Abstract
The aim of the study was to determine the effects of an antioxidant supplementation, which includes coenzyme Q10, on plasma and neutrophil oxidative stress and the antioxidant response after a soccer match. Nineteen voluntary male pre-professional footballers were randomly and double-blinded treated with either a multivitamin and mineral supplement (n = 8) or a placebo (n = 11). After the 3 months of supplementation, the sportsmen played a friendly soccer match of 60 min. The 3-month supplementation induced higher plasma ascorbate and coenzyme Q levels when compared to the placebo group. Antioxidant supplementation influenced plasma oxidative stress markers because they were lower in the supplemented group than in the placebo one after the match. The football match induced decreased neutrophil vitamin E levels and catalase and glutathione peroxidase activities but increased glutathione reductase activity. Antioxidant diet supplementation prevented plasma oxidative damage but did not influence the neutrophil response to a football match.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Coenzyme Q10 is an endogenous enzyme cofactor that is produced in all living cells in humans. It functions as a catalyst in proton/electron translocation in mitochondria and lysosomes, protects mitochondria from free radical damage (Lass and Sohal 2000) and is thought to be capable of preventing programmed cell death or apoptosis (Kagan et al. 1999). Furthermore, coenzyme Q10 has a primarily function as antioxidant and is carried mainly by lipoproteins in the circulation (Alleva et al. 1997). Recent evidence has indicated that coenzyme Q10 may recycle α-tocopherol (Lass and Sohal 2000) and ascorbate (Crane 2001), may prevent prooxidant effects of α-tocopherol (Thomas et al. 1996), and may provide lipoproteins with increased resistance to oxidation. Cell signalling and gene expression have also been described as potential functions of coenzyme Q10 (Crane 2001).
Dietary coenzyme Q10 supplements contain also vitamins as vitamin E, ascorbate, and riboflavin, and some oil to facilitate the bioavailability of liposoluble compounds. Potential benefits of coenzyme Q10 supplementation have been recognised with particular reference to cardiovascular and neurodegenerative diseases (Langsjoen and Langsjoen 1999; Overvad et al. 1999).
Exhaustive exercise induces oxidative stress and it may impair immune response (Nieman 1994). Exercise-related immunological acute changes include release of cytokines (Petersen et al. 2001), activation of immunocompetent cell lines (Suzuki et al. 1999), neutrophil priming for acute phase response (Cannon and Blumberg 2000; Suzuki et al. 1999) and lower antioxidant enzyme levels in neutrophils (Tauler et al. 2002a). The effects of nutritional antioxidants on the endogenous antioxidant response to oxidative stress as well as on exercised-induced acute changes in immune cell function have been pointed out (Krause et al. 2001; Morante et al. 2005; Sastre et al. 1992; Tauler et al. 2003a, b). Recent data indicate that contraction-induced ROS modulates at least some of the adaptative responses that occur in skeletal muscle following contractile activity (McArdle et al. 2005). This process involves activation of redox regulated transcription factors, such as NF-κΒ, leading to an increase of the expression of enzymes such as iNOS, NOS and Mn-SOD in response to intense exercise (Cases et al. 2006; Gomez-Cabrera et al. 2005; McArdle et al. 2005). High-dose antioxidant supplementation could prevent this antioxidant endogenous response to oxidative stress (Gomez-Cabrera et al. 2005). However, it is important to maintain an adequate vitamin E consumption not only to prevent liver oxidative damage but also because vitamin E plays an essential role modulating signal transduction (Morante et al. 2005). Furthermore, we have previously evidenced enhanced basal antioxidant enzyme activities in neutrophils after 3 months of antioxidant supplementation (Tauler et al. 2002b).
Because of the interest in using antioxidant nutrients as a preventive and therapeutic tool in clinical medicine and in physical activity, the aim of this study was to determine the effects of an antioxidant cocktail supplementation, which includes coenzyme Q10, on plasma and neutrophil oxidative stress markers and on the antioxidant response after a football match. The influence of the 3-month supplementation on the basal levels of plasma and neutrophil antioxidant defences as well as on the basal oxidative stress markers was also analysed. In order to ensure an adequate intake of other vitamins and minerals we used capsules containing a multivitamin and mineral supplement. The use of capsules allows us to provide together adequate amounts of several vitamins and minerals, which could be very difficult by means of a simple diet manipulation.
Materials and methods
Subjects and protocol
Nineteen voluntary male pre-professional footballers participated in this study. All the subjects were informed of the purpose and demands of the study before giving their written consent to participate. The protocol was in accordance with the Declaration of Helsinki for research on human subjects and was approved by the Ethical Committee of Clinical Investigation of CAR-Sant Cugat (Barcelona). The mean weight of the sportsmen was 75.2 ± 1.3 kg, height 178 ± 5 cm and VO2 max 56.6 ± 3.4 mL kg−1 min−1.
The 19 football players were randomly and double-blinded treated with either a multivitamin and mineral supplement or placebo. Compositions of the supplement are shown in Table 1. The dose of antioxidants used was the same as their RDA (when established). The eight players of the supplemented group took a capsule containing coenzyme Q and a tablet containing minerals and vitamins (Pharma Nord, Denmark) for 90 days. The 11 players of the placebo group took the same capsules and tablets as the supplemented group, but containing the placebo (Pharma Nord, Denmark). In order to ensure the compliance tablets and capsules were administered directly by one of the researchers on a daily basis. The supplement ensured the recommended allowance intake of vitamins C, E, D3, folate, biotin, pantotenic acid, niacin, cobalamin, piridoxin and the minerals Se, Zn, Cu, Mg and Mn.
Determinations of basal haematological parameters, antioxidant enzyme activities, antioxidant vitamin levels and oxidative stress markers were made before and after the 3 months of supplementation. After the 3 months of supplementation, the sportsmen played a friendly football match (60 min), and samples were taken to determine the same parameters before and after the match. The goal-keeper did not participate in the study.
The soccer players were monitored using a pulsometer during the match. As the cardiac heart rate increases linearly to the oxygen consumption (Karvonen and Vuorimaa 1988) we can indirectly evaluate the work done during a maximal and intervallic exercise through the heart rate (Balsom et al. 1992). The relationship between the power output, the heart rate and the oxygen uptake is linear not only for the maximal values but also for the percentual, and both can be used to monitor the training sessions (Arts and Kuipers 1994). We categorised the subjects under the perspective of the work performed during the training sessions and the competition in relation to the reference values of a progressive and maximal exercise test. Five metabolic zones are usually considered. From zone one (Z1) to zone five (Z5), the relationships to the maximal oxygen consumption were, respectively <70, 70–80, 80–90, 90–100 and 100% or higher. The pulsometer allowed to obtain the heart rate each time, which enabled us to calculate the time in which each individual worked at each intensity (zone).
Blood sampling
Blood samples were obtained from the antecubital vein of sportsmen after overnight fasting in suitable vacutainers with EDTA as anticoagulant. Neutrophils were purified following an adaptation of the method described by Boyum (1964). Blood was centrifuged at 900×g, at 4°C for 30 min after carefully introducing on Ficoll in a proportion of 1.5:1. The precipitate containing erythrocytes and neutrophils was incubated with ammonium chloride 0.15 M at 4°C to haemolyse erythrocytes. The suspension was centrifuged at 750×g, 4°C for 15 min and the supernatant was then discarded. The neutrophil phase at the bottom was washed first with ammonium chloride 0.15 M and then with PBS. Finally, neutrophils were lysed with distilled water. Neutrophil number was quantified in an automatic flow cytometer analyser Techicon H2 (Bayer) VCS system.
Plasma was obtained after centrifugation (30 min, 1,000g, 4°C) of another blood sample obtained as above.
Enzymatic determinations
Catalase, glutathione peroxidase, glutathione reductase and superoxide dismutase activities were determined in neutrophils using a Shimadzu UV-2100 spectrophotometer at 37°C.
Catalase activity was measured by the spectrophotometric method of Aebi (1984) based on the decomposition of H2O2. Glutathione reductase activity was measured by a modification of the Goldberg and Spooner (1985) spectrophotometric method. Glutathione peroxidase (GPx) activity was measured by an adaptation of the spectrophotometric method of Flohé and Gunzler using H2O2 as the substrate (Flohe and Gunzler 1984). SOD activity was measured by an adaptation of the method of McCord and Fridovich (1969).
Plasma vitamins, carotenes and coenzyme Q determination
Vitamin E was determined in plasma and neutrophils. Carotenes were determined in plasma. The deep-frozen plasma or neutrophil suspensions were thawed and mixed to disperse possible precipitates. The extraction of liposoluble vitamins and carotenoids was carried out using n-hexane after deproteinisation with ethanol containing 0.2% BHT. Liposoluble vitamins and carotenoids were determined by HPLC in the n-hexane extract of plasma after drying under a nitrogen current and redissolving in ethanol. The mobile phase consisted of 550:370:80 acetonitrile:tetrahydrofuran:H2O. The HPLC was a Shimadzu with a diode array detector and the column was a Nova Pak, C18, 3.9 × 150 mm. α-tocopherol was determined at 290 nm. β-carotene and lycopene were determined at 460 and 470 nm, respectively.
Coenzyme Q was determined in another plasma extract obtained as above following an adaptation of a HPLC method previously described (Podda et al. 1999). A gradient is used consisting of a mixture of 31.7 mM ammonium formate in 80:20 methanol:H2O and 31.7 mM ammonium formate in ethanol. The HPLC system was a Shimadzu with a diode array detector and a Nova Pak, C18, 3.9 × 300 mm column. Due to the sample treatment and storage conditions all the coenzyme Q was determined as ubiquinone at 275 nm.
Plasma and neutrophil ascorbate were determined by an HPLC method with electrochemical detection (Tsao and Salimi 1982) after deproteinisation with ortho-phosphoric acid. The mobile phase consisted of 0.05 M sodium phosphate, 0.05 M sodium acetate, 189 μM dodecyltrimethylammonium chloride and 36.6 μM tetraoctylammonium bromide in 25:75 methanol: H2O, pH 4.8. The HPLC system was a Shimadzu with a Waters Inc. electrochemical detector and a Nova Pak, C18, 3.9 × 300 mm column. The potential of the chromatographic detector was set at 0.7 V versus an Ag/AgCl reference electrode.
MDA determination
The MDA as a marker of lipid peroxidation was analysed in plasma and neutrophils by a colorimetric assay kit (Calbiochem, San Diego, CA, USA). The method used is specific for MDA determination.
Carbonyl derivatives determination
Protein carbonyl derivatives were measured in plasma by an adaptation of the method of Levine et al. (1994). After deproteinising the samples with trichloroacetic acid, precipitates were resuspended with 2,4-dinitrophenylhydrazine (DNPH) 10 mM, and incubated for 60 min at 37°C. Then, samples were precipitated with 20% trichloroacetic acid and centrifuged for 10 min at 1,000g and 4°C. The precipitate was washed twice with ethanol:ethyl acetate (1:1) to remove free DNPH. Guanidine 6 M in phosphate buffer 2 mM, pH 2.3 was added to the precipitate, and samples were incubated for 40 min at 37°C. Finally, samples were centrifuged for 5 min at 3,000g at 4°C to clarify the supernatant and the absorbance was measured at 360 nm.
Statistical analysis
Statistical analysis was carried out by using a statistical package for social sciences (SPSS 10 for Windows). Results are expressed as means ± s.e.m. and p < 0.05 was considered statistically significant. All the data were tested for homogeneity of variance. Student’s t test for unpaired data was used to identify differences in the physical activity performed during the soccer match (Table 2). The effects of the antioxidant supplementation on the changes induced by the football match were tested by a two-way ANOVA with antioxidant diet supplementation (S) and the football match (M) as ANOVA factors. The effects of the antioxidant supplementation and the training season on the basal parameters were also tested using a two-way ANOVA with antioxidant diet supplementation (S) and the 3 months of training (T) as factors. The sets of data in which there were significant effects were tested by the ANOVA one-way test.
Results
The physical activity performed by the football players during the match was determined (Table 2). No differences were observed during the match between the placebo and the supplemented group in the energy consumed, the mean cardiac frequency and the time expended in each exercise intensity zone (from Z1 to Z5). Furthermore, no difference was found in the time expended at 80–100% VO2 max (the anaerobic metabolism zones, Z3–Z5) which was about 70% in both groups. These observations allowed us to compare the results obtained in the placebo and in the supplemented groups.
Table 3 shows the effect of diet supplementation on the plasma and neutrophil basal levels of antioxidant metabolites. The 3-month supplementation induced higher basal plasmatic levels of coenzyme Q (29%) in the supplemented group than in the placebo one. No significant changes were observed in the basal plasmatic levels of vitamin E, carotene and lycopene.
The ANOVA analysis of the plasmatic antioxidant levels before and after the match (Table 4) revealed a significant effect of the supplementation on the ascorbate and coenzyme Q levels. As a consequence of the supplementation, the basal coenzyme Q levels in the supplemented group were higher than in the placebo one. On the other hand, the non-significant increases observed after the game in the ascorbate levels induced higher final levels in the supplemented group than in the placebo one.
A significant effect of the time factor was observed in the basal neutrophil ascorbate levels (Table 3). Significant decreases were observed after the 3 months of study both in the placebo (28%) and in the supplemented (16%) groups. The basal neutrophil vitamin E concentrations did not change throughout the study. However, the match induced significant decreases in neutrophil vitamin E in the placebo and in the supplemented groups (Table 3).
Basal neutrophil counts are maintained during the 3 months of training and competition (Table 5). When changes in the basal neutrophil antioxidant enzyme activities were analysed, a significant effect of the time factor was observed on the glutathione reductase activity, decreasing this activity in both groups at the end of the study. Basal catalase and glutathione peroxidase activities did not change along the study.
The football match performed after the supplementation induced higher neutrophil counts (Table 6). The circulating number of neutrophils increased about 67% in the placebo group and about 85% in the supplemented one. The football match influenced the neutrophil activities of catalase, glutathione peroxidase and glutathione reductase. Catalase and glutathione peroxidase activities decreased significantly in both placebo and supplemented groups. An increase in the neutrophil glutathione reductase activity was observed after the football game. This increase was significant only in the placebo group. SOD activity did not change along the study.
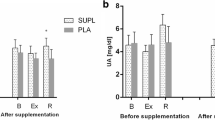
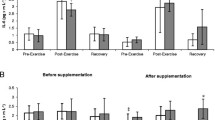
Figure 1 shows the basal MDA levels in plasma and neutrophils before and after the supplementation period. Basal plasma MDA levels increased both in the placebo (165%) and in the supplemented (189%) groups at the end of the study. The supplementation did not influence these basal plasma MDA levels because they were similar in both groups after the 3 months of study. When the changes in plasma MDA levels during the football match were analysed (Fig. 2) a significant effect of the supplementation was found: the values after the match were higher (100%) in the placebo than in the supplemented group. Neutrophil MDA levels did not change throughout the study.
Effects of the antioxidant supplementation on basal plasma and neutrophil MDA levels. Two way ANOVA. S supplementation; T time (3 months supplementation). S × T supplementation time interaction. Hash symbol indicates significant differences placebo versus supplemented; ambersand indicates significant differences initial versus final
Effects of a football match and of the antioxidant supplementation of the diet on the oxidative damage markers in plasma and neutrophils. Two way ANOVA. S supplementation; T time (3 months supplementation). S × T supplementation time interaction. Hash symbol indicates significant differences placebo versus supplemented; ambersand indicates significant differences initial versus final
Plasma protein carbonyl derivatives, as oxidative stress marker, were also measured before and after the football match (Fig. 2). The supplementation did not induce any differences in the pre-match protein carbonyl concentrations. However, concentrations were significantly lower in the supplemented group than in the placebo one after the match.
Discussion
Plasma antioxidant vitamins and other nutrient levels of all sportsmen participating in the study were within the range of well-nourished people (Tauler et al. 2002b). Diet supplementation with the antioxidant cocktail induced a real increase in the plasma levels of coenzyme Q. Under normal conditions plasma coenzyme Q concentrations are not significantly affected by dietary components such as dairy products, eggs, fish and vegetables. The nutritional habits of the football players participating in the study did not influence the basal levels of antioxidant nutrients in plasma. Coenzyme Q supplementation leads to increases in plasma coenzyme Q concentrations, the extent of which depends upon the dosage, duration and also the type of formulation (Bhagavan and Chopra 2006). The controlled trials seems to report a dose-dependent increase in plasma coenzyme Q in function of the chronic daily dose of coenzyme Q administered up to the daily dose of 200 mg (Bhagavan and Chopra 2006). Sportsmen in the present study took a 100 mg/day dose of coenzyme Q, but plasma concentration increased only about 25%; this increase is lower than those described by others (Kaikkonen et al. 2002; Niklowitz et al. 2004). However, and in accordance with the lower increases in other antioxidant nutrients as vitamin E and vitamin C observed after their supplementation (Tauler et al. 2002b), sportsmen present higher resistance to increase plasma coenzyme Q10 concentration than general population. It has been postulated that the reduced form of coenzyme Q10, together with α-tocopherol, prevents lipid peroxidation in plasma lipoproteins and biological membranes (Ernster and Forsmark-Andree 1993). The lower increase in coenzyme Q10 observed in plasma of sportsmen could indicate higher coenzyme Q10 cellular utilization by sportsmen than by general population.
The training and competition sessions resulted in increased basal oxidative stress as indicated by the increased MDA plasma levels after the 3 months of study. The antioxidant supplementation did not prevent this basal increased oxidative stress because similar increases were observed in both groups. It has been previously reported that an antioxidant supplementation with vitamin C, E and β-carotene decreased the lipoperoxide levels in basketball players (Schroder et al. 2000). Differences in the supplementation, in the oxidative stress markers analysed and in the competition and training sessions developed by the sportsmen could explain the differences in the results obtained. The molecular damage produced by ROS is parallel to the activation of the endogenous antioxidant defences (Gomez-Cabrera et al. 2005; Jackson 1999; Sureda et al. 2005). In a similar way, free radicals could be involved in the muscle adaptations to exercise in skeletal muscle; some ROS production is needed to attain optimal muscular isometric force production (Reid 2001). The basal plasma molecular damage increased during the study; this increase could be related with the muscle adaptations to exercise mediated by ROS. The surplus intake of antioxidants with the supplement did not influence the adaptations to exercise.
DT-diaphorase (NAD(P)H: quinone acceptor oxidoreductase) is an inducible antioxidant enzyme that maintains the reduced antioxidant form of coenzyme Q10 in membrane systems and to protect against xenobiotics which could generate ROS (Radak et al. 2000). DT-diaphorase activity increased in response to regular exercise in rats (Radak et al. 1999, 2000) and in response to chronic administration of hydrogen peroxide (Radak et al. 2000). In the present study DT-diaphorase activity has not been determined. Recent studies reported that antioxidant supplementation could prevent endogenous antioxidant adaptations to increased ROS production (Gomez-Cabrera et al. 2005). However, it has been also indicated that molecular damage produced by ROS is parallel to the activation of the endogenous antioxidant defences (Gomez-Cabrera et al. 2005; Jackson 1999; Sureda et al. 2005). Because a similar increase in basal plasma MDA levels had been found in both groups after the 3 months of supplementation in the present study, we could suppose that DT-diaphorase activity could be increased in both groups in parallel to increased MDA levels as a result of regular exercise as it has been indicated previously (Radak et al. 1999, 2000). However, additional studies are necessary in order to determine the effects of a supplementation with coenzyme Q on DT-diaphorase activity.
Increases in plasma MDA levels after exercise are widely shown in the literature (Miyazaki et al. 2001; Tauler et al. 2006). We appreciate a high variability in the post-exercise MDA values between sportsmen, probably because the intensity of the exercise developed by the football players depends on their position. The maintenance of protein carbonyl derivatives after the football match is in agreement with the findings of others (Miyazaki et al. 2001). A strong correlation between serum and urinary protein carbonyl derivatives was found in a previous study (Radak et al. 2003), suggesting that the filtration of carbonylated proteins could prevent its accumulation in plasma. Furthermore, results obtained in the present study revealed an influence of antioxidant levels because carbonyl derivatives were lower in the supplemented group than in the placebo one after the match. In fact, antioxidant supplementation influenced not only carbonyl derivative but also MDA levels in plasma. Taken together, we can suggest that even with low values of oxidative stress markers, the moderate antioxidant supplementation induced beneficial effects as indicated by the lower post-exercise MDA and carbonyl plasma levels in the supplemented group.
The football match decreased the neutrophil α-tocopherol but maintained the plasma concentrations in both groups. Changes in neutrophil tocopherol levels could be related with its availability but also with the exercise-induced oxidative stress. Previous studies have shown increases in neutrophil tocopherol after an acute bout of exercise, especially in supplemented sportsmen, but related to increases in plasma tocopherol (Cases et al. 2005). Increases in plasma tocopherol are, in turn, related with the VLDL output from the liver and with the triglyceride mobilisation when the intensity and duration of the exercise activate these processes (Aguilo et al. 2005). Higher oxidative stress levels or longer exercises could activate mechanisms leading to increased neutrophil vitamin E levels. As far as we know mechanisms underlying the neutrophil vitamin E uptake are not well known. Decreased vitamin E levels could be related to the maintenance of MDA levels in neutrophils. Vitamin E could play an essential role in neutrophils preventing higher oxidative damage as it is indicated by the lack of increases in MDA levels. It seems that neutrophil antioxidant defences are effective preventing exercise-induced oxidative damage because no changes in oxidative stress markers have been reported after several exercises (Sureda et al. 2005).
Interactions between neutrophil antioxidant enzyme activities and training but also with antioxidant supplementations have been previously reported (Tauler et al. 2002b). A significant decrease was observed in the basal neutrophil glutathione reductase activity during the study. We previously reported a significant decrease in neutrophil glutathione reductase activity after 3 months of training and competitions (Tauler et al. 2002b). However, this activity decreased only in the placebo group whereas it was maintained in the antioxidant supplemented one (Tauler et al. 2002b). In the present study the moderate antioxidant supplementation did not prevent the decrease in glutathione reductase activity. Decreases in glutathione reductase activities have been related to increased requirements of riboflavin in trained sportsmen (Ohno et al. 1988), because this enzyme is highly dependent on this vitamin. This is rather unlikely in this study because we ensured that the demands of riboflavin were covered with the supplementation. Thus, other causes such as oxidative modifications in the enzymatic protein should be considered as the main factor inducing the activity decrease (Tauler et al. 2002b).
Exercise induces an acute phase immune response (APIR) similar to the one induced by an infection (Cannon and Blumberg 2000). Increased neutrophil circulating counts as well as decreased neutrophil antioxidant enzyme activities (Tauler et al. 2002a) have been reported during this APIR. The football match induced an APIR but not as important as the ones observed after more intense exercises (Tauler et al. 2002a) as it is indicated by the slight changes observed in parameters such as the neutrophil number. The antioxidant diet supplementation influenced the APIR induced by exercise, producing higher decreases of antioxidant enzyme activities in neutrophils (Tauler et al. 2003b). However, the moderate antioxidant diet supplementation in this study did not influence neutrophil antioxidant response to the exercise because similar pictures were observed after the match in both the placebo and the supplemented groups. Catalase and glutathione peroxidase, the hydrogen peroxide removing enzymes, decreased their activities after the football match. However, glutathione reductase increased its activity, indicating enhanced glutathione regeneration in neutrophils after the football match. This increased glutathione reductase activity after the match could evidence a good availability of riboflavin; then, the basal decrease in this enzyme activity after the period of training and competition must be attributed to decreased enzyme levels. The increased glutathione reductase and the decreased glutathione peroxidase activities after the match could indicate that this enhanced glutathione regeneration is not induced by a higher rate in its consumption to remove H2O2, suggesting another role for glutathione in the neutrophil antioxidant defence during exercise. This role could be related to the recycling of ascorbate from its oxidised form, dehydroascorbate. Neutrophils preferentially uptake dehydroascorbate and then it is reduced to ascorbate by both glutathione-dependent and glutathione-independent systems (Welch et al. 1995). Then, the maintenance of neutrophil ascorbate concentration after the football match is in accordance with this potential role for glutathione and with the increased glutathione reductase activity.
In summary, the moderate antioxidant supplementation of the diet for 3 months using a multivitamin and mineral cocktail prevented the plasma oxidative molecular damage induced by a football match without influencing the antioxidant adaptations induced by exercise. The supplementation did not influence the antioxidant response or the oxidative stress makers in neutrophils.
References
Aebi HE (1984) Catalase. In: Bergmeyer HU (ed) Methods in enzymatic analysis. Verlag Chemie, Basel, pp 273–286
Aguilo A, Tauler P, Fuentespina E, Tur JA, Cordova A, Pons A (2005) Antioxidant response to oxidative stress induced by exhaustive exercise. Physiol Behav 84:1–7. doi:10.1016/j.physbeh.2004.07.034
Alleva R, Tomasetti M, Bompadre S, Littarru GP (1997) Oxidation of LDL and their subfractions: kinetic aspects and CoQ10 content. Mol Aspects Med 18(Suppl):S105–S112. doi:10.1016/S0098-2997(97)00039-3
Arts FJ, Kuipers H (1994) The relation between power output, oxygen uptake and heart rate in male athletes. Int J Sports Med 15:228–231
Balsom PD, Seger J, Sjodin B, Ekblom B (1992) Physiological response to maximal intensity intermittent exercise. Eur J Appl Physiol Occup Physiol 65:144–149. doi:10.1007/BF00705072
Bhagavan HN, Chopra RK (2006) Coenzyme Q10: absorption, tissue uptake, metabolism and pharmacokinetics. Free Radic Res 40:445–453. doi:10.1080/10715760600617843
Boyum A (1964) Separation of white blood cells. Nature 204:793–794. doi:10.1038/204793a0
Cannon J, Blumberg JB (2000) Acute phase immune response in exercise. In: Sen CK, Packer L, Hänninen O (eds) Handbook of oxidants and antioxidants in exercise. Elsevier , Amsterdam, pp 177–194
Cases N, Aguilo A, Tauler P, Sureda A, Llompart I, Pons A et al (2005) Differential response of plasma and immune cell’s vitamin E levels to physical activity and antioxidant vitamin supplementation. Eur J Clin Nutr 59:781–788. doi:10.1038/sj.ejcn.1602143
Cases N, Sureda A, Maestre I, Tauler P, Aguilo A, Cordova A et al (2006) Response of antioxidant defences to oxidative stress induced by prolonged exercise: antioxidant enzyme gene expression in lymphocytes. Eur J Appl Physiol 98:263–269. doi:10.1007/s00421-006-0273-y
Crane FL (2001) Biochemical functions of coenzyme Q10. J Am Coll Nutr 20:591–598
Ernster L, Forsmark-Andree P (1993) Ubiquinol: an endogenous antioxidant in aerobic organisms. Clin Investig 71:S60–S65. doi:10.1007/BF00226842
Flohe L, Gunzler WA (1984) Assays of glutathione peroxidase. Methods Enzymol 105:114–121. doi:10.1016/S0076-6879(84)05015-1
Goldberg DM, Spooner RJ (1985) Glutathione reductase. In: Bergmeyer HU (ed) Methods in enzymatic analysis. Verlag Chemie, Basel, pp 258–265
Gomez-Cabrera MC, Borras C, Pallardo FV, Sastre J, Ji LL, Vina J (2005) Decreasing xanthine oxidase-mediated oxidative stress prevents useful cellular adaptations to exercise in rats. J Physiol 567:113–120. doi:10.1113/jphysiol.2004.080564
Jackson MJ (1999) Free radicals in skin and muscle: damaging agents or signals for adaptation? Proc Nutr Soc 58:673–676. doi:10.1017/S0029665199001317
Kagan T, Davis C, Lin L, Zakeri Z (1999) Coenzyme Q10 can in some circumstances block apoptosis, and this effect is mediated through mitochondria. Ann NY Acad Sci 887:31–47
Kaikkonen J, Tuomainen TP, Nyyssonen K, Salonen JT (2002) Coenzyme Q10: absorption, antioxidative properties, determinants, and plasma levels. Free Radic Res 36:389–397. doi:10.1080/10715760290021234
Karvonen J, Vuorimaa T (1988) Heart rate and exercise intensity during sport activities. Practical application. Sports Med 5:303–311. doi:10.2165/00007256-198805050-00002
Krause R, Patruta S, Daxbock F, Fladerer P, Biegelmayer C, Wenisch C (2001) Effect of vitamin C on neutrophil function after high-intensity exercise. Eur J Clin Invest 31:258–263. doi:10.1046/j.1365-2362.2001.00797.x
Langsjoen PH, Langsjoen AM (1999) Overview of the use of CoQ10 in cardiovascular disease. Biofactors 9:273–284
Lass A, Sohal RS (2000) Effect of coenzyme Q(10) and alpha-tocopherol content of mitochondria on the production of superoxide anion radicals. FASEB J 14:87–94
Levine RL, Williams JA, Stadtman ER, Shacter E (1994) Carbonyl assays for determination of oxidatively modified proteins. Methods Enzymol 233:346–357. doi:10.1016/S0076-6879(94)33040-9
McArdle F, Pattwell DM, Vasilaki A, McArdle A, Jackson MJ (2005) Intracellular generation of reactive oxygen species by contracting skeletal muscle cells. Free Radic Biol Med 39:651–657. doi:10.1016/j.freeradbiomed.2005.04.010
McCord JM, Fridovich I (1969) Superoxide dismutase. An enzymic function for erythrocuprein (hemocuprein). J Biol Chem 244:6049–6055
Miyazaki H, Oh-ishi S, Ookawara T, Kizaki T, Toshinai K, Ha S et al (2001) Strenuous endurance training in humans reduces oxidative stress following exhausting exercise. Eur J Appl Physiol 84:1–6. doi:10.1007/s004210000342
Morante M, Sandoval J, Gomez-Cabrera MC, Rodriguez JL, Pallardo FV, Vina JR et al (2005) Vitamin E deficiency induces liver nuclear factor-kappaB DNA-binding activity and changes in related genes. Free Radic Res 39:1127–1138. doi:10.1080/10715760500193820
Nieman DC (1994) Exercise, upper respiratory tract infection, and the immune system. Med Sci Sports Exerc 26:128–139. doi:10.1249/00005768-199402000-00002
Niklowitz P, Menke T, Andler W, Okun JG (2004) Simultaneous analysis of coenzyme Q10 in plasma, erythrocytes and platelets: comparison of the antioxidant level in blood cells and their environment in healthy children and after oral supplementation in adults. Clin Chim Acta 342:219–226. doi:10.1016/j.cccn.2003.12.020
Ohno H, Yahata T, Sato Y, Yamamura K, Taniguchi N (1988) Physical training and fasting erythrocyte activities of free radical scavenging enzyme systems in sedentary men. Eur J Appl Physiol Occup Physiol 57:173–176. doi:10.1007/BF00640658
Overvad K, Diamant B, Holm L, Holmer G, Mortensen SA, Stender S (1999) Coenzyme Q10 in health and disease. Eur J Clin Nutr 53:764–770. doi:10.1038/sj.ejcn.1600880
Petersen EW, Ostrowski K, Ibfelt T, Richelle M, Offord E, Halkjaer-Kristensen J et al (2001) Effect of vitamin supplementation on cytokine response and on muscle damage after strenuous exercise. Am J Physiol Cell Physiol 280:C1570–C1575
Podda M, Weber C, Traber MG, Milbradt R, Packer L (1999) Sensitive high-performance liquid chromatography techniques for simultaneous determination of tocopherols, tocotrienols, ubiquinols and ubiquinones in biological samples. In: Packer L (ed) Methods in enzymology. Oxidants and antioxidants. Academic Press, San Diego, pp 330–341
Radak Z, Kaneko T, Tahara S, Nakamoto H, Ohno H, Sasvari M et al (1999) The effect of exercise training on oxidative damage of lipids, proteins, and DNA in rat skeletal muscle: evidence for beneficial outcomes. Free Radic Biol Med 27:69–74. doi:10.1016/S0891-5849(99)00038-6
Radak Z, Sasvari M, Nyakas C, Pucsok J, Nakamoto H, Goto S (2000) Exercise preconditioning against hydrogen peroxide-induced oxidative damage in proteins of rat myocardium. Arch Biochem Biophys 376:248–251. doi:10.1006/abbi.2000.1719
Radak Z, Ogonovszky H, Dubecz J, Pavlik G, Sasvari M, Pucsok J et al (2003) Super-marathon race increases serum and urinary nitrotyrosine and carbonyl levels. Eur J Clin Invest 33:726–730. doi:10.1046/j.1365-2362.2003.01202.x
Reid MB (2001) Invited review: redox modulation of skeletal muscle contraction: what we know and what we don’t. J Appl Physiol 90:724–731. doi:10.1063/1.1381002
Sastre J, Asensi M, Gasco E, Pallardo FV, Ferrero JA, Furukawa T et al (1992) Exhaustive physical exercise causes oxidation of glutathione status in blood: prevention by antioxidant administration. Am J Physiol 263:R992–R995
Schroder H, Navarro E, Tramullas A, Mora J, Galiano D (2000) Nutrition antioxidant status and oxidative stress in professional basketball players: effects of a three compound antioxidative supplement. Int J Sports Med 21:146–150. doi:10.1055/s-2000-8870
Sureda A, Tauler P, Aguilo A, Cases N, Fuentespina E, Cordova A et al (2005) Relation between oxidative stress markers and antioxidant endogenous defences during exhaustive exercise. Free Radic Res 39:1317–1324. doi:10.1080/10715760500177500
Suzuki K, Totsuka M, Nakaji S, Yamada M, Kudoh S, Liu Q et al (1999) Endurance exercise causes interaction among stress hormones, cytokines, neutrophil dynamics, and muscle damage. J Appl Physiol 87:1360–1367
Tauler P, Aguilo A, Cases N, Sureda A, Gimenez F, Villa G et al (2002a) Acute phase immune response to exercise coexists with decreased neutrophil antioxidant enzyme defences. Free Radic Res 36:1101–1107. doi:10.1080/1071576021000028334
Tauler P, Aguilo A, Fuentespina E, Tur JA, Pons A (2002b) Diet supplementation with vitamin E, vitamin C and beta-carotene cocktail enhances basal neutrophil antioxidant enzymes in athletes. Pflugers Arch 443:791–797. doi:10.1007/s00424-001-0770-0
Tauler P, Aguilo A, Gimeno I, Fuentespina E, Tur JA, Pons A (2003a) Influence of vitamin C diet supplementation on endogenous antioxidant defences during exhaustive exercise. Pflugers Arch 446:658–664. doi:10.1007/s00424-003-1112-1
Tauler P, Aguilo A, Gimeno I, Noguera A, Agusti A, Tur JA et al (2003b) Differential response of lymphocytes and neutrophils to high intensity physical activity and to vitamin C diet supplementation. Free Radic Res 37:931–938. doi:10.1080/1071576031000150454
Tauler P, Sureda A, Cases N, Aguilo A, Rodriguez-Marroyo JA, Villa G et al (2006) Increased lymphocyte antioxidant defences in response to exhaustive exercise do not prevent oxidative damage. J Nutr Biochem 17:665–671. doi:10.1016/j.jnutbio.2005.10.013
Thomas SR, Neuzil J, Stocker R (1996) Cosupplementation with coenzyme Q prevents the prooxidant effect of alpha-tocopherol and increases the resistance of LDL to transition metal-dependent oxidation initiation. Arterioscler Thromb Vasc Biol 16:687–696
Tsao CS, Salimi SL (1982) Differential determination of L-ascorbic acid and D-isoascorbic acid by reversed-phase high-performance liquid chromatography with electrochemical detection. J Chromatogr A 245:355–358. doi:10.1016/S0021-9673(00)88023-1
Welch RW, Wang Y, Crossman A, Park JB, Kirk KL, Levine M (1995) Accumulation of vitamin C (ascorbate) and its oxidized metabolite dehydroascorbic acid occurs by separate mechanisms. J Biol Chem 270:12584–12592. doi:10.1074/jbc.270.13.7047
Acknowledgments
This work has been granted by the Spanish Ministry of Science and Education (DEP2005-00238-C04-01/EOU and DEP2005-00238-C04-02/EOU) and the FEDER funding.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tauler, P., Ferrer, M.D., Sureda, A. et al. Supplementation with an antioxidant cocktail containing coenzyme Q prevents plasma oxidative damage induced by soccer. Eur J Appl Physiol 104, 777–785 (2008). https://doi.org/10.1007/s00421-008-0831-6
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00421-008-0831-6