Abstract
Intense exercise is directly related to muscular damage and oxidative stress due to excessive reactive oxygen species (ROS) in both, plasma and white blood cells. Nevertheless, exercise-derived ROS are essential to regulate cellular adaptation to exercise. Studies on antioxidant supplements have provided controversial results. The purpose of this study was to determine the effect of moderate antioxidant supplementation (lemon verbena extract) in healthy male volunteers that followed a 90-min running eccentric exercise protocol for 21 days. Antioxidant enzymes activities and oxidative stress markers were measured in neutrophils. Besides, inflammatory cytokines and muscular damage were determined in whole blood and serum samples, respectively. Intense running exercise for 21 days induced antioxidant response in neutrophils of trained male through the increase of the antioxidant enzymes catalase, glutathione peroxidase and glutathione reductase. Supplementation with moderate levels of an antioxidant lemon verbena extract did not block this cellular adaptive response and also reduced exercise-induced oxidative damage of proteins and lipids in neutrophils and decreased myeloperoxidase activity. Moreover, lemon verbena supplementation maintained or decreased the level of serum transaminases activity indicating a protection of muscular tissue. Exercise induced a decrease of interleukin-6 and interleukin-1β levels after 21 days measured in basal conditions, which was not inhibited by antioxidant supplementation. Therefore, moderate antioxidant supplementation with lemon verbena extract protects neutrophils against oxidative damage, decreases the signs of muscular damage in chronic running exercise without blocking the cellular adaptation to exercise.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Exercise can have both positive and negative effects on inflammatory and redox status. While moderate activity may enhance immune function over sedentary levels, excessive, prolonged and high-intensity exercise may impair immune function (Gleeson 2007). Intense physical activity increases oxygen consumption and the formation of reactive oxygen species (ROS) (Ji 1995) and induces an acute phase immune response similar to infection. Oxidative stress associated with exhaustive exercise may also impair the immune response increasing athletes’ susceptibility to upper respiratory tract infections (Nieman 1994). Exercise-related immunological acute changes include signs of inflammation such as release of cytokines (Petersen et al. 2001), activation of immunocompetent cell lines and neutrophil priming for acute phase response (Suzuki et al. 1999). In addition, higher oxidized glutathione levels and lower antioxidant enzymes levels in neutrophils (Tauler et al. 2002a), and the induction of acute phase proteins such as elastase or myeloperoxidase (MPO) are related to exhaustive exercise (Yamada et al. 2000; Morozov et al. 2003).
It is well accepted that health benefits of exercise are enhanced by positive dietary modification. The influence of the consumption of antioxidant supplements in health and disease, as well as athletic performance and adaptation to training and their impact on inflammation and oxidative stress has brought up much attention (Pedersen and Hoffman-Goetz 2000). However, attention has more recently shifted toward the specific interaction between antioxidant nutrients, redox-sensitive signaling pathways and inflammatory responses to exercise, e.g. it has been demonstrated that endogenous antioxidant enzymes and dietary antioxidant supplements can potentially attenuate cytokine production after exercise (Vassilakopoulos et al. 2003; Nieman et al. 2007).
Although there is large evidence on the positive effects of the supplementation containing antioxidant nutrients associated with exercise (Tauler et al. 2002b, 2008; Fischer et al. 2004; Sureda et al. 2004a; Su et al. 2008), some studies using high doses of antioxidant vitamins have shown that antioxidant supplementation may counteract the up-regulation of endogenous antioxidant capability in response to exercise (Gómez-Cabrera et al. 2008; Ristow et al. 2009). While some supplements may just demonstrate chemical antioxidants properties, which may indeed counteract the up-regulation of beneficial adaptive events (i.e. endogenous antioxidant capability) within a cell and therefore have a detrimental action, some natural antioxidants utilized at adequate doses may actually complement the action of exercise.
Therefore, there is no consensus regarding the need of antioxidant supplementation for athletes who have a balanced diet. On the other hand, physical training associated to a low intake of antioxidant nutrients may represent a period of greater vulnerability to oxidative stress. Thus, the intake of a diet enriched in antioxidants at moderate level is still the most cautious recommendation to minimize the deleterious actions of free radicals resulting from exercise (Urso and Clarkson 2003). According to this hypothesis, several studies have reported that supplementation with dietary polyphenols diminishes the exercise-induced oxidative stress and decreased inflammatory markers (McAnulty et al. 2004; Morillas-Ruiz et al. 2005, 2006; Nieman et al. 2007; Panza et al. 2008).
Lemon verbena (Aloysia triphylla, Lippia citriodora) is a widely used herb for food and medicinal purposes (Newall et al. 1996). Flavones, iridoids and phenylpropanoids represent the main class of compounds of this plant, being verbascoside the most abundant one (Funes et al. 2009; Quirantes-Piné et al. 2009). The strong antioxidant properties of this compound have been studied in detail (Valentao et al. 2002; Liu et al. 2003; Funes et al. 2009). In addition, this compound has also shown anti-inflammatory activity through different in vitro assays and animal models (Díaz et al. 2004; Lee et al. 2005; Lin et al. 2006; Hausmann et al. 2007; Korkina et al. 2007).
The aim of this study was to determine the influence of moderate antioxidant supplementation (lemon verbena extract containing 10% verbascoside, w/w) on neutrophils’ antioxidant response, muscular damage markers and cytokines’ profile of healthy male volunteers subjected to intense training sessions of 90-min running exercise protocol. The effects of exercise and supplement on immune cells adaptive response to exercise and activation of antioxidant defence systems were also studied.
Materials and methods
HPLC analysis of lemon verbena extract
Lemon verbena extract solution of 1 mg/ml was prepared in water:methanol 1:1, centrifuged and filtered through 0.45 μm polycarbonate filters. A sample of 10 μl was analyzed by HPLC with diode array detection coupled to electrospray and ion-trap mass spectrometry system (HPLC–DAD–ESI–MS/MS) under identical detection and operating conditions to those previously described (Funes et al. 2009). The LC/MS system consisted of an Agilent LC 1100 series (Agilent Technologies Inc., Palo Alto, CA, USA) controlled by the Chemstation software. The HPLC instrument was coupled to an Esquire 3000+ (Bruker Daltonics, GmbH, Germany) mass spectrometer equipped with an ESI source and ion-trap mass analyzer, and controlled by Esquire control and data analysis software. Briefly, a Merck LiChrospher 100 RP-18, 5 μm, 250 × 4 mm (i.d.) column was used for the analysis. The HPLC protocol consisted on a linear gradient: 0 min, 5% B; 20 min, 30% B; 30 min, 90% B; 40 min, 5% B. The initial conditions were held for 10 min. Flow rate was 0.5 ml/min and mobile phase was water:acetonitrile (90:10, v/v) with 1% of formic acid (A) and acetonitrile (B). Identification and quantification of the major constituents of lemon verbena extract were performed by HPLC–DAD– and –MS/MS analysis, comparing the retention time, UV and MS spectra of the peaks in the samples with those of authentic commercially available standards (verbascoside) or data reported in the literature (Funes et al. 2009).
Subjects and study protocol
The study was conducted on 15 young healthy and moderately trained males from the Sport Sciences degree of the Miguel Hernandez University (Elche, Spain), who gave their written informed consent. The experimental procedures were approved by the corresponding Ethics Committee (reference number IB 544/05 PI) and complied with the respective legal requirements for this purpose. The inclusion criteria involved absence of any chronic disease (hypertension, diabetes, cardiovascular disease, alcohol, drug dependence) or other deviation from normal food habits. Diet and exercise routine were monitored with twice a week meetings. Subjects agreed to avoid the use of vitamin/mineral supplements, nutritional or antioxidant supplements, herbs and medication.
Prior to the assay, a selection of the volunteers was made in order to obtain the most possible homogeneous group in sport and physical performance. For that, a 2,000 m run test was done and only those volunteers who achieved it under 10 min were chosen (Table 1). This selection reduced considerably the sample size.
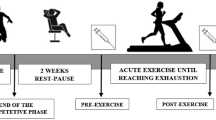
The volunteers were instructed by a qualified dietician to record food intakes and followed an adapted diet with personalized caloric expenditure. The diet was designed by software Dietsource Novartis and was rich in carbohydrates (60% carbohydrate, 25% lipids and 15% protein). In a randomized and double-blind procedure, the 15 participants were assigned either to extract group (EXT, n = 8) or placebo group (PLB, n = 7). The participants took three hard gelatin capsules/day (1,800 mg/day) containing lemon verbena extract (10% verbascoside, w/w) or placebo (microcrystalline cellulose) during 21 days, which were provided by Monteloeder, S.L. (Elche, Spain). To homogenize food habits and trained status of the participants, a 3-week washout period was used before supplementation period. Participants were regularly trained people before the study but in a much less extent and intensity than that one of the trial.
During the trial, the volunteers performed training sessions of 90-min eccentric running (14.5–16.5 km, pedometer determined) three times a week (non-consecutive days) during 21 days. The physical characteristics of the participants are shown in Table 1 (initial anthropometry parameters). All blood samples were collected between 9 and 11 am, before (0 day) and after (21 days) of training and supplementation period. Blood samples were taken more than 24 h after any physical activity, i.e. basal condition. The antecubital vein was punctured using a hypodermic needle and blood was collected in suitable tubes with EDTA as anticoagulant. The total volume of blood drawn did not exceed 30 ml.
Biochemical serum parameters
Part of blood was used for routine biochemical analyses, which was performed by a clinical hematology laboratory and included serum enzymes activities of creatine phosphokinase (CK), myoglobin (Mgb), aspartate aminotransferase (AST), alanine aminotransferase (ALT) and γ-glutamyltransferase (GGT). The remaining blood was used to make additional determinations in our laboratory as described below.
Neutrophils’ purification
The neutrophils’ fraction was purified following a previously reported method (Tauler et al. 2002a; Ferrer et al. 2009). Blood was carefully introduced on Ficoll in a proportion of 1.5:1 and then was centrifuged at 900g and 4°C for 30 min. The precipitate containing the erythrocytes and neutrophils was incubated at 4°C with 0.15 M ammonium chloride to hemolyse the erythrocytes. The suspension was centrifuged at 750g, at 4°C for 15 min and the supernatant was discarded. The neutrophils phase at the bottom was washed first with ammonium chloride and then with phosphate-buffered saline, pH 7.4. One sample of the precipitated neutrophils’ obtained from a known blood volume was used directly for RNA extraction. Other fraction was resuspended in water to determine enzyme activities, oxidative stress markers such as malondialdehyde (MDA) and protein carbonyl derivatives.
Enzymatic determinations in neutrophils
Catalase (CAT), glutathione peroxidase (GPx), glutathione reductase (GRed), superoxide dismutase (SOD), and MPO were determined in neutrophils using a microplate reader (SPECTROstar Omega, BMG LabTech GmbH, Offenburg, Germany) at 37°C. CAT, GPx, GRed and SOD activities were measured by spectrophotometric methods of Aebi (1984), Flohe and Gunzler (1984), Golberg and Spooner (1983) and McCord and Fridovich (1969), respectively (McCord and Fridovich 1969; Goldberg and Spooner 1983; Aebi 1984; Flohe and Gunzler 1984), according to previously published adaptations (Tauler et al. 2008). MPO activity of neutrophils was measured by guaiacol oxidation, under identical conditions to those previously described (Sureda et al. 2004a).
HPLC analysis of neutrophils’ malondialdehyde
First, 100 μl of neutrophils’ lysate was mixed with 50 μl of 0.05% butylated hydroxytoluene (BHT) in ethanol and 50 μl of trichloroacetic acid 20% in HCl 0.6 M. The samples were incubated 15 min on ice and then centrifuged at 5,000g during 15 min at 4°C. Then 100 μl of thiobarbituric acid (TBA) 0.6% in water was added to 100 μl of supernatant. At follow, the mixture was incubated at 97°C for 1 h, let to cool down and extracted with 300 μl of n-butanol through vigorous shaking, and then samples were centrifuged at 10,000g for 3 min. The TBA–MDA chromogen was determined using HPLC and fluorescence detection system as previously described (Funes et al. 2009). Promptly, the analysis was conducted by injecting 20 μl of sample into a reverse phase column LiChrospher® 100 RP-18 (5 μm, 250 × 4 mm i.d.) from Merck KGaA (Darmstadt, Germany) using isocratic mode with methanol–50 mM potassium phosphate buffer, pH 6.8 (40:60, v/v), and a flow rate of 1 ml/min. The TBA–MDA product was monitored by fluorescence detection with excitation at 515 nm and emission at 553 nm. Results were expressed in micromoles MDA/109 neutrophils.
Protein carbonyl derivatives determination in neutrophils
Protein carbonyl derivatives were measured in neutrophils by an adaptation of the method of Levine et al. (1994) using the precipitates of deproteinized samples. Precipitates were resuspended with 2,4-dinitrophenylhydrazine (DNPH) 10 mM and incubated for 60 min at 37°C. Then, samples were precipitated with 20% TCA and centrifuged for 10 min at 1,000g and 4°C. The precipitate was washed twice with ethanol:ethyl acetate (1:1; v/v) to remove free DNPH. Guanidine (6 M) in 2 mM phosphate buffer, pH 2.3, was added to the precipitate, and samples were incubated for 40 min at 37°C. Finally, samples were centrifuged for 5 min at 3,000g and 4°C to clarify the supernatant and absorbance was measured at 360 nm. The molar absorption of 22,000 M−1 cm−1 was used to quantify the levels of protein carbonyl derivatives. Samples were analyzed against a blank of guanidine solution (Sureda et al. 2004b).
Whole blood cytokines assay
Within 2 h after collection, the blood samples were cultured in a whole blood assay, following a previously reported method with some modifications (Baum et al. 1999). A volume of 100 μl of blood was added to 900 μl of Roswell Park Memorial Institute medium (RPMI) 1640 (Invitrogen Life Technologies, Carlsbad, CA, USA) in a 24 well plate. The culture medium was supplemented with 1% of penicillin/streptomycin obtained from Invitrogen Life Technologies (Carlsbad, CA, USA). Cells were cultured in the absence and in the presence of lipopolysaccharide 10 ng/ml (LPS, E. coli 0111:B4; Sigma, Munich, Germany) for 12 h in a humidified atmosphere at 37°C in 5% CO2. The supernatants of the cultures were collected after plate centrifugation and frozen at −80°C. Cytokine concentrations, specifically, interleukin-1β (IL-1β), interleukin-6 (IL-6) and tumor necrosis factor-α (TNF-α), were determined by standard enzyme-linked immunosorbent assay (ELISA) technique, according to the manufacturer’s instructions (Peprotech Inc., UK and U-Cytech biosciences, The Netherlands). The lower limits of detection were: 32 pg/ml for TNF-α, 5 pg/ml for IL-1β, and 32 pg/ml for IL-6.
Neutrophil RNA extraction and relative quantitative RT-PCR assay
Glutathione reductase mRNA expression was determined by real-time RT-PCR with human 36B4 rRNA as reference gene. mRNA isolation from neutrophils and quantitative PCR was performed as described in Sureda et al. (2007a). The primers used were: GRed, forward: 5′-CAA GGA AGA AAA GGT GGT TGG GAT C-3′ and reverse: 5′-GTC AAA GTC TGC CTT CGT TGC TCC-3′. h36B4-rib, forward: 5′-ATG TGA AGT CAC TGT GCC AG-3′ and reverse: 5′-GTG TAA TCC GTC TCC ACA GA-3′. The relative quantification was performed by standard calculations considering 2(−ΔΔCt) method. Initial mRNA levels at the beginning of the study were arbitrarily referred to as 1.
Statistical analysis
Statistical analysis was performed using the Statistical Package for Social Sciences (SPSS, v. 13.0 for Windows). The results were expressed as mean and standard errors of the mean (mean ± SEM). Statistical significance was set at P < 0.05, unless explicit indication. All the data were tested for normality. The statistical significance of the data were assessed using two-way analysis of variance (ANOVA). The ANOVA factors were antioxidant extract supplementation (S) and exercise training (T). The sets of data for which there were effects of supplementation, exercise training or a significant interaction between these two factors were tested by one-way ANOVA. Student’s t test for unpaired data were used to identify differences in the results of Table 1 and Fig. 2.
Results
Lemon verbena extract composition
The identification of the peaks was based on the analysis of their retention time, UV spectra and MS/MS data as mentioned in “Materials and methods”. Figure 1 shows the high performance liquid chromatography profile at 330 nm of lemon verbena extract (Fig. 1a) and its corresponding base peak chromatogram (BPC) (Fig. 1b). Among the phenylpropanoids, verbascoside was clearly the most abundant compound (approximately 10%, w/w). Minor compounds of the extract were other phenylpropanoids (isoverbascoside and martynoside) and several diglucuronidated flavones.
Characteristics of participants
In order to assure the homogeneity of the volunteers, a strict selection was performed based on the physical characteristics and performance of the participants after a 2,000 m run test (see “Materials and methods”). Table 1 shows the initial values of the sportsmen participating in the study. No significant differences in age, body characteristics or performance were observed between placebo and extract supplemented groups before supplementation and training period.
Muscular damage markers measurement
Table 2 shows the initial and final values of muscular damage markers found in serum samples of the volunteers in basal conditions. The activity of CK and Mgb was not significantly influenced by ANOVA factors. Nevertheless, the ANOVA analysis revealed a significant effect of the extract supplementation (S) factor on the enzymatic activities of ALT, AST, and GGT. Serum ALT activity increased after the study in the PLB group (+12.5%), whereas ALT activity was maintained constant in the EXT group. Moreover, serum AST activity showed significant lower values in the EXT group compared to the PLB at the end of the training period (−22%). With respect to GGT values, whereas the supplementation induced a significant effect on the EXT group (31% decrease), no significant effect was observed for the PLB group, as derived from ANOVA analysis.
Neutrophils antioxidant enzymes and myeloperoxidase activities
Table 3 shows the effect of extract supplementation (S) and training sessions (T) in neutrophils enzymatic activities for all the antioxidant enzymes studied (CAT, GPx, GRed and SOD) and MPO activity before and after the intervention study in basal conditions.
The two-way ANOVA analysis showed that the activities of CAT, GPx and GRed were influenced by the training factor. Besides, one-way ANOVA analysis was performed and significant differences were observed for these activities in both groups (EXT and PLB), when comparing between the initial and final values. CAT and GPx increased 67 and 110% in the PLB group, and 32 and 57% in the EXT group, respectively. Neutrophils’ GRed activity also exhibited a dramatic increase, i.e. 2.9-fold for PLB group and 4.2-fold for EXT group.
A strong interaction was observed on the effects of supplementation and exercise training in the MPO activity expressed on the basis of neutrophils’ number. A striking increase in the MPO activity was detected for the placebo group when values before and after the training period were compared, i.e. more than twofold. In contrast, in the supplemented group, the MPO activity at the final stage was substantially lower than the corresponding one determined in the PLB group.
Neutrophils’ oxidative stress markers
Figure 2 shows the values of MDA (a) and protein carbonyl derivatives (b) in neutrophils for PLB and EXT groups before and after the supplementation period. Neutrophils’ MDA level increased significantly (P = 0.05) when final values of PLB and EXT groups were compared. The behavior observed for the protein carbonyl derivatives was similar to that of MDA. This parameter underwent a significant increase (P = 0.08) in the placebo group through the study (+68%), whereas no significant change was observed for the EXT group.
Effects of extract supplementation and training sessions on oxidative damage markers in neutrophils. Concentration of MDA (a) and protein carbonyl derivatives (b) before (white) and after (light gray) supplementation period. The results are the mean ± SEM (EXT, n = 8; PLB, n = 7). For the analysis Student’s t test for unpaired data were used. &Significant difference between EXT and PLB after supplementation period. #Significant difference between initial and final values
Cytokines determination
The results obtained in the present study derived from whole blood stimulation experiments, which is a system reported to be closer to in vivo conditions (Gleeson 2007). As shown in Fig. 3a, IL-6 decreased in both groups in a significant manner. Moreover, a more significant decrease was observed for the EXT group when the final values of the two groups were compared (−63% EXT vs. −45% PLB). The ANOVA analysis showed that IL-6 level was affected by exercise training and by the interaction between supplementation and exercise. Regarding IL-1β (Fig. 3b), a significant decrease of the level of this cytokine was also observed in both groups (−29% EXT and −26% PLB), and ANOVA analysis showed a significant effect of T. TNF-α concentration (Fig. 3c), exhibited a weak decrease in both groups throughout the study, but the change was not significant regardless the ANOVA factor considered.
Effects of extract supplementation and training sessions on IL-6 (a), IL-1β (b), and TNF-α (c) cytokines release from whole blood stimulated with LPS, before (white) and after (light gray) supplementation period. The results are the mean ± SEM (EXT, n = 8; PLB, n = 7). Two-way ANOVA, P < 0.05. S extract supplementation, T training sessions, S × T interaction. Capital letters significant effects (S or T) or significant interaction (S × T). One-way ANOVA, P < 0.05. &Significant difference between EXT and PLB after supplementation period. #Significant difference between initial and final values
Neutrophil RNA extraction and relative quantitative RT-PCR assay
The values of GRed expression in neutrophils at the end of the study were 1.23 ± 1 and 1.31 ± 1 for EXT and PLB group, respectively. The ANOVA analysis of results showed that gene expression of this antioxidant enzyme was affected neither by exercise training (T) nor by extract supplementation (S) ANOVA factors.
Discussion
It has been established that exhaustive exercise leads to an excessive production of ROS causing cellular damage (Ji 1995; Sureda et al. 2008, 2009). Nevertheless, exercise-derived ROS are required for the cellular adaptation consisting of an increased expression of antioxidant proteins through the activation of the nuclear factor κΒ (NF-κΒ) (Ji et al. 2004). Recently, a high dose of antioxidant vitamins supplementation has shown a preventive effect on this positive cellular adaptation mechanism (Ristow et al. 2009). In the present study the effect of a moderate dose of an antioxidant supplementation consisting of a polyphenolic extract on exhaustive exercise (approx. 15.5 km run) is discussed.
A significant increase in the neutrophils’ CAT and GPx activities was observed in both placebo and supplemented groups, revealing the metabolic activation of these enzymes in order to detoxify peroxides (H2O2, ROOH) by the type of physical exercise performed. Previous studies have also reported an increase of post-exercise GPx activity after different types of exercise (Tauler et al. 2004; Sureda et al. 2007a; Ferrer et al. 2009). The increase observed for both groups must be interpreted as a beneficial regulatory effect of subtoxic levels of ROS (Ji 2008). GRed activity also showed a noteworthy increase indicating a higher regeneration of the reduced glutathione. This result is in agreement to those found after a football match (Tauler et al. 2008) and in lymphocytes GRed 3 h after a cycling stage (Tauler et al. 2006). Anyhow, our results showed that moderate lemon verbena supplementation do not affect the increase of the endogenous antioxidant enzyme response promoted by exercise. On the contrary, GRed activity was even higher in the group supplemented with the lemon verbena extract (up to 4.2-fold). Surprisingly, glutathione gene expression was maintained after the training period, indicating that this up-regulation could be at post-transcriptional level.
In agreement to that recently reported using a vitamins-enriched drink (Sureda et al. 2007b), in our study, a significant inhibitory effect on neutrophils’ MPO activity was detected in the EXT group. Several studies have shown that physical exercise promotes neutrophils activation through enzymatic activities such as MPO (Yamada et al. 2000; Morozov et al. 2003, 2006), which may lead into excessive ROS production. This enzyme is the main source for hypochlorous acid, which induces a transitory oxidative stress concomitant to the respiratory burst (Sureda et al. 2005). Therefore, our results support that MPO activity is strongly regulated by the supplementation with antioxidants and this fact may offer an additional protective effect of neutrophils against oxidative damage.
High levels of MDA and protein carbonyl derivatives in plasma, neutrophils and lymphocytes have been reported as oxidative stress markers derived from acute bouts of exercise (Sureda et al. 2005, 2007a, b, 2008; Tauler et al. 2006). These are signs of lipid and protein peroxidation and may compromise the function of white blood cells. Our results corroborate this statement, since consistent increases of neutrophils’ MDA and protein carbonyl derivatives were observed in the placebo group as a consequence of the 21 days exercise period. On the contrary, oxidative damage level did not increase for the Lippia supplemented group, which suggests that antioxidant supplementation protects neutrophils against exercise-induced damage, as reported for lymphocytes (Sureda et al. 2008). Moreover, neutrophils’ oxidative damage induced by acute exercise has been related to impaired immune function through the decrease of neutrophil chemotaxis and phagocytosis indexes (Ferrer et al. 2009). Therefore, supplementation with lemon verbena extract could improve the effectiveness of neutrophils immune function in exhaustive exercise.
Endurance physical exercise significantly increases serum enzymatic activities such as CK, Mgb, ALT, AST and GGT. Hence these have been considered as markers for the muscular damage derived from intense exercise (Fallon et al. 1999; Suzuki et al. 1999; Sureda et al. 2004b, 2007a; Miura et al. 2005; Kim et al. 2007). The presence of some of these markers in plasma has been used to relate the positive influence of antioxidants’ supplementation on muscular damage (Morillas-Ruiz et al. 2005, 2006; Sureda et al. 2007b; Panza et al. 2008; Su et al. 2008). The increase of plasmatic CK or Mgb has been reported just after exhausting bouts of exercise (Tauler et al. 2006; Sureda et al. 2007a, b) probably through muscle microruptures, evoking an inflammatory response. Nevertheless, no changes for CK or Mgb levels were observed in the present study when values before the study (basal conditions) and after the study in the recovery phase were compared, which would probably indicate an adaptation phenomenon, as reported (Noakes 1987).
On the contrary ALT, AST and GGT levels were significantly reduced in the supplemented group. The increase of the activity of some transaminases has been correlated to muscular, joint, hepatic or even osseous damage, which may be induced by extreme exercise conditions such as ultramarathon (Fallon et al. 1999; Kim et al. 2007). ALT and AST are present in muscle and liver, but GGT is a rather-specific hepatic enzyme. Little information is available on the relationship between exercise, antioxidants’ supplementation and transaminases’ activities. Anyhow, it was reported that the increase in AST and oxidative stress markers induced by exercise was inhibited by an antioxidant green tea beverage, in agreement to our results (Panza et al. 2008). Regarding ALT and GGT transaminases, there is no information available. However, the results obtained in the present study lead us to postulate that the consumption of Lippia extract provides a protecting effect against physical exercise damage.
Physical exercise induces immunological acute changes in the cytokines level. After an acute bout of exercise, immunological functions normally return to pre-exercise values within 3–24 h (Gleeson 2007). An increase in IL-6 levels, which seems to derive from skeletal muscle, is observed when basal conditions are compared to those immediately post-exercise (Pedersen and Hoffman-Goetz 2000). However, light-moderate exercise (1 h/day walking) does not affect IL-6 circulating levels (Gleeson 2007). In the present work, exercise induced a significant decrease of IL-6 and IL-1β levels after 21 days measured in basal conditions. Moreover the decrease of IL-6 was also influenced by the supplementation with the antioxidant extract.
Whereas a large body of studies has focused on the immune response upon acute exercise, just little information is available concerning the chronic effects on the adaptive immune function (Pedersen and Hoffman-Goetz 2000). Neutrophils’ count increase induced by acute exercise is dependent on catecholamines such as epinephrine and cortisol. Nevertheless, this effect disappears some days after adaptation, when this exercise becomes repetitive, then hormonal regulation seems to be involved (Suzuki et al. 1999; Pedersen and Hoffman-Goetz 2000; Pedersen and Toft 2000; Miura et al. 2005). Our results on IL-6 are in agreement to those previously reported indicating that the immune adaptive response induced by chronic exercise is not blocked by moderate antioxidant supplementation. Attenuation in IL-6 level has been also related to lower exercise-induced depression of immune function, and hence lower incidence of upper respiratory tract infections symptoms in ultramarathon runners supplemented with vitamins (Fischer et al. 2004).
Little information is available about the effect of antioxidant supplementation on the cytokines profile during exercise (Fischer et al. 2004; Tauler et al. 2006; Davison et al. 2007; Su et al. 2008). Anyhow, our results on IL-6 levels are in agreement to those reported after long-term antioxidant supplementation on individuals performing moderate exercise (Phillips et al. 2003; Fischer et al. 2004; Nieman et al. 2007), indicating that moderate antioxidant supplementation (vitamins or polyphenols) do not affect immune adaptive mechanisms of long-term exercise.
Conclusions
In conclusion, intense running exercise for 21 days by trained male induced antioxidant response in neutrophils through the increase of the antioxidant enzymes CAT, GPx and GRed. Supplementation with moderate levels of an antioxidant lemon verbena extract did not block this cellular adaptive response and also reduced exercise-induced oxidative damage of proteins and lipids in neutrophils. Moreover, supplementation maintained or decreased the level of serum transaminases activity indicating a protection of muscular tissue.
Finally, exercise induced a decrease of IL-6 and IL-1β levels after 21 days measured in basal conditions. The decrease of IL-6 was also influenced by the supplementation with the antioxidant extract. Therefore, moderate antioxidant supplementation with lemon verbena extract protects neutrophils against oxidative damage, decreases the signs of muscular damage and acute inflammation in chronic running exercise and do not block the cellular adaptation to exercise.
References
Aebi H (1984) Catalase in vitro. Methods Enzymol 105:121–126
Baum M, Köpping-Menke K et al (1999) Increased concentrations of interleukin 1-beta in whole blood cultures supernatants after 12 weeks of moderate endurance exercise. Eur J Appl Physiol Occup Physiol 79(6):500–503
Davison G, Gleeson M et al (2007) Antioxidant supplementation and immunoendocrine responses to prolonged exercise. Med Sci Sports Exerc 39(4):645–652
Díaz AM, Abad MJ et al (2004) Phenylpropanoid glycosides from Scrophularia scorodonia: in vitro anti-inflammatory activity. Life Sci 74(20):2515–2526
Fallon KE, Sivyer G et al (1999) The biochemistry of runners in a 1600 km ultramarathon. Br J Sport Med 33(4):264–269
Ferrer MD, Tauler P et al (2009) Antioxidant regulatory mechanisms in neutrophils and lymphocytes after intense exercise. J Sports Sci 27(1):49–58
Fischer CP, Hiscock NJ et al (2004) Supplementation with vitamins C and E inhibits the release of interleukin-6 from contracting human skeletal muscle. J Physiol 558(2):633–645
Flohe L, Gunzler WA (1984) Assays of glutathione peroxidase. Methods Enzymol 105:114–121
Funes L, Fernández-Arroyo S et al (2009) Correlation between plasma antioxidant capacity and verbascoside levels in rats after oral administration of lemon verbena extract. Food Chem 117(4):589–598
Gleeson M (2007) Immune function in sport and exercise. J Appl Physiol 103(2):693–699
Goldberg DM, Spooner RJ (1983) Glutathione reductase. In: Bergmeyer HU, Bergmeyer J, Grabl M (eds) Methods of enzymatic analysis. Verlag Chemie, Basal, pp 258–265
Gómez-Cabrera M-C, Domenech E et al (2008) Oral administration of vitamin C decreases muscle mitochondrial biogenesis and hampers training-induced adaptations in endurance performance. Am J Clin Nutr 87(1):142–149
Hausmann M, Obermeier F et al (2007) In vivo treatment with the herbal phenylethanoid acteoside ameliorates intestinal inflammation in dextran sulphate sodium-induced colitis. Clin Exp Immunol 148(2):373–381
Ji LL (1995) Oxidative stress during exercise: implication of antioxidant nutrients. Free Radic Biol Med 18:1079–1086
Ji LL (2008) Modulation of skeletal muscle antioxidant defense by exercise: role of redox signaling. Free Radic Biol Med 44(2):142–152
Ji LL, Gómez-Cabrera MC et al (2004) Acute exercise activates nuclear factor (NF)-kappaB signaling pathway in rat skeletal muscle. Faseb J 18:1499–1506
Kim H, Lee Y et al (2007) Biomarkers of muscle and cartilage damage and inflammation during a 200 km run. Eur J Appl Physiol 99(4):443–447
Korkina LG, Mikhal’chik EV et al (2007) Molecular mechanisms underlying wound healing and anti-inflammatory properties of naturally occurring biotechnologically produced phenylpropanoid glycosides. Cell Mol Biol 53(5):84–91
Lee JY, Woo ER et al (2005) Inhibition of lipopolysaccharide-inducible nitric oxide synthase expression by acteoside through blocking of AP-1 activation. J Ethnopharmacol 97(3):561–566
Levine RL, Williams JA et al (1994) Carbonyl assays for determination of oxidatively modified proteins. Methods Enzymol 233:346–357
Lin L-C, Wang Y-H et al (2006) The inhibitory effect of phenylpropanoid glycosides and iridoid glucosides on free radical production and beta2 integrin expression in human leucocytes. J Pharm Pharmacol 58(1):129–135
Liu MJ, Li JX et al (2003) The effects of verbascoside on plasma lipid peroxidation level and erythrocyte membrane fluidity during immobilization in rabbits: a time course study. Life Sci 73(7):883–892
McAnulty SR, McAnulty LS et al (2004) Consumption of blueberry polyphenols reduces exercise-induced oxidative stress compared to vitamin C. Nutr Res 24(3):209–221
McCord JM, Fridovich I (1969) Superoxide dismutase. An enzymic function for erythrocuprein (hemocuprein). J Biol Chem 244(22):6049–6055
Miura M, Umeda T et al (2005) Effect of 6 months’ training on the reactive oxygen species production capacity of neutrophils and serum opsonic activity in judoists. Luminescence 20(1):1–7
Morillas-Ruiz J, Zafrilla P et al (2005) The effects of an antioxidant-supplemented beverage on exercise-induced oxidative stress: results from a placebo-controlled double-blind study in cyclists. Eur J Appl Physiol 95(5–6):543–549
Morillas-Ruiz JM, Villegas García JA et al (2006) Effects of polyphenolic antioxidants on exercise-induced oxidative stress. Clin Nutr 25(3):444–453
Morozov VI, Pryatkin SA et al (2003) Effect of exercise to exhaustion on myeloperoxidase and lysozyme release from blood neutrophils. Eur J Appl Physiol 89(3–4):257–262
Morozov VI, Tsyplenkov PV et al (2006) The effects of high-intensity exercise on skeletal muscle neutrophil myeloperoxidase in untrained and trained rats. Eur J Appl Physiol 97(6):716–722
Newall CA, Anderson LA, Phillipson JD (1996) Herbal medicines: a guide for health-care professionals. The Pharmaceutical Press, London, p 263
Nieman D (1994) Exercise, upper respiratory tract infection, and the immune system. Med Sci Sports Exerc 26:128–139
Nieman DC, Henson DA et al (2007) Quercetin’s influence on exercise-induced changes in plasma cytokines and muscle and leukocyte cytokine mRNA. J Appl Physiol 103(5):1728–1735
Noakes TD (1987) Effect of exercise on serum enzyme activities in humans. Sports Med 4(4):245–267
Panza VSP, Wazlawik E et al (2008) Consumption of green tea favorably affects oxidative stress markers in weight-trained men. Nutrition 24(5):433–442
Pedersen BK, Hoffman-Goetz L (2000) Exercise and the immune system: regulation, integration, and adaptation. Physiol Rev Suppl 80(3):1055–1081
Pedersen BK, Toft AD (2000) Effects of exercise on lymphocytes and cytokines. Br J Sport Med 34(4):246–251
Petersen EW, Ostrowski K et al (2001) Effect of vitamin supplementation on cytokine response and on muscle damage after strenuous exercise. Am J Physiol Cell Physiol 280:C1570–C1575
Phillips T, Childs AC et al (2003) A dietary supplement attenuates IL-6 and CRP after eccentric exercise in untrained males. Med Sci Sports Exerc 35(12):2032–2037
Quirantes-Piné R, Funes L et al (2009) High-performance liquid chromatography with diode array detection coupled to electrospray time-of-flight and ion-trap tandem mass spectrometry to identify phenolic compounds from a lemon verbena extract. J Chromatogr A 1216(28):5391–5397
Ristow M, Zarse K et al (2009) Antioxidants prevent health-promoting effects of physical exercise in humans. Proc Natl Acad Sci USA 106(21):8665–8670
Su Q-S, Tian Y et al (2008) Effects of allicin supplementation on plasma markers of exercise-induced muscle damage, IL-6 and antioxidant capacity. Eur J Appl Physiol 103(3):275–283
Sureda A, Batle JM et al (2004a) Hypoxia/reoxygenation and vitamin C intake influence no synthesis and antioxidant defenses of neutrophils. Free Radic Biol Med 37(11):1744–1755
Sureda A, Batle JM et al (2004b) Neutrophil tolerance to oxidative stress induced by hypoxia/reoxygenation. Free Radic Res 38(9):1003–1009
Sureda A, Tauler P et al (2005) Relation between oxidative stress markers and antioxidant endogenous defences during exhaustive exercise. Free Radic Res 39(12):1317–1324
Sureda A, Ferrer MD et al (2007a) Intense physical activity enhances neutrophil antioxidant enzyme gene expression. Immunocytochemistry evidence for catalase secretion. Free Radic Res 41(8):874–883
Sureda A, Tauler P et al (2007b) Antioxidant supplementation influences the neutrophil tocopherol associated protein expression, but not the inflammatory response to exercise. Cent Eur J Biol 2(1):56–70
Sureda A, Tauler P et al (2008) Influence of an antioxidant vitamin-enriched drink on pre- and post-exercise lymphocyte antioxidant system. Ann Nutr Metab 52:233–240
Sureda A, Ferrer MD et al (2009) Effects of exercise intensity on lymphocyte H2O2 pro-duction and antioxidant defences in soccer players. Br J Sport Med 43:186–190
Suzuki K, Totsuka M et al (1999) Endurance exercise causes interaction among stress hormones, cytokines, neutrophil dynamics, and muscle damage. J Appl Physiol 87(4):1360–1367
Tauler P, Aguiló A et al (2002a) Acute phase immune response to exercise coexists with decreased neutrophil antioxidant enzyme defences. Free Radic Res 36(10):1101–1107
Tauler P, Aguiló A et al (2002b) Diet supplementation with vitamin E, vitamin C and beta-carotene cocktail enhances basal neutrophil antioxidant enzymes in athletes. Pflugers Arch 443(5–6):791–797
Tauler P, Aguiló A et al (2004) Different effects of exercise tests on the antioxidant enzyme activities in lymphocytes and neutrophils. J Nutr Biochem 15(8):479–484
Tauler P, Sureda A et al (2006) Increased lymphocyte antioxidant defences in response to exhaustive exercise do not prevent oxidative damage. J Nutr Biochem 17(10):665–671
Tauler P, Ferrer MD et al (2008) Supplementation with an antioxidant cocktail containing coenzyme Q prevents plasma oxidative damage induced by soccer. Eur J Appl Physiol 104(5):777–785
Urso ML, Clarkson PM (2003) Oxidative stress, exercise, and antioxidant supplementation. Toxicology 189(1–2):41–54
Valentao P, Fernandes E et al (2002) Studies on the antioxidant activity of Lippia citriodora infusion: scavenging effect on superoxide radical, hydroxyl radical and hypochlorous acid. Biol Pharm Bull 25(10):1324–1327
Vassilakopoulos T, Karatza MH et al (2003) Antioxidants attenuate the plasma cytokine response to exercise in humans. J Appl Physiol 94(3):1025–1032
Yamada M, Suzuki K et al (2000) Effect of exhaustive exercise on human neutrophils in athletes. Luminescence 15(1):15–20
Acknowledgments
We thank Monteloeder, S.L. for providing the lemon verbena extract and liability insurance. This investigation was awarded with the 2nd National Prize of Research in Sports Medicine 2009 (CajAstur), Spain. This investigation has been supported by Grants ACOMP/2010/107 from GV, and AGL2007-60778, AGL2007-62806, and FPI fellowship to L. Funes from Ministerio de Educación y Ciencia (MEC).
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by William Kraemer.
Rights and permissions
About this article
Cite this article
Funes, L., Carrera-Quintanar, L., Cerdán-Calero, M. et al. Effect of lemon verbena supplementation on muscular damage markers, proinflammatory cytokines release and neutrophils’ oxidative stress in chronic exercise. Eur J Appl Physiol 111, 695–705 (2011). https://doi.org/10.1007/s00421-010-1684-3
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00421-010-1684-3