LSD. This outcome contrasts that of Roecker et al. (2005), who found significantly elevated hepcidin levels 24 h after the completion of exercise. This difference may be explained by the duration and intensity of exercise completed, since the participants of Roecker and colleagues ran four times the distance covered during the T2LSD run, at an average speed that was 26% slower, and for a duration averaging 3 h 44 min longer. Therefore, a relationship may exist between the duration and intensity of exercise, and the half life of hepcidin activity post-exercise. However, further investigation is needed to verify this assertion.
Iron status
A second explanation for the increased post-exercise hepcidin activity may be the significant increases in serum iron. In addition to free Hb and serum Hp, post-exercise increases in serum iron also reflect a hemolytic stimulus (Buchman et al. 1998). As previously mentioned, increased iron levels are also involved in the up-regulation of hepcidin activity (Nemeth et al. 2004b). As such, the post-exercise hepcidin response is likely homeostatic in nature, to help control and reduce the elevated levels of iron resulting from the exercise-induced hemolysis. However, it is possible that elevations in post-exercise hepcidin activity after the increases in serum iron have been controlled may potentially affect the ability to absorb dietary iron, thereby reducing the athletes’ potential to replenish their daily iron requirements. Such an outcome would elicit an opposite regulatory process whereby the heightened hepcidin activity begins to decrease the body’s iron store. However, the long-term effect of this hepcidin hyperactivity on an athlete’s iron status remains to be investigated.
Finally, it should be considered that although significant increases in post-exercise serum iron and ferritin levels were seen here, there were no cumulative effects of two training sessions within 12 h, since there were no changes to serum iron or ferritin at the conclusion of the T2LSD run. Furthermore, the T2INT changes that did occur were not different to those of the T1INT. The lack of change after the T2LSD run might be explained by the significantly lower amount of hemolysis seen in response to the LSD run, since the changes in serum Hp and free Hb were not as pronounced. Previously, Pattini et al. (1990) showed serum iron and ferritin responses to be dependent upon the intensity and duration of exercise, with more intense and longer duration efforts causing greater changes. As such, an intensity-dependent relationship may underscore the lack of change seen at the conclusion of the T2LSD run.
Practical application
The results of this investigation would suggest that current training methodologies (i.e. multiple daily training sessions separated by 10–12 h) are relatively safe for high level athletes to complete when considering the cumulative effect on acute inflammation and hepcidin responses on iron metabolism. However, the cumulative increases in hemolysis must be considered by coaches working with athletes that struggle to maintain a healthy iron balance, with the possibility of non-weight-bearing, cross-training alternatives in place of a second running session on the same day, to avoid further taxing already compromised iron stores. Furthermore, although no cumulative increases in hepcidin activity were shown here, athletes and sports nutritionists need to consider that there may exist a period of decreased dietary iron absorption 3 h post-exercise, therefore influencing the timing of consuming iron-containing foods. However, future research is warranted to further explore the time course of potential optimal feeding periods.
Conclusions
The cumulative effects of two running training sessions within 12 h of one another have been demonstrated on hemolysis in well trained male athletes. However, despite this effect, the inflammatory response (as shown by an increased IL-6 expression), the hepcidin activity, and iron status do not have the same cumulative response. The reaction of these variables and their interaction to a more chronic training stimulus remains to be investigated.
References
Babić Z, Papa B, Sikirika-Bosnjaković M et al (2001) Occult gastrointestinal bleeding in rugby players. J Sports Med Phys Fitness 41:399–402
Beard J, Tobin B (2000) Iron status and exercise. Am J Clin Nutr 72:594S–597S
Bruunsgaard H, Galbo H, Halkjaer-Kristensen J et al (1997) Exercise-induced increase in serum interleukin-6 in humans is related to muscle damage. J Physiol 499:833–841
Buchman AL, Keen C, Commisso J et al (1998) The effect of a marathon run on plasma and urine mineral and metal concentrations. J Am Coll Nutr 17:124–127
DeRuisseau KC, Cheuvront SN, Haymes EM et al (2002) Sweat iron and zinc losses during prolonged exercise. Int J Sport Nutr Exerc Metab 12:428–437
Giblett ER (1968) The haptoglobin system. Ser Haematol 1:3–20
Jones AM, Doust JH (1996) A 1% treadmill grade most accurately reflects the energetic cost of outdoor running. J Sports Sci 14:321–327. doi:10.1080/02640419608727717
Kemna E, Pickkers P, Nemeth E et al (2005a) Time-course analysis of hepcidin, serum iron, and plasma cytokine levels in humans injected with LPS. Blood 106:1864–1866. doi:10.1182/blood-2005-03-1159
Kemna E, Tjalsma H, Laarakkers C et al (2005b) Novel urine hepcidin assay by mass spectrometry. Blood 106:3268–3270. doi:10.1182/blood-2005-05-1873
Kemna EH, Tjalsma H, Podust VN (2007) Mass spectrometry-based hepcidin measurements in serum and urine: analytical aspects and clinical implications. Clin Chem 53:620–628. doi:10.1373/clinchem.2006.079186
Kristiansen M, Graversen JH, Jacobsen C (2001) Identification of the haemoglobin scavenger receptor. Nature 409:198–201. doi:10.1038/35051594
Margeli A, Skenderi K, Tsironi M et al (2005) Dramatic elevations of interleukin-6 and acute-phase reactants in athletes participating in the ultradistance foot race spartathlon: severe systemic inflammation and lipid and lipoprotein changes in protracted exercise. J Clin Endocrinol Metab 90:3914–3918. doi:10.1210/jc.2004-2346
McInnis MD, Newhouse IJ, von Duvillard SP et al (1998) The effect of exercise intensity on hematuria in healthy male runners. Eur J Appl Physiol Occup Physiol 79:99–105. doi:10.1007/s004210050480
Miller B, Pate RR, Burgess W (1988) Foot impact force and intravascular hemolysis during distance running. Int J Sports Med 9:56–60. doi:10.1055/s-2007-1024979
Nemeth E, Rivera S, Gabayan V et al (2004a) IL-6 mediates hypoferremia of inflammation by inducing the synthesis of the iron regulatory hormone hepcidin. J Clin Invest 113:1271–1276
Nemeth E, Tuttle MS, Powelson J et al (2004b) Hepcidin regulates cellular iron efflux by binding to ferroportin and inducing its internalisation. Science 306:2090–2093. doi:10.1126/science.1104742
Ostrowski K, Hermann C, Bangash A et al (1998) A trauma-like elevation of plasma cytokines in humans in response to treadmill running. J Physiol 513:889–894. doi:10.1111/j.1469-7793.1998.889ba.x
Ostrowski K, Schjerling P, Pedersen BK (2000) Physical activity and plasma interleukin-6 in humans—effect of intensity of exercise. Eur J Appl Physiol 83:512–515. doi:10.1007/s004210000312
Park CH, Valore EV, Waring AJ et al (2001) Hepcidin, a urinary antimicrobial peptide synthesized in the liver. J Biol Chem 276:7806–7810. doi:10.1074/jbc.M008922200
Pattini A, Schena F, Guidi GC (1990) Serum ferritin and serum iron changes after cross-country and roller ski endurance races. Eur J Appl Physiol Occup Physiol 61:55–60. doi:10.1007/BF00236694
Pedersen BK, Steensberg A, Schjerling P (2001) Exercise and interleukin-6. Curr Opin Hematol 8:137–141. doi:10.1097/00062752-200105000-00002
Peeling P, Dawson B, Goodman C et al (2008a) Athletic induced iron deficiency: new insights into the role of inflammation, cytokines and hormones. Eur J Appl Physiol 103:381–391. doi:10.1007/s00421-008-0726-6
Peeling P, Dawson B, Goodman C et al (2008b) Training surface and intensity: inflammation, hemolysis and hepcidin expression. Med Sci Sports Exerc (in press)
Poortmans JR, Haralambie G (1979) Biochemical changes in a 100 km run: proteins in serum and urine. Eur J Appl Physiol Occup Physiol 40:245–254. doi:10.1007/BF00421516
Roecker L, Meier-Buttermilch R, Brechtel L et al (2005) Iron-regulatory protein hepcidin is increased in female athletes after a marathon. Eur J Appl Physiol 95:569–571. doi:10.1007/s00421-005-0055-y
Ronsen O, Lea T, Bahr R et al (2002) Enhanced plasma IL-6 and IL-1ra responses to repeated vs. single bouts of prolonged cycling in elite athletes. J Appl Physiol 92:2547–2553
Schobersberger W, Tschann M, Hasibeder W et al (1990) Consequences of 6 weeks of strength training on red cell O2 transport and iron status. Eur J Appl Physiol Occup Physiol 60:163–168. doi:10.1007/BF00839152
Selby GB, Eichner ER (1986) Endurance swimming, intravascular hemolysis, anemia, and iron depletion, new perspective on athlete’s anemia. Am J Med 81:792–794. doi:10.1016/0002-9343(86)90347-5
Swinkels DW, Girelli D, Laarakkers C et al (2008) Advances in quantitative hepcidin measurements by time of flight mass spectrometry. PLoS ONE 3:e2706. doi:10.1371/journal.pone.0002706
Telford R, Sly GJ, Hahn AG et al (2003) Footstrike is the major cause of hemolysis during running. J Appl Physiol 94:38–42
Van Dijk BA, Kemna EH, Tjalsma H et al (2007) Effect of the new HJV-L165X mutation on penetrance of HFE. Blood 109:5525–5526. doi:10.1182/blood-2006-11-058560
Van Vlierberghe H, Langloisb M, Delanghec J (2004) Haptoglobin polymorphisms and iron homeostasis in health and in disease. Clin Chim Acta 345:35–42. doi:10.1016/j.cccn.2004.03.016
Zoller H, Vogel W (2004) Iron supplementation in athletes—first do no harm. Nutrition 20:615–619. doi:10.1016/j.nut.2004.04.006
Acknowledgments
The authors wish to acknowledge the grant funding received from the Fremantle Hospital Medical Research Foundation. The authors would also like to acknowledge the grant funding received from the University of Western Australia’s Research Grant Scheme. Grant funding for this project was received from the Fremantle Hospital Medical Research Foundation, and the University of Western Australia’s Research Grant Scheme.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Peeling, P., Dawson, B., Goodman, C. et al. Cumulative effects of consecutive running sessions on hemolysis, inflammation and hepcidin activity. Eur J Appl Physiol 106, 51–59 (2009). https://doi.org/10.1007/s00421-009-0988-7
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00421-009-0988-7