Abstract
Purpose
Adverse cardiovascular events are more prevalent during winter and in people that exercise/work in cold temperatures. Since pulse wave analysis indices, aortic systolic blood pressure (ASBP), augmentation index (AIx), and wasted left ventricular pressure energy (ΔE w), are stronger predictors of cardiovascular events and myocardial performance than brachial blood pressure (BP), we sought to evaluate the aortic hemodynamic responses during cold exposure with concurrent isometric handgrip exercise (IHG).
Methods
In a crossover randomized fashion, 20 healthy normotensive men (22.1 ± 2 years) were evaluated, by means of radial applanation tonometry, inside an environmental chamber in the supine position at cold (4 °C) and temperate (24 °C) conditions. Following a 30-min equilibration period, measurements were performed during pre-exercise baseline (REST), in the last 90 s of a 3-min IHG at 30 % maximal voluntary contraction, and 3 min immediately after the finalization of IHG bout (recovery, REC).
Results
At REST, brachial systolic BP (BSBP) (12 ± 2 mmHg; P < 0.01), ASBP (14 ± 3 mmHg; P < 0.01), AIx (11 ± 3 %; P < 0.05), and ΔE w (737 ± 128 dynes s/cm2; P < 0.01) were higher in 4 °C compared to 24 °C trial. Compared to REST, IHG significantly increased (P < 0.01) BSBP, ASBP, AIx, and ΔEw, while BSBP and ASBP remained elevated during REC (P < 0.01). Compared to REST and temperate, AIx (11 ± 3 %) and ΔE w (793 ± 145 dynes s/cm2; P < 0.01) were higher during IHG and cold, while BSBP and ASBP were elevated during REC and cold. AIx and ΔE w returned to REST values in both trials, but AIx (11 ± 4 %; P < 0.05) and ΔE w (656 ± 132 dynes s/cm2; P < 0.05) were higher in the cold.
Conclusions
Cold exposure with concurrent IHG induces a significant increase in aortic hemodynamic markers, which may evoke adverse cardiovascular events.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Converging evidence from epidemiological studies indicates that the incidence of cardiovascular morbidity and mortality reaches a peak during the winter months (Analitis et al. 2008; Rocklov and Forsberg 2008). It has been suggested that low environmental temperatures, typical of the winter season, may induce increased cardiovascular stress resulting in hypertension (Adamopoulos et al. 2010). In addition, hypertension- and cardiovascular-related visits to emergency rooms are more prevalent during cold spells (Cheng and Su 2010), and in those individuals working inside cold rooms (Kim et al. 2003). In fact, cold temperatures per se have been implicated as a putative factor associated with increased adverse cardiovascular events such as angina, myocardial infarction, and stroke (Woodman et al. 2006; Reinhard et al. 2010; Wolf et al. 2009; Hong et al. 2003). However, the hemodynamic mechanisms that may explain this association remain poorly understood.
Previous research has consistently documented the cold-induced pressor response by means of different cold exposure models such as the cold pressor test, cold air inhalation, regional cooling, and whole-body exposure (Makinen et al. 2008; Bokenes et al. 2004; Korhonen 2006; Edwards et al. 2008b; Muller et al. 2011). This is worth noting because interpretation of acute hemodynamic responses, including cold-induced hypertension, between the different cold exposure models may yield conflicting results. Interestingly, the application of cold exposure in addition to physical activities that may simulate physical activities, such as snow shoveling and/or heavy lifting performed inside cold rooms, may add to our understanding of how the combination of exercise and cold may precipitate adverse cardiovascular events. Since exercise with concurrent cold exposure imposes an additional cardiovascular stress (Geleris et al. 2004), which may ultimately impair central hemodynamics (Adamopoulos et al. 2010), the use of isometric handgrip (IHG) exercise has been proposed as a valuable test for evaluating hemodynamic and autonomic responses during cold stimulation (Makinen et al. 2008; Geleris et al. 2004). It is worth mentioning that regional cooling, such as cold pressor test, induces a higher pressor response compared with whole-body cold exposure (Korhonen 2006). For this reason, the application of whole-body cold exposure may be a more appropriate model to study the effects of cold with concurrent exercise.
Indices of pulse wave analysis, including aortic systolic blood pressure (ASBP), aortic augmentation index (AIx; surrogate of wave reflection), and wasted left ventricular (LV) pressure energy (ΔE w), are superior predictors of cardiovascular events and cardiovascular disease progression than brachial blood pressure (BP) (O’Rourke and Adji 2005; Safar et al. 2008; Vlachopoulos et al. 2010). Accordingly, increased AIx, a surrogate of wave reflection, has been associated with high rates of cardiovascular morbidity and mortality (Vlachopoulos et al. 2010; Roman et al. 2009) owing to an increased LV afterload and ΔEw (Hashimoto et al. 2008). Consistent with this view, previous research has documented that local cooling and whole-body cold exposure increase AIx and central BP to a greater magnitude than brachial BP (Edwards et al. 2006; Hess et al. 2009). These cold-induced increases in wave reflection have also been shown during cold pressor test with concurrent IHG. For instance, Geleris et al. (2004) reported a greater increase in AIx when performing IHG during cold pressor test compared to either condition alone. Together these studies suggest that evaluation of pulse wave analysis during cold exposure with concurrent exercise may be a more practical tool for detecting cardiovascular anomalies that may be unnoticeable using brachial BP.
Accordingly, the aim of the present study was to evaluate the aortic hemodynamic responses during cold exposure with concurrent IHG exercise. We hypothesized that the combination of cold exposure with IHG would evoke a greater acute increase in peripheral and central BP, wave reflection, and ΔEw than cold exposure or IHG alone. In addition, we anticipated a greater increase in central BP compared to peripheral BP in response to cold exposure.
Methods
Subjects
Twenty healthy men young adults (age 18–35 years) were enrolled in this study. Males were selected for study inclusion to eliminate the potential effect of gender on the study variables. Subjects were nonsmokers and were not regular exercisers (<120 min per week) 6 months prior to the study. Subjects were excluded from the study if they had BP ≥ 140/90 mmHg, chronic diseases, taking medications (e.g., beta blockers, antidepressants, and stimulants) and/or nutritional supplements (e.g., l-citrulline, l-arginine, and Creatine) that would influence our outcome variables. All participants were recruited from a university sample and gave their written consent prior to the experiments as approved by The Florida State University institutional review board. This study was part of the experimental procedures that comprised clinical trial NCT01462591.
Study design and experimental protocol
This study was undertaken in Tallahassee, FL, USA (30.46 N, 84.28 W) between the months of June and August (mean temperature 29.5 °C). In a crossover randomized design, cardiovascular functioning of eligible subjects was evaluated inside an environmental chamber (Heinicke HU 682 Lancaster, PA, USA) either at cold (4 °C) or temperate (24 °C) environmental conditions with humidity set at 65 % and air velocity of <0.5 m/s for both trials.
Height and weight were assessed during the familiarization day. The maximal voluntary contraction (MVC) of the dominant arm was measured by having subjects squeeze a Lafayette hand dynamometer model 78010 (Lafayette Instrument Company, Indiana, USA) for 3 s using the best of three attempts separated by 1 min. The MVC was used to calculate their individual 30 % for the experimental protocol.
Trials
The hemodynamic responses to cold or temperate with concurrent IHG exercise were evaluated on 2 separate days at least 48 h apart. The experiments were conducted in the afternoon hours at the same time (±1 h) and in a fasted state (>4 h) to avoid potential circadian and/or food ingestion influences on BP and vascular reactivity. Subjects were instructed to enter in an environmental chamber and rest in the supine position on an air filled mattress, wearing shorts and t-shirt, for 30 min of temperature equilibration. Thereafter, cardiovascular measurements were performed, in duplicates and averaged, during the last 90 s of a 5-min pre-exercise baseline (REST), the last 90 s of a 3 min IHG at 30 %MVC, and the last 90 s of 3 min immediately after the finalization of IHG bout (post IHG recovery, REC). The subjects were exposed for a total of 41 min to both environmental conditions. The rationale for allowing 30 min equilibration time was based on a prior study showing that this time evokes an increase in aortic systolic blood pressure (Edwards et al. 2006).
Anthropometrics
Height was measured using a stadiometer to the nearest 0.5 cm, and body weight was measured using a SECA scale (Sunbeam Products Inc., Boca Raton, FL, USA) to the nearest 0.1 kg. Body mass index (BMI) was calculated as kg/m2. The subject characteristics age, height, weight, and BMI were 22.1 ± 2 years, 1.77 ± 0.01 m, 84.7 ± 3.12 kg, and 27.1 ± 1.0 kg/m2, respectively.
Cardiovascular measurements
Brachial BP was recorded using an automated oscillometric device (HEM-705CP; Omron healthcare, Vernon hill, Illinois, USA) in the non-dominant arm. Brachial systolic BP (BSBP) and diastolic BP (BDBP) were used to calibrate radial waveforms obtained from a 10-s epoch using a high-fidelity tonometer (SPT-301B; Millar instruments, Houston, TX, USA) also in the non-dominant arm. Aortic BP waveforms were derived using a validated generalized transfer function (SphygmoCor, AtCor Medical, Sydney, Australia) (Chen et al. 1997; Gallagher et al. 2004). The aortic BP wave comprises a forward wave (P1), caused by stroke volume ejection, and a reflected wave (P2) that returns to the aorta from peripheral sites (Nichols and O’Rourke 1998). The AIx is defined as the augmented pressure (AP = P2−P1) expressed as a percentage of the aortic pulse pressure (APP). Transit time of the reflected wave (Tr) indicates the round-trip travel of the forward wave to the peripheral reflecting sites and back to the aorta. Since there is an inverse relationship between AIx and heart rate (HR), it was automatically adjusted by the software at 75 bpm (AIx@75) (Wilkinson et al. 2002). HR was obtained from the time between pulse waveforms. The ΔE w was calculated as 1.333 × AP [ventricular ejection duration (ED)−Tr] × π/4, where 1.333 is the conversion factor for mm Hg/s to dynes s/cm2. The ΔE w is considered an estimate of additional workload generated by the myocardium to overcome increased ASBP and wave reflection indicative of ventricular work and myocardial oxygen demand (Hashimoto et al. 2008; Casey et al. 2008b).
Statistical analysis
A repeated-measures analysis of variance (ANOVA) with Bonferoni’s adjustment was used to test the effects of temperature (4 versus 24 °C) and condition (REST versus IHG versus REC) for all cardiovascular parameters. Significant main effects of the repeated-measures ANOVA were followed up with simple effects test via univariate contrasts. The difference between the peripheral and central systolic BP response to cold exposure at REST was analyzed using Student’s t test. Statistical significance was accepted at P < 0.05. All statistical analyses were performed using SPSS Version 19 (SPSS, Inc., Chicago, IL, USA). Since our primary outcome variable is aortic SBP a priori power calculation determined that 16 subjects would enable to observe a difference of 3–5 % between the trials (cold versus temperate) with a power of 80 %.
Results
All data are presented as mean ± SE. Figure 1 shows examples of typical radial and aortic BP waveforms at 24 and 4 °C trials, respectively. Data showing peripheral and central hemodynamics at REST, IHG and during REC are shown in Table 1.
Effects of cold environmental conditions on cardiovascular variables at rest
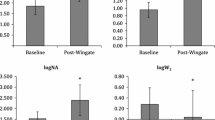
Compared with 24 °C, BSBP, BDBP, brachial mean arterial pressure (BMAP), ASBP (Fig. 2a), aortic diastolic BP (ADBP; Fig. 2b), and aortic mean arterial pressure (AMAP; Fig. 2c) were higher (P < 0.01) at REST in the 4 °C trial. Indices of pulse wave analysis, AP, AIx (P < 0.05), AIx@75 (P < 0.05), P1, P2, and ΔE w were higher (P < 0.01) whereas Tr and ED were lower (P < 0.01) in the 4 °C compared with the 24 °C trial. Brachial pulse pressure (BPP), aortic pulse pressure (APP; Fig. 2d), and HR were not different at REST between the trials. The magnitude of the cold-induced change (4 versus 24 °C) in ASBP (24.0 ± 1.7 mmHg) was greater (P < 0.01) than the change in BSBP (19.3 ± 1.9 mmHg).
Aortic blood pressure responses to isometric handgrip exercise in the cold (4 °C) and temperate (24 °C) trials (n = 20). Data are mean ± SE. Temp temperate, REST pre-exercise baseline, IHG isometric handgrip exercise, REC post-exercise recovery, ASBP aortic systolic blood pressure, ADBP aortic diastolic blood pressure, AMAP aortic mean arterial pressure, APP aortic pulse pressure. † P < 0.05, ‡ P < 0.01 different than cold (4 °C). *P < 0.05, **P < 0.01 different than REST
Effects of cold environmental conditions on cardiovascular variables during exercise
During IHG there were significant increases (P < 0.01) in HR, BSBP, BDBP, BMAP (P < 0.05), ASBP (Fig. 2a), ADBP (P < 0.05; Fig. 2b), AMAP (Fig. 2c), AP, AIx, AIx@75 (P < 0.05), P1, P2, and ΔEw whereas Tr and ED decreased (P < 0.01) compared with REST in both trials. However, compared to the 24 °C trial, AP, AIx (Fig. 3a), AIx@75 (P < 0.05), and ΔE w (Fig. 3c) were higher (P < 0.01) at 4 °C during IHG, whereas Tr (Fig. 3b) and ED were lower (P < 0.01), at 4 °C compared to the 24 °C trial, during IHG exercise.
Effects of cold environmental conditions on cardiovascular variables during post-exercise recovery
Compared with REST, BSBP, BDBP, BMAP, ASBP, ADBP, AMAP, P1, and P2 remained elevated (P < 0.01) in the 24 °C trial only during REC. When comparing these same variables between 4 and 24 °C, all variables were higher (P < 0.01) in the 4 °C compared to the 24 °C. Indices of pulse wave analysis, AP, AIx, AIx@75, ΔE w, and Tr, returned to REST values during the REC period in both trials. However at 4 °C, AP, AIx, AIx@75, and ΔEw were higher (P < 0.05) while Tr was lower (P < 0.01) when compared with the 24 °C trial. During REC, ED returned to the REST level in the 24 °C trial, but remained increased in the 4 °C trial. During REC, HR fully recovered in both trials.
Discussion
We sought to evaluate the acute aortic hemodynamic responses to IHG during whole-body cold exposure and thermoneutral environmental conditions. The main findings of the present study are the following (i) cold exposure increases both peripheral and central BP, but as opposed to our hypothesis the BP during IHG is similar between 24 and 4 °C, and (ii) cold exposure with concurrent IHG evokes a significant increase in AIx and ΔE w when compared with IHG at 24 °C. Our findings suggest that cold exposure imposes an additional increase in myocardial work at rest and during exercise that may ultimately evoke adverse cardiovascular events. Pulse wave analysis could provide a better understanding of the potential adverse effects of cold exposure during physical activity.
Our results are in agreement with those of Edwards et al. (2006) that showed greater changes in central systolic blood pressure (SBP) (~13 %) compared to the changes in peripheral SBP (~3 %) after whole-body cold exposure suggesting that peripheral vasoconstriction, and its profound effect on central pressure pulse wave characteristics, may not be effectively detected by brachial BP measurements alone. The low sensitivity of peripheral BP measurements to detect central BP changes in response to stressors might be associated to the fact that during cold exposure ASBP is mostly determined by reflected wave pressure (P2) whereas BSBP is more dependent on incident wave pressure (P1) (Takazawa et al. 1995; Edwards et al. 2006). In the present study, both aortic P1 and P2 were higher at REST and REC during the 4 °C trial compared to the 24 °C. However, in 4 °C, P2 was higher than P1 suggesting that the cold-induced pressor response is more dependent upon peripheral arteriolar vasoconstriction rather than to central arterial stiffening per se (Nichols 2005; Edwards et al. 2006). This phenomenon could be attributed to the fact that central arteries have smaller quantity of smooth muscle fibers and lower sympathetic innervation than muscular and resistance vessels and therefore arteriolar vasoconstriction may be more relevant contributor of wave reflection in the context of whole-body cold exposure (Kienecker and Knoche 1978).
The exact underlying hemodynamic mechanisms that may explain how cold exposure and exercise increase the likelihood of cardiovascular events are poorly understood. However, an increased pressor response and impaired cardiovagal reactivation resulting from exercising in a cold environment appear to be pivotal contributing factors (Sanchez-Gonzalez and Figueroa 2013; Mercer et al. 1999). In accordance with Figueroa et al. (2010a) and Edwards et al. (2008a), we showed that an acute bout of IHG increases HR (~12 bpm), BSBP (~20 mmHg), and ASBP (~28 mmHg) in 24 °C conditions. Nevertheless, the exercise pressor response during cold exposure was not higher than those during temperate conditions. Previous studies have demonstrated an exaggerated pressor exercise response (peripheral pressure) when performing IHG during cold pressor test (Geleris et al. 2004; Peikert and Smolander 1991). However, this is not a universal finding since other studies have failed to show the additive effect of cold exposure on the exercise pressor response (Makinen et al. 2008; Kahn et al. 1993; Muller et al. 2011). Nevertheless, we found that APP, but not BPP, was increased during IHG only in the cold condition. This finding is in accordance with prior studies that reported a higher increase in central pulse pressure (APP) than peripheral PP in young healthy adults during cold exposure (Casey et al. 2008a; Edwards et al. 2006). This differential response to exercise in cold temperature is important since increased central pressures, and more importantly APP, are associated with adverse cardiovascular events (Roman et al. 2007, 2009). Our findings suggest that the concurrent effects of IHG and cold exposure are more influential on arterial stiffening than BP.
In the present study, we have demonstrated that cold exposure with concurrent IHG increases the AIx to a greater magnitude than IHG in temperate conditions. Our results are somewhat in agreement with those of Geleris et al. (2004) that showed the additive effects of cold with concurrent IHG on the aortic AIx and pulse wave velocity (PWV, gold standard measure of arterial stiffness). However, we used whole-body cold exposure as opposed to cold pressor test to examine this response and hence we avoided potential effects of pain on the exercise pressor response to IHG. In addition, the Tr was reduced and APP was increased in the cold trial during the performance of IHG, to a greater extent than in the 24 °C trial, suggesting an additive effect on aortic stiffness. Interestingly, we observed a significant difference in wave reflection (AIx, AP) and estimated aortic stiffness (Tr, APP) during IHG between the trials in the absence of a significant differences in BP and HR. It is known that increased HR or reduced LV ED can reduce AIx (Wilkinson et al. 2002). However, cold exposure increases AIx due to faster wave reflection despite a small reduction in ED (Casey et al. 2008a), indicating that Tr is a rather more important determinant of AIx than ED. Therefore, during cold exposure with concurrent IHG exercise the responses in AIx are more likely to be driven by the timing (Tr) rather than the amplitude (P2) of the reflected wave, HR or ED. Interestingly, cold exposure increases peripheral arterial stiffness, but it does not increase central PWV suggesting a peripheral-mediated arterial tree stiffness (King et al. 2013). It could be that when performed concurrently IHG and cold exposure impact aortic stiffness to a greater extent than either condition alone and hence would explain higher AIx, Tr, and ΔE w in the cold trial than the temperate trial during IHG.
In the present study, we used ΔE w to estimate myocardial oxygen demand since this is a factor less influenced by peripheral BP and/or HR. The ΔE w has been shown to increase in response to the cold pressor test (Casey et al. 2008a). Despite displaying similar responses in BP and HR during exercise between the trials, we observed that ΔEw was increased with IHG in cold to a greater magnitude than IHG in temperate conditions. This is a novel finding because we demonstrated that IHG increases ΔE w which is further amplified during cold exposure. These results suggest that cold exposure with concurrent IHG increases myocardial work more than either cold or IHG alone even in the absence of detectable changes in peripheral or central rate pressure product (RPP). Therefore, ΔE w may be an additional measure to detect the effects of cold exposure with concurrent exercise on myocardial performance that may compliment peripheral BP, HR or RPP measurements.
The potential mechanisms that may explain increased aortic hemodynamic indices during whole-body cold exposure with concurrent exercise may be associated with sympathetic-mediated peripheral vasoconstriction and wave reflection. Previously, we have shown an association among IHG, cold exposure, and impaired cardiovagal modulation (Sanchez-Gonzalez and Figueroa 2013). Since the hemodynamic cardiovascular responses to IHG during cold exposure are driven by increased adrenergic stimulation (Makinen et al. 2008) and untimely increased smooth muscle vascular tone, this effect would have significantly impacted P2 more than P1 resulting in a greater increase in AP. In addition, sympathetic-mediated vasoconstriction induces a faster return of the reflected waveº (reduced Tr) from peripheral arteries to the aorta and its fusion with the forward wave during late systole increases APP and AIx during IHG and cold pressor test (Figueroa et al. 2010a, b; Lydakis et al. 2008; Casey et al. 2008a).
We recognize that the present study has some limitations including limited sample size, only male participants were recruited, the lack of endothelial function and direct arterial stiffness measurements, and the absence of skin and core temperature measurements. Applanation tonometry of peripheral arteries could hinder potential effects of cold exposure with concurrent exercise owing to vasoconstriction. We used Tr in our measurements which may not be an accurate measure of estimated arterial stiffness. Our study evaluated cardiovascular function in healthy young adults and hence we cannot generalize our results to other populations. Our study was not aimed to unveil the mechanisms that explain impaired aortic hemodynamics during cold exposure but rather to identify and define the responses.
In conclusion, whole-body cold exposure with concurrent IHG induces a significant increase in cardiovascular stress that is more pronounced when markers of aortic hemodynamics are measured. Our results demonstrate that the effects of increased wave reflection and myocardial demand during cold exposure with concurrent exercise may lead to exacerbation of adverse cardiovascular events. Further research is warranted to evaluate aortic hemodynamics during cold exposure with concurrent exercise in populations at increased cardiovascular risk.
Abbreviations
- ∆E w :
-
Wasted left ventricular pressure energy
- ADBP:
-
Aortic diastolic blood pressure
- AIx:
-
Aortic augmentation index
- AIx@75:
-
Aortic augmentation index adjusted to a heart rate of 75 bpm
- AMAP:
-
Aortic mean arterial pressure
- AP:
-
Augmented pressure
- APP:
-
Aortic pulse pressure
- ASBP:
-
Aortic systolic blood pressure
- BDBP:
-
Brachial diastolic blood pressure
- BMAP:
-
Brachial mean arterial pressure
- BMI:
-
Body mass index
- BP:
-
Blood pressure
- BPP:
-
Brachial pulse pressure
- BSBP:
-
Brachial systolic blood pressure
- HR:
-
Heart rate
- IHG:
-
Isometric handgrip
- LV:
-
Left ventricular
- MVC:
-
Maximal voluntary contraction
- P2:
-
Second systolic peak pressure
- P1:
-
First systolic peak pressure
- PP:
-
Pulse pressure
- PWV:
-
Pulse wave velocity
- REC:
-
Post-exercise recovery
- REST:
-
Pre-exercise baseline
- RPP:
-
Rate pressure product
- SBP:
-
Systolic blood pressure
- Tr :
-
Transit time of the reflected wave
References
Adamopoulos D, Vyssoulis G, Karpanou E, Kyvelou SM, Argacha JF, Cokkinos D, Stefanadis C, van de Borne P (2010) Environmental determinants of blood pressure, arterial stiffness, and central hemodynamics. J Hypertens 28(5):903–909
Analitis A, Katsouyanni K, Biggeri A, Baccini M, Forsberg B, Bisanti L, Kirchmayer U, Ballester F, Cadum E, Goodman PG, Hojs A, Sunyer J, Tiittanen P, Michelozzi P (2008) Effects of cold weather on mortality: results from 15 European cities within the PHEWE project. Am J Epidemiol 168(12):1397–1408
Bokenes L, Alexandersen TE, Tveita T, Osterud B, Mercer JB (2004) Physiological and hematological responses to cold exposure in young subjects. Int J Circumpolar Health 63(2):115–128
Casey DP, Braith RW, Pierce GL (2008a) Changes in central artery blood pressure and wave reflection during a cold pressor test in young adults. Eur J Appl Physiol 103(5):539–543
Casey DP, Nichols WW, Braith RW (2008b) Impact of aging on central pressure wave reflection characteristics during exercise. Am J Hypertens 21(4):419–424
Chen CH, Nevo E, Fetics B, Pak PH, Yin FC, Maughan WL, Kass DA (1997) Estimation of central aortic pressure waveform by mathematical transformation of radial tonometry pressure. validation of generalized transfer function. Circulation 95(7):1827–1836
Cheng X, Su H (2010) Effects of climatic temperature stress on cardiovascular diseases. Eur J Intern Med 21(3):164–167
Edwards DG, Gauthier AL, Hayman MA, Lang JT, Kenefick RW (2006) Acute effects of cold exposure on central aortic wave reflection. J Appl Physiol 100(4):1210–1214
Edwards DG, Mastin CR, Kenefick RW (2008a) Wave reflection and central aortic pressure are increased in response to static and dynamic muscle contraction at comparable workloads. J Appl Physiol 104(2):439–445
Edwards DG, Roy MS, Prasad RY (2008b) Wave reflection augments central systolic and pulse pressures during facial cooling. Am J Physiol Heart Circ Physiol 294(6):H2535–H2539
Figueroa A, Hooshmand S, Figueroa M, Bada AM (2010a) Cardiovagal baroreflex and aortic hemodynamic responses to isometric exercise and post-exercise muscle ischemia in resistance trained men. Scand J Med Sci Sports 20(2):305–309
Figueroa A, Trivino JA, Sanchez-Gonzalez MA, Vicil F (2010b) Oral l-citrulline supplementation attenuates blood pressure response to cold pressor test in young men. Am J Hypertens 23(1):12–16
Gallagher D, Adji A, O’Rourke MF (2004) Validation of the transfer function technique for generating central from peripheral upper limb pressure waveform. Am J Hypertens 17(11 Pt 1):1059–1067
Geleris P, Stavrati A, Boudoulas H (2004) Effect of cold, isometric exercise, and combination of both on aortic pulse in healthy subjects. Am J Cardiol 93(2):265–267
Hashimoto J, Nichols WW, O’Rourke MF, Imai Y (2008) Association between wasted pressure effort and left ventricular hypertrophy in hypertension: influence of arterial wave reflection. Am J Hypertens 21(3):329–333
Hess KL, Wilson TE, Sauder CL, Gao Z, Ray CA, Monahan KD (2009) Aging affects the cardiovascular responses to cold stress in humans. J Appl Physiol 107(4):1076–1082
Hong YC, Rha JH, Lee JT, Ha EH, Kwon HJ, Kim H (2003) Ischemic stroke associated with decrease in temperature. Epidemiology 14(4):473–478
Kahn JF, Piton A, Lepage S, Brunet A, Lagha A, Monod H (1993) Cardiovascular changes during an isometric contraction combined to a cold pressor test. Acta Physiol Scand 149(1):7–13
Kienecker EW, Knoche H (1978) Sympathetic innervation of the pulmonary artery, ascending aorta, and coronar glomera of the rabbit. a fluorescence microscopic study. Cell Tissue Res 188(2):329–333
Kim JY, Jung KY, Hong YS, Kim JI, Jang TW, Kim JM (2003) The relationship between cold exposure and hypertension. J Occup Health 45(5):300–306
King SG, Ahuja KD, Wass J, Shing CM, Adams MJ, Davies JE, Sharman JE, Williams AD (2013) Effect of whole-body mild-cold exposure on arterial stiffness and central haemodynamics: a randomised, cross-over trial in healthy men and women. Eur J Appl Physiol 113(5):1257–1269
Korhonen I (2006) Blood pressure and heart rate responses in men exposed to arm and leg cold pressor tests and whole-body cold exposure. Int J Circumpolar Health 65(2):178–184
Lydakis C, Momen A, Blaha C, Gugoff S, Gray K, Herr M, Leuenberger UA, Sinoway LI (2008) Changes of central haemodynamic parameters during mental stress and acute bouts of static and dynamic exercise. J Hum Hypertens 22(5):320–328
Makinen TM, Mantysaari M, Paakkonen T, Jokelainen J, Palinkas LA, Hassi J, Leppaluoto J, Tahvanainen K, Rintamaki H (2008) Autonomic nervous function during whole-body cold exposure before and after cold acclimation. Aviat Space Environ Med 79(9):875–882
Mercer JB, Osterud B, Tveita T (1999) The effect of short-term cold exposure on risk factors for cardiovascular disease. Thromb Res 95(2):93–104
Muller MD, Gao Z, Drew RC, Herr MD, Leuenberger UA, Sinoway LI (2011) Effect of cold air inhalation and isometric exercise on coronary blood flow and myocardial function in humans. J Appl Physiol 111(6):1694–1702
Nichols WW (2005) Clinical measurement of arterial stiffness obtained from noninvasive pressure waveforms. Am J Hypertens 18(1 Pt 2):3S–10S
Nichols WW, O’Rourke MF (1998) McDonald’s blood flow in arteries. theoretical, experimental and clinical principles. Arnold, London
O’Rourke MF, Adji A (2005) An updated clinical primer on large artery mechanics: implications of pulse waveform analysis and arterial tonometry. Curr Opin Cardiol 20(4):275–281
Padilla J, Young CN, Simmons GH, Deo SH, Newcomer SC, Sullivan JP, Laughlin MH, Fadel PJ (2010) Increased muscle sympathetic nerve activity acutely alters conduit artery shear rate patterns. Am J Physiol Heart Circ Physiol 298(4):H1128–H1135
Peikert D, Smolander J (1991) The combined effect of the cold pressor test and isometric exercise on heart rate and blood pressure. Eur J Appl Physiol Occup Physiol 62(6):445–449
Reinhard H, Jacobsen PK, Lajer M, Pedersen N, Billestrup N, Mandrup-Poulsen T, Parving HH, Rossing P (2010) Multifactorial treatment increases endothelial progenitor cells in patients with type 2 diabetes. Diabetologia 53(10):2129–2133
Rocklov J, Forsberg B (2008) The effect of temperature on mortality in Stockholm 1998–2003: a study of lag structures and heatwave effects. Scand J Public Health 36(5):516–523
Roman MJ, Devereux RB, Kizer JR, Lee ET, Galloway JM, Ali T, Umans JG, Howard BV (2007) Central pressure more strongly relates to vascular disease and outcome than does brachial pressure: the strong heart study. Hypertension 50(1):197–203
Roman MJ, Devereux RB, Kizer JR, Okin PM, Lee ET, Wang W, Umans JG, Calhoun D, Howard BV (2009) High central pulse pressure is independently associated with adverse cardiovascular outcome the strong heart study. J Am Coll Cardiol 54(18):1730–1734
Safar ME, Blacher J, Protogerou A, Achimastos A (2008) Arterial stiffness and central hemodynamics in treated hypertensive subjects according to brachial blood pressure classification. J Hypertens 26(1):130–137
Sanchez-Gonzalez MA, Figueroa A (2013) Cold exposure attenuates post exercise cardiovagal reactivation and sympathetic withdrawal. Auton Neurosci 176(1–2):95–97
Takazawa K, Tanaka N, Takeda K, Kurosu F, Ibukiyama C (1995) Underestimation of vasodilator effects of nitroglycerin by upper limb blood pressure. Hypertension 26(3):520–523
Vlachopoulos C, Aznaouridis K, O’Rourke MF, Safar ME, Baou K, Stefanadis C (2010) Prediction of cardiovascular events and all-cause mortality with central haemodynamics: a systematic review and meta-analysis. Eur Heart J 31(15):1865–1871
Wilkinson IB, Mohammad NH, Tyrrell S, Hall IR, Webb DJ, Paul VE, Levy T, Cockcroft JR (2002) Heart rate dependency of pulse pressure amplification and arterial stiffness. Am J Hypertens 15(1 Pt 1):24–30
Wolf K, Schneider A, Breitner S, von Klot S, Meisinger C, Cyrys J, Hymer H, Wichmann HE, Peters A (2009) Air temperature and the occurrence of myocardial infarction in Augsburg, Germany. Circulation 120(9):735–742
Woodman RJ, Playford DA, Watts GF (2006) Basal production of nitric oxide (NO) and non-NO vasodilators in the forearm microcirculation in type 2 diabetes: associations with blood pressure and HDL cholesterol. Diabetes Res Clin Pract 71(1):59–67
Acknowledgments
We would like to express our gratitude to the participants.
Conflict of interest
The authors declare no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by George Havenith.
Rights and permissions
About this article
Cite this article
Koutnik, A.P., Figueroa, A., Wong, A. et al. Impact of acute whole-body cold exposure with concurrent isometric handgrip exercise on aortic pressure waveform characteristics. Eur J Appl Physiol 114, 1779–1787 (2014). https://doi.org/10.1007/s00421-014-2897-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00421-014-2897-7