Abstract
Purpose
Rigorous exercise is known to generate reactive oxygen species (ROS) and inflict inflammatory response. The present study investigated whether dietary supplementation of avenanthramides (AVA) in oats would increase antioxidant protection and reduce inflammation in humans after an acute bout of eccentric exercise.
Methods
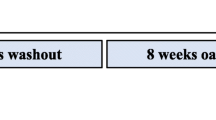
Young women (age 18–30 years, N = 16) were randomly divided into two groups in a double-blinded fashion, receiving two cookies made of oat flour providing 9.2 mg AVA (AVA) or 0.4 mg AVA (Control, C) each day for 8 weeks. Before and after the dietary regimen each group of subjects ran downhill (DR) on a treadmill at −9 % grade for 1 h at a speed to elicit 75 % of maximal heart rate. Blood samples were collected at rest, immediately and 24 h post-DR.
Results
Before dietary supplementation plasma creatine kinase activity and tumor necrosis factor (TNF)-α concentration were increased immediately after DR (P < 0.05), whereas neutrophil respiratory burst (NRB) was elevated 24 h post-DR (P < 0.05). CK and TNF-α response to DR was abolished during post-supplementation tests in both AVA and C groups, whereas NRB was blunted only in AVA but not in C. Plasma interleukin-6 level and mononuclear cell nuclear factor (NF) κB activity were not affected by DR either before or after dietary supplementation, but were lowered 24 h post-DR in AVA versus C (P < 0.05). Both groups increased plasma total antioxidant activity following 8-week dietary regimen (P < 0.05), whereas only AVA group increased resting plasma glutathione (GSH) concentration (P < 0.05), decreased glutathione disulfide response to DR, and lowered erythrocyte GSH peroxidase activity (P < 0.05).
Conclusions
Our data of pre- and post-supplementation difference reflect an interaction between repeated measure effect of eccentric exercise and AVA in diet. Long-term AVA supplementation can attenuate blood inflammation markers, decrease ROS generation and NFkB activation, and increased antioxidant capacity during an eccentric exercise bout.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
With the demand for increasing physical activity to reduce risks of certain chronic diseases, maintaining proper antioxidant defense during exercise and sports is an important issue for musculoskeletal health. Overproduction of reactive oxygen species (ROS) not only causes subtle cellular damage to muscle but also increases the risk of systemic inflammatory response following rigorous physical exertion (Proske and Allen 2005; Ji et al. 2009). However, recent research indicates that supplementation of large doses of antioxidants of pharmaceutical source can be problematic as it interferes with intrinsic adaptive responses and sometimes abolishes the benefit of exercise (Ristow et al. 2009; Gomez-Cabrera et al. 2008). Thus, exercise and nutrition scientists are increasingly interested in phytochemicals that demonstrate antioxidant and anti-inflammatory properties for potential dietary supplementation.
Avenanthramides (AVA) are a group of diphenolic acids that are found only in oats (Avena sativa) (Collins 1989). Consisting of an anthranilic acid and a hydroxycinnamic acid derivative linked by a pseudo-peptide bond, these compounds play the role of intrinsic antioxidants in oats, and share structural similarity to the pharmaceutical antioxidant Tranilast (Isaji et al. 1998). Of all the AVA that have been identified, three stand out due to their abundance and have been labeled as AVA-A, -B, and -C, which differ by a single moiety on the hydroxycinnamic acid ring. All three AVA demonstrate clear antioxidant activity in vitro, with AVA-C being the most potent (Peterson et al. 2002). In vitro studies have shown that AVA have anti-inflammatory and anti-atherogenic effects of decreasing monocyte adhesion to human aortic endothelial cells (HAEC), as well as suppressing adhesion molecule and pro-inflammatory cytokine production (Liu et al. 2004). AVA-C displayed further anti-atherogenic potential by inhibiting vascular smooth muscle cell (SMC) proliferation and enhancing nitric oxide production in both SMC and HAEC, along with up-regulation of endothelial nitric oxide synthase and decreased nuclear factor (NF) κB activity (Nie et al. 2006a, b). However, the aforementioned in vitro data were derived with AVA doses not possible to reach with human dietary studies; therefore, their implication to health benefit remains unclear.
The ability of AVA to reduce exercise-induced ROS and oxidative damage has been reported sparsely in the literature. Ji et al. (2003) showed that rats fed AVA-C at the dose of 0.1 g/kg for 50 days displayed lower ROS generation and lipid peroxidation in the soleus muscle after an acute bout of treadmill running. Furthermore, AVA-supplemented rats showed higher levels of antioxidant enzyme activities in the muscle, heart, liver and kidney. It is important to note that after rats were orally gavaged a mixture of synthetic AVA, all three fractions of AVA appeared in significant quantity in the plasma, heart, liver and skeletal muscle during a 48 h period, indicating AVA are bioavailable to tissues (Koenig et al. 2011). The above studies have provided some promise that dietary supplementation of AVA could be a potential protector against muscle oxidative stress and inflammatory response during and following rigorous muscle contraction. However, no study to date has examined the potential anti-oxidant and anti-inflammatory effect of AVA in human subjects.
Thus, the purpose of the present study is to examine whether long-term dietary supplementation of an oat product rich in AVA content could enhance blood antioxidant capacity and reduce blood inflammatory markers after a downhill running (DR) protocol in young female subjects. We tested the following hypotheses: (1) In healthy human subjects, high concentration of AVA supplementation would significantly decrease DR-induced muscle damage and subsequent inflammatory responses in blood circulation; (2) the protective effects of AVA supplementation are due to its ability to enhance blood antioxidant defense and to inhibit NFκB activation in the immune-active blood cells.
Materials and methods
Subjects
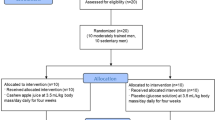
Young women aged 18–30 years were recruited from the Madison, Wisconsin, community. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. The study was approved by the Institutional Review Board of University of Wisconsin-Madison Health Science (protocol number H-2007-0078-2).
Informed consent was obtained from all individual participants included in the study. The subjects also completed a Health History Survey to ensure that they were eligible for the study and healthy enough to exercise. During the initial visit subjects were instructed to complete the International Physical Activity Questionnaire (IPAQ) and health history questionnaire. Major exclusion criteria were cardiovascular disease, diabetes, asthma, smoking or other tobacco use, drinking alcohol in excess of 5 drinks per week, use of nutraceuticals (e.g., St. John’s Wort), blood pressure medication, non-steroidal anti-inflammatory drugs (NSAIDs), anticoagulants, antidiabetic and hypoglycemic drugs. Those subjects who had been routinely engaged in high level of physical training were also excluded. Subjects were told to refrain from heavy exercise with heart rate over 120 BPM. A daily was provided to each subject to register any physical activity that exceeds the above level. We found that exercise habits during the course of the study did not diff between treatment groups over time.
Dietary supplementation
The subjects were randomly assigned to one of following two groups (N = 8 per group) receiving either a high dose of AVA supplementation in the diet, or a low dose of AVA. Other than this difference, the groups were treated exactly the same in a double-blind fashion. Both dietary groups of subjects received cookies made of oat flours with known AVA concentration, provided courtesy of Ceapro Inc. (Edmonton, AB, Canada). AVA concentrations were verified using high-performance liquid chromatography (HPLC) in our laboratory (see below). The high-AVA oat flour contained 190 mg/kg, and the low-AVA control group flour contained 8 mg/kg. We used this line of oat as control because it contained the lowest amount of AVA concentration among all oat lines available, but contained identical levels of starch, lipid, protein and other nutrients such as tocopherols, flavonoids and phenolic acids, as the high-AVA oat. The recipe for each type of cookie was identical except in the type of flour used. Each cookie contained 30 g flour (AVA or Control), 5.91 mL unsweetened apple sauce (Surefine, USA), 12.32 mL artificial sweetener (Natrataste Gold, NY), 0.616 mL baking soda, and 0.0308 mL table salt, with a total of 125 kcal per cookie. They were baked in a low temperature oven (121° C) for 15 min to ensure that AVA was not broken down or produced by the oat during the process (Dimberg et al. 2001). Final AVA concentration in the high- and low-AVA cookie was 4.6 mg/cookie (9.2 mg/day) and 0.2 mg/cookie (0.4 mg/day), respectively. Subjects were instructed to consume two cookies per day: one in the morning with breakfast and one in the evening with dinner. All subjects were instructed to maintain their normal dietary habit and not to take oat products throughout the experimental period. They were also asked to keep a record of any drug or antioxidant intake, which was periodically checked by the study administrator. No such violation was found for any of the subjects.
Exercise protocol
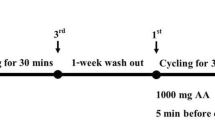
Before and after the 8-week dietary regimen, each subject performed a DR consisted of four bouts of 15 min of treadmill running at −9 % grade, separated by three sessions of 5 min rest. Subject wore a heart rate monitor and the treadmill speed was manually adjusted at 5-min intervals to keep the heart rate at 75 % of its maximum (220—age). Three blood samples were taken prior to DR at the resting state, immediately after DR, and 24 h after DR. All blood samples were taken in the post-absorptive state at 7:00–10:00 am. Subjects were told to terminate cookie consumption at least 24 h prior to the visit when the resting blood sample was taken. In order to control for the potential effect of hormone levels, DR was performed between days 7 and 10 of the participant’s menstrual cycle, determined by self-report.
Blood sample collection and preparation
Mixed venous blood was drawn from an antecubital vein into 4 EDTA-coated Vacutainer tubes (7 mL each). One tube of whole blood was immediately centrifuged at 500×g at 4° C for glutathione assay. The remaining blood volume was gently pipetted over two layers of density gradient (Histopaque and Ficoll-Paque) for isolation of blood cells. After centrifugation at 500×g for 30 min at 20 °C, plasma was removed by aspiration and frozen at −80 °C. A band of monocytes was then removed by aspiration, washed with phosphate buffered saline (PBS), and frozen at −80 °C. The remaining fluid was removed and washed with ice cold PBS to attain neutrophils. Packed erythrocytes were removed and stored immediately at −80 °C.
Biological measurements
Elisa
Plasma IL-1B, IL-6, TNFa, and CRP were analyzed in duplicate via commercially available ELISA kits (eBioscience, Read-Set-Go! ELISA. San Diego, CA). All samples were measured in duplicate using 96-well plates coated with various capture antibodies.
Nuclear factor-kappaB binding to DNA was measured by ELISA in nuclear extracts of mononuclear cells using antibody against p65 (eBioscience InstantOne ELISA. San Diego, CA).
High-performance liquid chromatography (HPLC)
Plasma concentration of reduced (GSH) and oxidized glutathione GSSG) were measured by HPLC (Shimadzu Scientific, Columbia, MD) equipped with a UV–VIS detector set at 365 nm wavelength (Ji and Fu 1992). AVA concentrations in the oat cookie were measured using HPLC equipped with a Supelco C-18 column and a UV–VIS detector set at 330 nm absorption wavelength according to Chen et al. (2004) with some modifications (Koenig et al. 2014).
Neutrophil respiratory burst assay
Neutrophils respiratory burst activity was measured using a procedure according to Benbarek et al. (1996) with modifications. Three concentrations of phorbol myristate acetate (PMA) were used to evaluate the effects of maximal (160 µmol/L), moderate (16 µmol/L), and minimal (1.6 µmol/L) stimulation to the cells. A total of 1 × 106 neutrophils were used in each trial. The respiratory burst chemiluminescence was tracked for 30 min by a luminometer (Turner Biosystems, Madison, WI).
Spectrophotometric assays
Plasma total antioxidant capacity (TAC) was measured by spectrophotometer by monitoring the attenuation of 2,2′-azinobis-3-ethylbenzothiazoline-6-sulfonic acid (ABTS) oxidation at 734 nm according to Re et al. (1999). A solution of 7 mmol/L ABTS and 2.45 mmol/L aluminum potassium sulfate (APS) was made immediately before the conducting of the assay and kept in the dark. An aliquot of 100 µL plasma was added to a final volume of 1 mL with ABTS/APS solution. The cuvette was mixed by inversion and then incubated at 37 °C for 5 min. The cuvettes were then read against a Trolox (6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid) standard curve using a spectrophotometer (Shimadzu UV160).
Plasma creatine kinase (CK) activity was measured as a marker of eccentric muscle damage according to the procedure of Tanzer and Gilvarg (1959). The CK reaction was coupled to NADH conversion to NAD by lactate dehydrogenase (LDH), which, along with pyruvate kinase (PK), phosphoenol pyruvate (PEP), and NADH, was present in the reaction mixture. The decrease in NADH concentration was tracked using a spectrophotometer. Erythrocyte superoxide dismutase (SOD) activity was measured spectrophotometrically by tracking the decrease in auto-oxidation of epinephrine to adrenochrome according to Sun and Zigman (1978). Epinephrine autoxidation rate was measured at 320 nm for 3 min in the presence of an aliquot of erythrocyte lysate. The slope of the linear portion of the absorption graph was used to determine SOD activity by determining the percent inhibition of epinephrine autoxidation via comparison to the blank. Erythrocyte glutathione peroxidase (GPx) activity was measured by monitoring the change in NADPH concentration in a system with excess GSH and glutathione reductase (GR) in the presence of H2O2 (Flohe and Gunzler 1984). SOD and GPx activities were normalized to hemoglobin concentration.
Statistical analysis
Data were shown as mean ± SEM and analyzed using the Planned Comparison method. A three-way repeated measures ANOVA was first conducted using R (version 2.14.1) statistical software. The three main factors are (a) post- versus pre-AVA supplementation, (b) timing with respect to DR test, and (c) high-AVA versus low-AVA supplementation. The standard error of estimate of the ANOVA was used to complete a priori planned comparisons. Significance for each comparison was set at P < 0.05.
Results
Participant data
The age, height, weight, and body mass index (BMI) of the study participants are displayed in Table 1. There were no significant differences between groups for any of the parameters measured. Body weight and BMI were unchanged following dietary supplementation.
Muscle damage caused by Down-hill running
During the pre-supplementation trials, plasma CK activity increased sharply (P < 0.05) after DR and returned to baseline 24 h post-DR (Fig. 1). After 8-week dietary supplementation, CK response to DR was abolished in both high- and low-AVA groups. Furthermore, post-DR CK levels were significantly lower after supplementation compared to pre-supplementation. However, there was no AVA dose difference at any time point.
Inflammatory markers
NRB activity immediately post-DR was not significantly different from the resting level, but showed an increase 24 h post-DR (P < 0.05) (Fig. 2). After supplementation, NRB activity was increased after DR and remained elevated 24 h post-DR in low-AVA group (P < 0.05). In high-AVA group NRB activity was not altered by DR, and the levels of post-DR and 24 h post-DR were lower than their counterparts during pre-supplementation trial (P < 0.05).
Plasma TNF-α concentration was increased after DR (P < 0.05), but returned to the resting level after 24 h during the pre-supplementation trials (Fig. 3). After dietary supplementation TNF-α concentration was no longer altered by DR regardless of AVA dose, whereas post-DR levels were lower compared to pre-supplementation values (P < 0.05). Also, there was a trend towards a lower TNF-α levels in high-AVA versus low-AVA group (0.05 < P < 0.1).
Plasma IL-6 concentration was not affected by DR during pre-supplementation trials (Fig. 4). However, after supplementation IL-6 level was significantly lower at post-DR and 24 h post-DR in high-AVA group than low-AVA group (P < 0.05, AVA-DR interaction). Plasma IL-1β showed no significant change in response to DR or AVA supplementation (not shown). Although CRP levels appeared to be elevated in 24 h post-DR prior to AVA supplementation, the change was not significance due to high variation (Fig. 5).
Mononuclear cell NFκB activation
Nuclear factor-kappaB binding did not change significantly in response to DR before or after dietary regimen (Fig. 6). ANOVA revealed a main effect that NFκB binding was reduced after supplementation compared to before supplementation (P < 0.05). Post-hoc test showed that NFκB binding was lower in AVA versus C at 24 h post-DR (P < 0.05).
Plasma antioxidant capacity
Total antioxidant capacity was not affected by DR before oat supplementation, but elevated significantly after the dietary treatment regardless AVA dose (P < 0.05; main effect) (Fig. 7).
Erythrocyte SOD activity was not affected by DR or diet. Erythrocyte GPx activity showed no change following DR and 24 h post-DR before supplementation. However, after dietary supplementation GPx activity was lowered in high-AVA compared to low-AVA group and the difference was significant immediately post-DR (P < 0.05).
Plasma GSH concentration was not altered immediately after DR, but significantly elevated 24 h post-DR both before and after dietary supplementation (P < 0.05, Table 2). Following supplementation, resting plasma GSH was significantly higher in high- versus low-AVA group (P < 0.05). During pre-supplementation trials plasma GSSG concentration was increased above resting levels immediately after DR (P < 0.05). This change was again observed in the low-AVA group after supplementation, but not in the high-AVA group. The GSH:GSSG ratio was elevated above resting levels at 24 h post-DR (P < 0.05) in both AVA groups before supplementation, but after supplementation GSH: GSSG was increased only in high- AVA group (P < 0.05).
Discussion
Several previous studies have reveled AVA as an inhibitor for inflammatory cytokine and chemokine production and cell proliferation in vitro (Liu et al. 2004; Nie et al. 2006a, b; Guo et al. 2008; Sur et al. 2008). We hypothesized that human subjects engaged in eccentric exercise or exercise containing eccentric component could benefit from dietary oat supplementation for some protection. Our data partially supported our hypothesis, but were complicated by a well-documented repeated measure effect of eccentric exercise that also predicts an attenuation of stress markers when two exercise testing protocols are repeated between a few months. Thus, given the current experimental design, this observed protection may reflect an interaction between the antioxidant and anti-inflammatory effect of AVA and the repeated measure effect.
The repeated measure effect was evidenced by the finding that plasma CK activity, a classic muscle damage marker, was elevated by DR but blunted after subjects had consumed two oat cookies daily for 8 weeks regardless of AVA concentration. In addition, DR-inflicted plasma TNF-α level was also blunted by the oat supplementation regimen in both high-AVA and Control group, again suggesting a possible repeated measure effect (Fig. 3). However, since oats contain several antioxidants unrelated to AVA, such as phenolic acids, flavonoids and tocopherols, it cannot be ruled out that these antioxidants exerted an inhibitory effect on the production of this pro-inflammatory cytokine.
Avenanthramides supplementation at high concentration reduced ROS production during neutrophil respiratory burst, a hallmark response after neutrophil infiltration into damaged muscle fibers. While NRB was elevated by 80 % at 24 h post DR prior to supplementation, this effect was completely abolished after high-AVA supplementation, whereas Control group still showed elevated NRB (Fig. 2). Furthermore, NFκB binding was significantly decreased by oat supplementation, and high-AVA group showed significantly lower binding than the Control group (Fig. 6). These data were consistent with the findings of Sur et al. (2008) showing that an AVA concentration of 1 part per billion was sufficient to reduce the breakdown of IκB from NFκB complex in cultured keratinocytes and provided evidence that this mechanism might be effective in vivo.
One of the most significant consequences of NFκB signaling is the production of pro-inflammatory cytokines such as TNF-α, IL-1, -6, and -8 with their DNA containing consensus NFκB binding sites. Elevated plasma TNF-α concentration and enhanced TNF-α gene expression in skeletal muscle have been reported following DR in rats (Liao et al. 2010) and in human (Nieman et al. 2001; Pedersen et al. 2001; Hamada et al. 2005). Pro-inflammatory function of TNF-α is at least in part attributed to its ability of elevating cellular reactive oxygen species (ROS) generation (Li et al. 1999). Specifically, TNF-α has been shown to stimulate mitochondrial electron transport chain (ETC) to produce superoxide radical (\(O_{2}^{\cdot -}\)) and H2O2, and to increase member-borne NADPH oxidase activity (Li et al. 2005). ROS can activate NFκB, mitogen-activated protein kinase (MAPK), and the heat shock response, which regulate the expression of other pro-inflammatory cytokines such as IL-1 and IL-6 forming a self-propelled vicious cycle. Elected plasma TNF-α also underlie the etiology of pathogeneses such as chronic infection, congestive heart failure, and cancer cachexia (Costelli et al. 1993). Our observation that plasma TNF-α concentration was decreased after the 8-week dietary regimen appeared consistent with the in vitro data reported by Guo et al. (2008) that AVA could inhibit IL-1β-induced TNF-α expression in endothelial cells. However, caution must be exercised to suggest that AVA supplementation inhibits TNF-α production in vivo, because repeated eccentric exercise is known to reduce certain blood stress markers. Another possible interpretation is that oat antioxidants unrelated to AVA might have exerted an inhibitory effect on blood TNF-α.
The source of elevated plasma TNF-α could not be confirmed at present but skeletal muscle has been considered a main production site when it performs demanding eccentric contraction (Liao et al. 2010; Nieman et al. 2001). A second source of TNF-α could be the plasma membrane of endothelial cells wherein NADPH oxidase complex is activated to produce ROS, and hence stimulates TNF-α expression via NFκB signaling (Frey et al. 2002). It is conceivable that inhibiting NFκB may represent a major cellular mechanism by which AVA reduces TNF-α production from both muscle cells and blood vessels in the damaging site.
After dietary oat supplementation, high-AVA group showed significantly lower IL-6 levels immediately and 24 h post-DR. IL-6 is considered a pleiotropic cytokine linked to not only pro- but also anti-inflammatory responses, such as suppressing TNF-α and IL-1β secretion in cultured monocytes (Tilg et al. 1994). Our finding that AVA reduces plasma IL-6 suggests that plasma inflammatory stimulus to IL-6 production was attenuated by AVA. However, plasma IL-1β concentration was unaltered by DR, which was consistent with several other reported studies (Bruunsgaard et al. 1997; Hirose et al. 2004). Taken together, our data provided new evidence that long-term dietary intake of AVA at the given dose can attenuate selective pro-inflammatory cytokine production caused by eccentric muscular contraction. However, whether or not people chronically consuming large dose of AVA in diet would suffer from potential negative effects, as has been reported with high dose of antioxidant supplementation, remains unknown.
Previous research has provided strong evidence that high dose of antioxidant supplementation may interfere with cellular redox signaling thereby compromising training adaptation of mitochondrial and antioxidant enzymes (Ristow et al. 2009; Gomez-Cabrera et al. 2008). In the current study, although plasma TAC was significantly elevated by high-AVA consumption, there was no sign of adverse effect resulting from dietary AVA supplementation. Erythrocyte SOD activity was not affected whereas GPx activity was decreased immediately post-DR. The main function of GPx in the red cell is to defend against H2O2 formed from SOD-catalyzed \(O_{2}^{\cdot -}\) dismutation, and its activity is one of the highest among all body tissues. A reduction of GPx activity might indicate a lower H2O2 production or lipid peroxide formation from lipid membrane of the red cell.
Eccentric exercise has been shown to induce disturbances to muscle glutathione homeostasis due to ROS generation and oxidation of GSH to GSSG, and increased export of GSSG into the blood circulation (Zembron-Lacny et al. 2010; Goldfarb et al. 2011). In the present study, resting plasma GSH content was elevated whereas GSSG response to DR was blunted in high-AVA group, which also showed a higher GSH:GSSG ratio 24 h post-DR. These protective effects caused by AVA consumption have never been reported and the reason is unknown. Since glutamyl-cysteine ligase (GCL), the rate-limiting step of GSH synthesis, is strongly inhibited by TNF-α (Rahman et al. 1999), it is possible that reduced plasma TNF-α level seen after AVA supplementation could lessen GCL inhibition and hence allowed for a greater hepatic GSH output during DR.
Previous animal and clinical studies indicated that AVA is bioavailable to body tissues after being orally ingested. For example, when synthetic AVA (6 mg/100 g body weight) was gavage-fed to rats, a maximum combined plasma AVA (a, b and c) concentration (C max) of 5.5 nmol/L was reached at 2 h post ingestion and an accumulation of AVA in liver, heart and skeletal muscle was observed at 2–12 h (Koenig et al. 2011). In human, after 60 and 120 mg of AVA enriched mixture was consumed, Cmax of total plasma AVA was reported to be 168 and 560 nmol/L, respectively, at 2 h and elevated AVA in plasma lasted for 10 h (Chen et al. 2007). The AVA dose in the current study at 9.2 mg/day was considerably lower compared with previous human or animal studies based on unit of body weight. However, high-AVA supplementation still increased plasma TAC and reduced selective inflammation and oxidative stress markers by DR, rendering promises for the efficacy of AVA supplementation.
In summary, our data indicate that a diet containing high daily dose of AVA may provide some protection to woman subjects who participate in eccentric muscle contraction that elicits an inflammatory response. Based on the current experimental design, some of the protective effects, such as reduced plasma CK activity and dose-unrelated TNF-α reduction, could result from the repeated measure effect, an adaptive muscle response previously documented. However, other effects such as DR-induced reduction of neutrophil respiratory burst, NFκB activation, plasma IL-6 concentration, and erythrocyte GPx activity, as well as a more reduced plasma glutathione status, were only observed in young women supplemented with high-AVA. These specific antioxidant and anti-inflammatory properties of AVA demonstrated in this study may have potential merit to consider AVA as a dietary supplement.
Abbreviations
- AVA:
-
Avenanthramides
- BMI:
-
Body mass index
- CK:
-
Ceatine kinase
- CRP:
-
C-reactive protein
- DR:
-
Downhill running
- GSH:
-
Glutathione
- GSSG:
-
Glutathione disulfide
- GPx:
-
Glutathione peroxidase
- IL:
-
Interleukin
- (NF) κB:
-
Nuclear factor-kappaB
- NRB:
-
Neutrophil respiratory burst
- ROS:
-
Reactive oxygen species
- SOD:
-
Superoxide dismutase
- TAC:
-
Total antioxidant capacity
- TNF-α:
-
Tumor necrosis factor-α
References
Benbarek H, Deby-Dupont G, Deby C, Caudron I, Mathy-Hartert M, Lamy M, Serteyn D (1996) Experimental model for the study by chemiluminescence of the activation of isolated equine leucocytes. Res Vet Sci 61:59–64
Bruunsgaard H, Galbo H, Halkjaer-Kristensen J, Johansen TL, MacLean DA, Pedersen BK (1997) Exercise-induced increase in serum interleukin-6 in humans is related to muscle damage. J Physiol 99:833–841
Chen CY, Milbury PE, Kwak HK, Collins FW, Samuel P, Blumberg JB (2004) Avenanthramides and phenolic acids from oats are bioavailable and act synergistically with vitamin C to enhance hamster and human LDL resistance to oxidation. J Nutr 143:1459–1466
Chen CY, Milbury PE, Collins FW, Blumberg JB (2007) Avenanthramides are bioavailable and have antioxidant activity in humans after acute consumption of an enriched mixture from oats. J Nutr 137:1375–1382
Collins FW (1989) Oat phenolics: avenanthramides, novel substituted N-cinnamoylanthranilate alkaloids from oat groats and hulls. J Agric Food Chem 37:60–66
Costelli P, Carbó N, Tessitore L, Bagby GJ, Lopez-Soriano FJ, Argilés JM, Baccino FM (1993) Tumor necrosis factor-alpha mediates changes in tissue protein turnover in a rat cancer cachexia model. J Clin Invest 92:2783–2789
Dimberg LH, Sunnerheim K, Sundberg B, Walsh K (2001) Stability of oat avenanthramides. Cereal Chem 78:278–281
Flohe L, Gunzler WA (1984) Assays of glutathione peroxidase. Methods Enzymol 105:114–121
Frey RS, Rahman A, Kefer JC, Minshall RD, Malik AB (2002) PKCzeta regulates TNF-alpha-induced activation of NADPH oxidase in endothelial cells. Circ Res 90:1012–1019
Goldfarb AH, Garten RS, Cho C, Chee PD, Chambers LA (2011) Effects of a fruit/berry/vegetable supplement on muscle function and oxidative stress. Med Sci Sports Exerc 43:501–508
Gomez-Cabrera MC, Domenech E, Romagnoli M, Arduini A, Borras C, Pallardo FV, Sastre J, Viña J (2008) Oral administration of vitamin C decreases muscle mitochondrial biogenesis and hampers training-induced adaptations in endurance performance. Am J Clin Nutr 87:142–149
Guo W, Wise ML, Collins FW, Meydani M (2008) Avenanthramides, polyphenols from oats, inhibit IL-1β-induced NF-κB activation in endothelial cells. Free Radic Biol Med 44:415–429
Hamada K, Vannier E, Sacheck JM, Witsell AL, Roubenoff R (2005) Senescence of human skeletal muscle impairs the local inflammatory cytokine response to acute eccentric exercise. FASEB J 19:264–266
Hirose L, Nosaka K, Newton M, Laveder A, Kano M, Peake J, Suzuki K (2004) Changes in inflammatory mediators following eccentric exercise of the elbow flexors. Exerc Immunol Rev 10:75–90
Isaji M, Miyata H, Ajisawa Y, Yoshimura N (1998) Inhibition by tranilast of vascular endothelial growth factor (VEGF)/vascular permeability factor (VPF)-induced increase in vascular permeability in rats. Life Sci 63:PL71–PL74
Ji LL, Fu R (1992) Responses of glutathione system and antioxidant enzymes to exhaustive exercise and hydroperoxide. J Appl Physiol 72:549–554
Ji LL, Lay D, Chung E, Fu Y, Parkin K, Peterson DM (2003) Effects of avenanthramides on oxidant and antioxidant status in exercised rats. Nutr Res 23:1579–1590
Ji LL, Gomez-Cabrera MC, Vina J (2009) Role of antioxidants in muscle health and pathology. Infectious disorders special issue. Infect Disord Drug Targets 9:428–444
Koenig R, Dickman JR, Ji LL (2011) Avenanthramides are bioavailable and accumulate in hepatic, cardiac, and skeletal muscle tissue following oral gavage in rats. J Agr Food Chem 59:6438–6443
Koenig R, Dickman JR, Kang C, Zhang T, Chu Y-F, Ji LL (2014) Avenanthramide supplementation attenuates exercise-induced inflammation in postmenopausal women. Nutr J 13:21–31
Li YP, Atkins CM, Sweatt JD, Reid MB (1999) Mitochondria mediate tumor necrosis factor-alpha/NF-kappaB signaling in skeletal muscle myotubes. Antioxid Redox Signal 1:97–104
Li JM, Fan LM, Christie MR, Shah AM (2005) Acute tumor necrosis factor alpha signaling via NADPH oxidase in microvascular endothelial cells: role of p47phox phosphorylation and binding to TRAF4. Mol Cell Biol 25:2320–2330
Liao P, Zhou J, Ji LL, Zhang Y (2010) Eccentric contraction induces inflammatory responses in rat skeletal muscle: role of tumor necrosis factor-α. Am J Physiol Regul Integr 298:R599–R607
Liu L, Zubik L, Collins FW, Marko M, Meydani M (2004) The antiatherogenic potential of oat phenolic compounds. Atherosclerosis 175:39–49
Nie L, Wise ML, Peterson DM, Meydani M (2006a) Avenanthramide, a polyphenol from oats, inhibits vascular smooth muscle cell proliferation and enhances nitric oxide production. Atherosclerosis 186:260–266
Nie L, Wise M, Peterson D, Meydani M (2006b) Mechanism by which avenanthramide-c, a polyphenol of oats, blocks cell cycle progression in vascular smooth muscle cells. Free Radic Biol Med 41:702–708
Nieman DC, Henson DA, Smith LL, Utter AC, Vinci DM, Davis JM, Kaminsky DE, Shute M (2001) Cytokine changes after a marathon race. J Appl Physiol 91:109–114
Pedersen BK, Steensber A, Schjerling P (2001) Muscle-derived interleukin-6: possible biological effects. J Physiol 536:329–337
Peterson DM, Hahn MJ, Emmons CL (2002) Oat avenanthramides exhibit antioxidant activities in vitro. Food Chem 79:473–478
Proske U, Allen TJ (2005) Damage to skeletal muscle from eccentric exercise. Exerc Sport Sci Rev 333:98–104
Rahman I, Antonicelli F, MacNee W (1999) Molecular mechanism of the regulation of glutathione synthesis by tumor necrosis factor-α and dexamethasone in human alveolar epithelial cells. J Biol Chem 274:5088–5096
Re R, Pellegrini N, Proteggente A, Pannala A, Yang M, Rice-Evans C (1999) Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic Biol Med 26:1231–1237
Ristow M, Zarse K, Oberbach A, Klöting N, Birringer M, Kiehntopf M, Stumvoll M, Kahn CR, Blüher M (2009) Antioxidants prevent health-promoting effects of physical exercise in humans. Proc Natl Acad Sci USA 106:8665–8670
Sun M, Zigman S (1978) An improved spectrophotometric assay for superoxide dismutase based on epinephrine autoxidation. Anal Biochem 90:81–89
Sur R, Nigam A, Grote D, Liebel F, Southall MD (2008) Avenanthramides, polyphenols from oats, exhibit anti-inflammatory and anti-itch activity. Arch Dermatol Res 300:569–574
Tanzer ML, Gilvarg C (1959) Creatine and creatine kinase measurement. J Biol Chem 234:3201–3204
Tilg H, Trehu E, Atkins MB, Dinarello CA, Mier JW (1994) Interleukin-6 (IL-6) as an anti-inflammatory cytokine: induction of circulating IL-1 receptor antagonist and soluble tumor necrosis factor receptor p55. Blood 83:113–118
Zembron-Lacny A, Naczk M, Gajewski M, Ostapiuk-Karolczuk J, Dziewiecka H, Kasperska A, Szyszka K (2010) Changes of muscle-derived cytokines in relation to thiol redox status and reactive oxygen and nitrogen species. Physiol Res 59:945–951
Acknowledgments
R.K. and L.L.J. designed research; R.K., J.R.D. and C.K. conducted research; R.K. analyzed data; R.K., C.K., Y.C., and L.L.J. wrote the paper; T.O.Z. formatted the paper; L.L.J. had primary responsibility for final content. All authors read and approved the final manuscript. This research was supported by a grant from the University of Wisconsin Foundation.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Fabio Fischetti.
Rights and permissions
About this article
Cite this article
Koenig, R.T., Dickman, J.R., Kang, CH. et al. Avenanthramide supplementation attenuates eccentric exercise-inflicted blood inflammatory markers in women. Eur J Appl Physiol 116, 67–76 (2016). https://doi.org/10.1007/s00421-015-3244-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00421-015-3244-3