Abstract
Introduction
Arterial dysfunction develops early in life even in individuals with modest cardiovascular risk. Stress is associated with increased risk in cardiovascular morbidity and mortality.
Aim
The main objectives of this study were to investigate the acute effects of moderate continuous training (MCT) on established markers of arterial stiffness and cardiovascular risk during standardized cold pressor stress testing (CPT).
Methods
29 young healthy male subjects (33.7 ± 8 years, BMI 24 ± 2 kg/m2) performed a 60-min period of moderate upright bicycle exercise with 65% of maximum heart rate. Before (t0), 45 (t45) as well as 60 (t60) min after exercise peripheral pulse pressure (PP) as well as augmentation index at a set heart rate (AIx@75) were assessed non-invasively at rest using an oscillometric device. Immediately after t0 and t60 PP and AIx@75 were registered at the end of a 2 min CPT.
Results
PP (p = 0.005) and AIx@75 (p = 0.04) were reduced below pre-exercise level at t60. In contrast to CPT before exercise, there were significant reductions in PP (p = 0.039) as well as AIx@75 (p = 0.002) during CPT after exercise. Additionally, there was a negative correlation between maximal oxygen consumption and AIx@75 (r = −0.42, p = 0.044).
Conclusions
Acute MCT decreased PP and reduces AIx@75 after 60 min of recovery. Furthermore, PP and AIx@75 showed reduced values after completion of MCT indicating attenuated hemodynamic response to stress testing after MCT. Moreover, higher physical conditioning status was associated with more favorable effects on stress test-related arterial compliance.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Cardiovascular diseases (CVD) have become a significant and growing health care problem [1, 2]. In this context it has been shown that arterial dysfunction develops early in life even in individuals with modest cardiovascular risk [3]. Therefore, lifestyle modifications might be one of the important targets to prevent future cardiovascular morbidity [4]. Convincing evidence demonstrates that regular aerobic exercise results in multiple health benefits [5–7]. The vascular effects of exercise interventions can be quantified by the use of vascular biomarkers.
Pulse pressure (PP), a simple correlate of conduit vessel stiffness, is associated with left ventricular hypertrophy. Increased PP has also been implicated in the development and progression of large-vessel atherosclerosis and small-vessel disease. Accumulating evidence indicates that PP (difference between systolic and diastolic blood pressure) may be an important predictor of future cardiovascular events [8, 9].
Augmentation index (AIx), a parameter analyzing the augmentation of systolic blood pressure by reflection of the peripheral pulse wave, is a validated parameter of arterial stiffness. It predicts cardiovascular events in the general population and in patients with cardiovascular diseases as well [10, 11]. A reduced augmentation index displays a lesser contribution of the reflected wave on the central systolic blood pressure, indicating reduced arterial stiffness and cardiac afterload as well as a favorably altered central blood pressure [12].
Regular moderate continuous exercise has been reported to reduce peripheral and central blood pressure as well as to improve arterial compliance in young normotensive and prehypertensive adults [13]. The effect of an acute bout of endurance exercise on PP and central systolic wave augmentation is less clear and is limited to measurements during subsequent resting periods. However, chronic as well as acute stress is an inherent element of everyday life and recent investigations underline the importance of vascular function during stress, which may predict future cardiovascular morbidity and mortality [14–16].
Therefore, a study was designed to investigate the acute hemodynamic effects of a single bout of moderate continuous exercise on PP and central pressure augmentation up to 60 min of recovery, but moreover during subsequent stress testing.
2 Methods
2.1 Setting and Participants
Volunteers were prospectively recruited from the local community. Inclusion criteria were an age between 18–50 years and male gender. Exclusion criteria (chronic cardiovascular, metabolic or pulmonary disease, current smoking, body mass index ≥30 kg m−2, resting blood pressure >140/90 mmHg and orthopaedic problems) were assessed using a medical history questionnaire [17]. Medical examination included a resting electrocardiogram (ECG). Females were excluded due to possible modification in wave reflection during the menstrual cycle [18]. The study was performed according to the Guidelines for Good Clinical Practice and in agreement with the Helsinki Declaration on the use of human subjects for research. Participation was voluntary and written informed consent was obtained from all subjects after they were well briefed about the nature and purpose of this study.
2.2 Study Design
Examination of participants took place at two visits, which were separated by at least 48 h and within a maximum of 10 days. Both sessions took place at the same time of day. During the first visit, medical history was assessed and baseline measurements of anthropometrics were recorded and calculated as per established guidelines by the same operator in each subject. Measurements were completed with participants wearing light-weight clothing using a stadiometer and physician’s balance scale. Additionally, each subject completed a continuous incremental to maximum cardiopulmonary exercise test (CPET) with direct gas analysis (MetaLyzer 3B-R2 Cortex) on an electronically braked bicycle ergometer (Customed Ergo Control 3000) to assess maximum aerobic capacity. Heart rate was monitored continuously with a twelve-lead electrocardiogram (Custo Cardio 200; Customed, Munich, Germany). To ensure that each participant attained a valid maximum aerobic capacity at least two of the following criteria had to be met by each subject: (1) plateau in oxygen uptake with increasing exercise intensity, (2) respiratory exchange ratio >1.1, (3) achievement of age-predicted maximum heart rate.
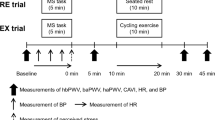
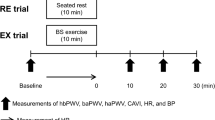
At the second visit, PP and the heart rate-corrected augmentation index (AIx@75) were registered simultaneously after 10 min of seated rest (t0), whereby the average of two recordings was documented. Thereafter, a Cold Pressor Test (CPT) was performed by immersing the left hand up to the wrist into an ice-cold water container with 6 °C for 2 min. Participants’ hemodynamics were registered at the end of the 2nd minute. After cold pressor stress testing subjects performed a supervised 60-min period of upright bicycle exercise with 65% of maximum heart rate corresponding to 45% of maximal oxygen consumption (VO2max), which were determined during previous CPET. After exercise performance, PP and AIx@75 were obtained again 45 (t45) and 60 (t60) min post-exercise and thereafter during a second CPT.
Participants were tested in the postprandial state (2 h) and were instructed to refrain from caffeine and alcoholic beverages for 12 h and avoid exercise for 24-h prior to testing. A quiet test environment was ensured with a consistent room temperature at 23±1 °C. Body weight was measured before and after exercise to control for possible volume changes due to fluid intake.
2.3 Hemodynamic Measurements
All hemodynamic measurements were obtained using an oscillometric Mobil-O-Graph® 24-h PWA Monitor (I.E.M GmbH, Germany) as a clinically validated device for hemodynamic measurements [19] with a novel transfer function-like algorithm, using brachial cuff-based waveform recordings. During hemodynamic measurements the right arm was extended and placed on a customized arm-support. The augmentation index from the central pulse is calculated as: AIx = 100 × (P2 − P1)/pulse pressure, where P2 is the peak of the reflected backward wave, P1 is the peak of the forward pressure wave and pulse pressure is the systolic pressure maximum minus the diastolic pressure. A positive AIx indicates an augmentation of peak systolic pressure by the reflected wave.
2.4 Statistical Analysis
Statistical analysis was performed using IBM SPSS Statistics 20.0. All data were inspected statistically for normality and are reported as means ± standard deviation (95% CI). For testing comparisons between baseline (t0) and different post-exercise time points repeated measurement ANOVAs were performed. Statistical inference was based on the F-test. Bonferroni post-hoc analyses were conducted to examine pair-wise mean differences. Paired Student’s t tests were used to compare pre- and post-exercise stress test-related values as well as subjects’ body weights. Statistical testing about the relationship between maximal oxygen consumption and AIx@75 measured during CPT before exercise was performed by applying partial correlation analysis adjusted for age, body mass index and height, because these parameters are known to influence wave reflection [20]. Standard Pearson correlation coefficients were determined and tested for statistical significance. Statistical significance was a probability value of less than 0.05 on two-sided tests. Prospective calculations of power were performed according to the Altman [21] method.
3 Results
In total, 29 healthy men (25–50 years) were recruited from the local community. On the basis of the medical history and examination as well as the ECG, none of the participants had increased cardiovascular risk. During the period of data collection (between first and last examination), subjects reported no major changes in their physical activity or dietary habits. There were no significant differences in body weights before and after exercise (weight loss 0.37 ± 0.39 kg, p > 0.05). Subjects’ characteristics including the endurance capacity according to the maximal oxygen consumption are presented in Table 1.
Regarding measurements in the time course of recovery (Table 2), PP showed reductions below pre-exercise level at t45 (47–43 mmHg, 95% CI 0.11–7.55, p = 0.042) and t60 (47–42 mmHg, 95% CI 1.37–8.74, p = 0.005). Compared to PP response and as shown in Fig. 1 AIx@75 declined also during recovery from moderate continuous training (MCT), but reached a significantly lower value compared to baseline only at t60 (7.3–4.2%, 95% CI 0.11–6.11, p = 0.040).
In contrast to CPT before exercise, there were significant reductions in PP (48±11–43±10 mmHg, p = 0.039) as well as AIx@75 (10.0±9–6.0±9%, p = 0.002) during CPT after exercise (Figs. 2, 3). Additionally, there was a negative correlation (r = −0.42, p = 0.044) between maximal oxygen consumption and AIx@75 during CPT before exercise (Fig. 4).
4 Discussion
To our knowledge, this is the first study investigating the effects of an acute bout of aerobic exercise on pulse pressure and wave reflection during recovery and moreover during subsequent stress testing. The salient findings are as follows: first, an acute 60-min bout of moderate aerobic exercise has a favorable effect on pulse pressure and wave reflection even after 60 min of recovery. Second, even 60 min after exercise, pulse pressure and augmentation during cold stress testing were reduced. Third, there was a negative correlation between maximal oxygen consumption and central wave reflection during stress testing.
Only few studies have investigated the acute physiologic effects of aerobic exercise on pulse pressure and central wave reflection during subsequent recovery [12, 22, 23]. Our results of a decreased PP after moderate continuous exercise are in line with previous findings by Sugawara et al. [23] and Hanssen et al. [12]. In their studies, peripheral PP remained significantly decreased up to 50 min of recovery. Thus, from our study and previous data it can be concluded that peripheral PP is reduced during immediate recovery from aerobic exercise.
Similar to our findings concerning Alx@75 after acute exercise, Dischl et al. [22] found a reduction of Alx@75 45 min following submaximal and maximal aerobic exercise. After prolonged exhaustive exercise such as a marathon run, AIx@75 has also been reported to be decreased 60 min after the marathon [24]. However, since variation of exercise mode and intensity affects the cardiovascular system differently in chronic exercise programs [3, 25], our results probably cannot be directly compared to those of previous groups. This may explain the contradictory results to Hanssen et al. [12], where AIx@75 was significantly increased immediately post-exercise with a gradual decline near baseline levels 50 min following exercise. This may suggests that heart rate corrected AIx@75 can be positively affected due to a previous exercise bout, but with delayed response [12].
It is well accepted that stress contributes to elevated blood pressure and to the development of hypertension and arterial stiffness. However, to our knowledge, this is the first study to quantify the changes in wave reflection response during post-exercise stress testing.
Therefore, the current study demonstrate that a single bout of moderate continuous exercise has the potential to attenuate PP and central wave reflection response to subsequent stress testing, whereby these findings extend those of previous reports relating to hemodynamic response to aerobic exercise. Furthermore, this study provides evidence for an inverse relationship between aerobic capacity, indexed by maximal oxygen consumption and stress test-related Alx@75 measured before exercise. Therefore, higher physical conditioning status seem to be associated with more favorable effects on arterial compliance during stress testing. Since CPT is known as a classic test of sympathetic activity, causing arteriolar vasoconstriction and thus resulting in an increase in BP [26, 27] these results can be interpreted as an attenuated global sympathetic response to hemodynamic stress, which is evident even 1 h after MCT. Considering recent results from Zhao et al. [14], where BP reactivity to a CPT has been shown to be significantly associated with hypertension incidence, moderate continuous training, an established suggestion to lower BP in both hypertensive and normotensive individuals [28], probably gains additional importance.
Moreover, since even slight BP reductions lead to significant decreases in cardiovascular risk [29], it is likely that the PP reduction in the present study is already epidemiologically and physiologically relevant. It also has been shown that a decrease in AIx, an increasingly powerful independent predictor of cardiovascular morbidity and mortality and significant reclassifier of cardiovascular risk [10], is associated with decreased cardiac afterload and left ventricular burden. An increase in AIx by 10% has previously been associated with a 31.8% increased risk for an adverse cardiovascular outcome [30]. Repeated exposure to exercise and, therefore, recurring periods of reduced wave reflection may cumulate and thus may have beneficial effects on hemodynamics and long-term arterial stiffness during stress.
The underlying mechanisms for the exercise-induced reduction in PP and pressure wave augmentation in the immediate recovery are complex. Previous studies have shown that aerobic exercise achieves this goal by an increase in blood flow and arterial vasodilatation [31]. Therefore, there has been evidence to suggest that shear stress-induced release of nitric oxide seems to be a key mechanism underlying the post-exercise reduction of pressure augmentation [32].
Acute exercise increases heart rate (HR), which is known to be inversely related to AIx [33]. In the current study Alx was calculated for a set HR of 75 beats per minute, which indicates that factors other than HR contribute to the decreased wave reflection during recovery from moderate continuous exercise. Furthermore, it is unlikely that the hemodynamic changes observed in this study were attributable to a weight reduction, because there was no significant change in body weight after exercise when compared to pre-exercise. However, several limitations of our study should be considered. First, the sample was not a random sample, as the participants were recruited from the surrounding area. Inclusion criteria in this study were purposely restrictive to eliminate or limit strong confounding effects such as gender, age or medication. Therefore, they limit the generalization of the findings to the studied population since all were healthy men.
5 Conclusions
In conclusion, the results of the study highlight that in healthy men an acute 60-min bout of moderate aerobic exercise as classified by the American College of Sports Medicine [34] has a favorable effect on pulse pressure and arterial wave reflection at rest, thereby reducing arterial stiffness and, eventually, myocardial burden. Furthermore, MCT is able to induce attenuated hemodynamic response to stress testing even 60 min after exercise. Moreover, a higher physical conditioning status seems to be associated with more favorable effects on stress test-related arterial compliance. Future research should address if regular MCT prolong the duration of these favorable effects on arterial compliance for more than 60 min and whether it can induce clinically relevant improvements of central and peripheral arterial properties in individuals of other age groups and in subjects with augmented arterial stiffness.
References
Heidenreich PA, Trogdon JG, Khavjou OA, Butler J, Dracup K, Ezekowitz MD, Finkelstein EA, Hong Y, Johnston SC, Khera A, Lloyd-Jones DM, Nelson SA, Nichol G, Orenstein D, Wilson PW, Woo YJ. Forecasting the future of cardiovascular disease in the United States: a policy statement from the American Heart Association. Circulation. 2011;123(8):933–44.
World Health Organization (WHO). Global status report on noncommunicable diseases 2010. Description of the global burden of NCDs, their risk factors and determinants.
Perk J, De Backer G, Gohlke H, Graham I, Reiner Z, Verschuren M, Albus C, Benlian P, Boysen G, Cifkova R, Deaton C, Ebrahim S, Fisher M, Germano G, Hobbs R, Hoes A, Karadeniz S, Mezzani A, Prescott E, Ryden L, Scherer M, Syvänne M, Scholte op Reimer WJ, Vrints C, Wood D, Zamorano JL, Zannad F. European guidelines on cardiovascular disease prevention in clinical practice (version 2012). The fifth joint task force of the European society of cardiology and other societies on cardiovascular disease prevention in clinical practice. Eur Heart J. 2012;2012(33):1635–701.
Lakatta EG, Levy D. Arterial and cardiac aging. Major shareholders in cardiovascular disease enterprises: Part I: aging arteries: a “set up” for vascular disease. Circulation. 2003;107:139–46.
Blair SN, Kohl HW 3rd, Paffenbarger RS Jr, Clark DG, Cooper KH, Gibbons LW. Physical fitness and all-cause mortality. A prospective study of healthy men and women. JAMA. 1989;262:2395–401.
Ketelhut RG, Franz IW, Scholze J. Regular exercise as an effective longterm approach in antihypertensive therapy. Med Sci Sports Exerc. 2004;1:4–8.
Cameron JD, Dart AM. Exercise training increases total systemic arterial compliance in humans. Am J Physiol. 1994;266:H693–701.
Benetos A, Safar M, Rudnichi A, Smulyan H, Richard JL, Ducimetieère P, Guize L. Pulse pressure: a predictor of long-term cardiovascular mortality in a French male population. Hypertension. 1997;30:1410–5.
Lee JW. Pulse pressure and systolic blood pressure. Korean Circ J. 2002;32:293–8.
Chirinos JA, Kips JG, Jacobs DR Jr, Brumback L, Duprez DA, Kronmal R, Bluemke DA, Townsend RR, Vermeersch S, Segers P. Arterial wave reflections and incident cardiovascular events and heart failure: MESA (Multiethnic Study of Atherosclerosis). J Am Coll Cardiol. 2012;60(21):2170–7.
Vlachopoulos C, Aznaouridis K, O’Rourke MF, Safar ME, Baou K, Stefanadis C. Prediction of cardiovascular events and all-cause mortality with central haemodynamics: a systematic review and meta-analysis. Eur Heart J. 2010;31(15):1865–71.
Hanssen H, Nussbaumer M, Moor C, Cordes M, Schindler C, Schmidt-Trucksass A. Acute effects of interval versus continuous endurance training on pulse wave reflection in healthy young men. Atherosclerosis. 2015;238(2):399–406.
Beck DT, Martin JS, Casey DP, Braith RW. Exercise training reduces peripheral arterial stiffness and myocardial oxygen demand in young prehypertensive subjects. Am J Hypertens. 2013;26(9):1093–102.
Zhao Q, Gu D, Lu F, Mu J, Wang X, Ji X, Hu D, Ma J, Huang J, Li J, Chen J, Cao J, Chen CS, Chen J, Rice TK, He J. Blood pressure reactivity to the cold pressor test predicts hypertension among chinese adults: the GenSalt Study. Am J Hypertens. 2015 (Epub ahead of print).
Vlachopoulos C, Kosmopoulou F, Alexopoulos N, Ioakeimidis N, Siasos G, Stefanadis C. Acute mental stress has a prolonged unfavorable effect on arterial stiffness and wave reflections. Psychosom Med. 2006;68:231–7.
Nomura K, Nakao M, Karita K, Nishikitani M, Yano E. Association between work-related psychological stress and arterial stiffness measured by brachial-ankle pulse-wave velocity in young Japanese males from an information service company. Scand J Work Environ Health. 2005;31:352–9.
Armstrong L, Balady GJ, Berry MJ, Davis SE, Davy BM, Davy KP, Franklin BA, Gordon NF, I-m L, McConnell T, Myers JN, Pizza FX, Rowland TW, Stewart K, Thompson PD, Wallace JP. ACSM’s guidelines for exercise testing and prescription. 7th ed. Philadelphia: Lippincott Williams & Wilkins; 2006.
Robb AO, Mills NL, Din JN, Smith IBJ, Paterson F, Newby DE, Denison FC. Influence of the menstrual cycle, pregnancy, and preeclampsia on arterial stiffness. Hypertension. 2009;53(6):952–8.
Papaioannou TG, Argyris A, Protogerou AD, Vrachatis D, Nasothimiou EG, Sfikakis PP, Stergiou GS, Stefanadis CI. Non-invasive 24 hour ambulatory monitoring of aortic wave reflection and arterial stiffness by a novel oscillometric device: the first feasibility and reproducibility study. Int J Cardiol. 2013;169(1):57–61.
Janner JH, Godtfredsen NS, Ladelund S, Vestbo J, Prescott E. Aortic augmentation index: reference values in a large unselected population by means of the SphygmoCor device. Am J Hypertens. 2010;23:180–5.
Altman DG. Statistics and ethics in medical research: III. How large a sample? Br Med J. 1980;281:1336–8.
Dischl B, Engelberger RP, Gojanovic B, Liaudet L, Gremion G, Waeber B, Feihl F. Enhanced diastolic reflections on arterial pressure pulse during exercise recovery. Scand J Med Sci Sports. 2011;21(6):325–33.
Sugawara J, Komine H, Miyazawa T, Imai T, Ogoh S. Influence of single bout of aerobic exercise on aortic pulse pressure. Eur J Appl Physiol. 2015;115:739–46.
Pressler A, Hanssen H, Dimitrova M, Krumm M, Halle M, Scherr J. Acute and chronic effects of marathon running on the retinal microcirculation. Atherosclerosis. 2011;219:864–8.
Ciolac EG. High-intensity interval training and hypertension: maximizing the benefits of exercise? Am J Cardiovasc Dis. 2012;2(2):102–10.
Benetos A, Safar ME. Response to the cold pressor test in normotensive and hypertensive patients. Am J Hypertens. 1991;4:627–9.
Victor RG, Leimbach WN, Seals DR, Wallin BG, Mark AL. Effects of the cold pressor test on muscle sympathetic nerve activity in humans. Hypertension. 1987;9:429–36.
Mancia G, Fagard R, Narkiewicz K, Redon J, Zanchetti A, Böhm M, Christiaens T, Cifkova R, De Backer G, Dominiczak A, Galderisi M, Grobbee DE, Jaarsma T, Kirchof P, Kjeldsen SE, Laurent S, Manolis AJ, Nilsson PM, Ruilope LM, Schmieder RE, Sirnes PA, Sleight P, Viigimaa M, Waeber B, Zannad F. 2013 ESH/ESC guidelines for the management of arterial hypertension: the Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). Eur Heart J. 2013;34:2159–219.
Cook NR, Cohen J, Hebert PR, Taylor JO, Hennekens CH. Implications of small reductions in diastolic blood pressure for primary prevention. Arch Intern Med. 1995;155:701–9.
Vlachopoulos C, Aznaouridis K, Stefanadis C. Prediction of cardiovascular events and all-cause mortality with arterial stiffness: a systematic review and meta-analysis. J Am Coll Cardiol. 2010;55(13):1318–27.
Munir S, Jiang B, Guilcher A, Brett S, Redwood S, Marber M, Chowienczyk P. Exercise reduces arterial pressure augmentation through vasodilation of muscular arteries in humans. Am J Physiol Heart Circ Physiol. 2008;294(4):H1645–50.
Halliwill JR, Buck TM, Lacewell AN, Romero SA. Postexercise hypotension and sustained postexercise vasodilatation: what happens after we exercise? Exp Physiol. 2013;98(1):7–18.
Wilkinson IB, MacCallum H, Flint L, Cockcroft JR, Newby DE, Webb DJ. The influence of heart rate on augmentation index and central arterial pressure in humans. J Physiol. 2000;525(1):263–70.
Garber CE, Blissmer B, Deschenes MR, Franklin BA, Lamonte MJ, Lee IM, Nieman DC, Swain DP. American College of Sports Medicine position stand. Quantity and quality of exercise for developing and maintaining cardiorespiratory, musculoskeletal, and neuromotor fitness in apparently healthy adults: guidance for prescribing exercise. Med Sci Sports Exerc. 2011;43:1334–59.
Acknowledgements
We would like to thank the participants without whom this study would not have been possible. This work has been funded by Takeda Pharma Company, Berlin, Germany, who provided the monitoring device.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Rights and permissions
About this article
Cite this article
Milatz, F., Ketelhut, S. & Ketelhut, R.G. Acute Effects of Moderate Continuous Training on Stress Test-Related Pulse Pressure and Wave Reflection in Healthy Men. High Blood Press Cardiovasc Prev 24, 61–67 (2017). https://doi.org/10.1007/s40292-017-0180-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40292-017-0180-9