Abstract
This study was conducted to determine whether ventilatory parameters would change in breath-hold divers (BHDs) after they performed the glossopharyngeal technique for lung insufflation. Fifteen elite BHDs, 16 non-expert BHDs and 15 control subjects participated in this cross-sectional study. Volumes and expiratory flow rates were measured twice, before and after the glossopharyngeal technique performed at rest. Before the technique, greater forced vital capacity (FVC) and forced expiratory volume in 1 s (FEV1) and lower FEV1/FVC were noted in the elite and non-expert BHDs compared with controls. No difference was noted regarding the other pulmonary parameters. After the technique, increases were noted in FVC, FEV1 and maximal voluntary ventilation in the elite BHDs (P < 0.001, respectively). The FEF25–75%/FVC ratios were lower in the BHDs both before and after the technique, indicating possible dysanapsis. The ventilatory parameters observed after the glossopharyngeal technique indicated (1) higher lung volumes in expert BHDs and (2) a correlation with BHD performance (maximal dynamic BH performance). This correlation became more significant after the technique, indicating a positive effect of glossopharyngeal insufflation on performance.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Glossopharyngeal breathing (GPB) relies on the glossopharyngeal muscles instead of the respiratory muscles to move air into the lungs (glossopharyngeal insufflation, GI) and out of them (glossopharyngeal exsufflation) (Collier et al. 1956; Lindholm and Nyren 2005, Nygren-Bonnier et al. 2009). The volume of each GI or gulp has been reported to be up to 200 ml (Astrand et al. 1963). GI was first described by Dail et al. (1955) as a technique for patients with poliomyelitis. These patients were able to augment their tidal breathing by repeatedly insufflating a few gulps of air, typically starting at around their functional residual capacity, while still relying on passive expirations. More recently, GI was used successfully for patients with cervical spinal cord injury (Nygren-Bonnier et al. 2009). After practicing GI for 8 weeks, the patients were able to improve pulmonary function and chest expansion (Nygren-Bonnier et al. 2009). GI has also been used in healthy breath-hold divers (BHDs) to increase air volume in the lungs above normal total lung capacity (TLC); by doing so, the intrapulmonary oxygen stores are increased, thereby preventing the lungs from dangerous compression at depth (Lindholm and Nyren 2005; Muth et al. 2005; Tetzlaff et al. 2008). Loring et al. (2007) reported that GI was able to increase TLC in elite BHDs by up to 47%. It has been suggested that breath-holding (BH) performances are related to lung volumes (Andersson and Schagatay 1998; Overgaard et al. 2006). Indeed, the volume added by GI may be used by BHDs to increase both diving depth and duration (Lindholm and Nyren 2005) or maximal BH at rest or during underwater swimming (Overgaard et al. 2006). Many competitive BHDs have large lung volumes (Lindholm and Nyren 2005), but it is not known whether this is solely the result of the selection of individuals with a genetic advantage or whether GPB plays a role. We hypothetized (1) that elite BHDs represent a particular group of BHDs with high capacity to achieve the GI and (2) that this capacity is correlated to their BH performance.
The aims of this cross-sectional study were (1) to detect whether GI would result in ventilatory changes in BHDs and/or controls, and (2) to specify the breath-hold training parameters associated with any observed changes.
Materials and methods
The cohort
Forty-six healthy men responded to an invitation to participate in this study. They were separated into three groups of 15 elite BHDs, 16 novice BHDs and 15 non-BHDs. The elite BHDs were professional competitors and this study was conducted during a training session 2 days before a world BH championship. To be selected for the national team participating in this championship, the elite BHDs had to have been among the top performers in static and dynamic BH in their respective countries. The BHDs were considered to be novices when they had been practicing BH for at least 6 months with static BH performances of less than 2 min and dynamic performances of less than 75 m. All subjects were non-smokers and they did not consume caffeinated beverages or heavy meals on the day of the experiment. Table 1 presents the baseline morphological characteristics and sports activities per week as assessed by questionnaire. The body mass index (BMI = weight/height2) was calculated. The percentage of fat mass was assessed by the skinfold method according to Durnin and Womersley (1974) using a calibrated skinfold calliper. Cumulative BH exposure documented years of BH practice (YBHP), maximal static breath-holding (MSBH) performance and maximal dynamic breath-holding (MDBH) performance. The experimental procedures were conducted in accordance with the Declaration of Helsinki and were approved by the local ethics committee. Methods were explained in detail and informed written consent was obtained from all subjects.
Glossopharyngeal insufflation
All subjects received instruction on the GI technique from the same physician. They watched an instructional video, reviewed written information and practiced GI with the physician. Each subject first inhaled from the spirometer filling up their lungs to TLC. Then, after additional filling by GI off the spirometer, the exhaled volume and flow profiles were measured to allow the volume of GI filling to be obtained. All subjects performed GI via the mouth and wore a nose-clip to avoid air leakage (Bach et al. 1987; Dail et al. 1955). All briefly warmed up with stretching exercises for the chest, then performed ten repetitions of GI in a sitting position until they felt “full enough” (Loring et al. 2007). The subjects were instructed to fill their lungs to maximal level with only these ten GI. It allows us to see what supplementary volume provided to the same number of gulps is possible to have and to reduce the great variability in number of gulps and volume per gulp that has been reported in a previous study (Tetzlaff et al. 2008). The air was then passively expelled and the subjects resumed normal respiration.
Pulmonary function tests
Several parameters were measured: forced vital capacity (FVC), forced expiratory volume in 1 s (FEV1), FEV1/FVC, peak expiratory flow, maximal expiratory flow rates at 75, 50 and 25% of FVC (MEF75%, MEF50%, MEF25%), and forced mid-expiratory flow rate (FEF25–75%). For each parameter, the best value was chosen from at least three consecutive maneuvers differing by not more than 5% (Quanjer et al. 1993). The FEF25–75%/FVC ratio was also calculated as an indicator of disproportionately small airways for a given lung size (Green et al. 1974; Martin et al. 1987; Parker et al. 2003). The forced expiratory time (FET) defined and recorded by the spirometer software was used (PFT suite version 8.1, Cosmed, Rome, Italy). The beginning point in time for the FET measurement was determined by the back extrapolation method according to the ATS/ERS 2005 standards (Pellegrino et al. 2005). The end-point in time for FET measurement was the beginning of the end-expiratory plateau. An end-expiratory plateau with zero flow for 1 s was required for the acceptable maneuvers, but this zero-flow time was not included in the measured FET. The spirometer system and time measurement were computer based. The predicted maximal voluntary ventilation (MVV) was calculated according the following equation: MVV (l min−1) = FEV1 × 40.
Ventilatory function variations (Δ) were calculated as the index of change (magnitude and direction) induced by GI [for example, ΔFVC = (FVCGI − FVC)/FVC) × 100)]. Δ values were negative or positive, depending on the parameter kinetics (increase or decrease). This method minimized the differences between before and after GI values of the ventilatory parameters. All of the parameters were measured using a Microquark spirometer (Cosmed, Rome, Italy) in the same conditions, with air temperature and hygrometry monitored by the same technician. The pulmonary function tests (before and after GI) were performed in a sitting position, with the subject breathing through the mouthpiece with a nose-clip. The spirometer volume was calibrated twice daily with a 3-l calibrated syringe. The results were corrected to BTPS conditions and compared with predicted values (Quanjer et al. 1993).
Statistics
The results are presented as means and standard deviations (±SD) and as percentages of predicted values according to Quanjer et al. (1993). Morphological characteristics, lung parameters (percentages of predicted, and before and after GI) and lung function changes (Δ: mean) were compared by a Wilcoxon signed rank test. Multiple linear regression analysis, performed in a stepwise backward fashion, was used to assess relevant correlations of age and BH exposure with the lung function parameters. Pearson correlations were also performed. Ancova analysis was performed to test differences on regression slopes. A P value <0.05 was considered significant. Analyses were performed with Statview software (Abacus Concepts, Inc., Berkeley, CA, USA; 1992).
Results
Because height was different between the groups, all lung parameters were expressed as % of predicted values for comparison. Table 2 shows the results of lung function testing for the three groups before GI and Table 3 after GI. Table 4 shows the lung function changes (Δ mean) between before and after GI for the three groups.
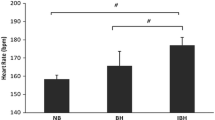
Before GI, the elite BHDs had the highest FVC values of all groups (P < 0.01). They also had lower FEV1/FVC than novice BHDs but higher FEV1/FVC and lower FEF25–75%/FVC than controls. The novice BHDs had higher FVC and FEV1 and lower FEV1/FVC than controls.
After GI, the elite BHDs had higher values of FVC, FEV1 and FET than the novice BHDs and the control group. They also had lower FEV1/FVC and FEF25–75%/FVC than these two groups. The novice BHDs had higher FVC and FET and lower FEV1/FVC than the controls. All subjects had increased their FET with GI.
The Δ changes in all lung function parameters are significant in controls (Table 4), except for FEF25–75%/FVC, which changed least in this group (P < 0.001). The Δ changes in FVC and FET were greater in the elite and non-expert BHDs. The latter also showed greater Δ changes in FEV1 and MVV. The volume per gulp was higher in elite BHDs than in novice and controls (136 ± 87 vs. 28 ± 62 vs. 20 ± 10 ml, P < 0.001 respectively).
In the BHDs (elite plus novice BHDs), YBHP was correlated with MSBH performance (r = 0.77, P < 0.0001) and MDBH performance (r = 0.76, P < 0.0001). Stepwise regression analyses of FVC and FEF25–75%/FVC before GI and the independent parameters (age, height, YBHP, MSBH, MDBH, hours of BH training and hours of sports activities) showed that the main factor contributing to the changes in FVC and FEF25–75%/FVC was the MDBH performance (r = 0.49 and r = −0.38, respectively; P < 0.01). After GI, FVC and FEF25–75%/FVC were also correlated with MDBH (r = 0.71 and r = −0.62, respectively; P < 0.001) (Figs. 1, 2). The FEF25–75%/FVC ratio before and after GI was positively correlated with FEF25–75% (P < 0.001) and FEV1/FVC (P < 0.001). FEV1/FVC showed a negative correlation with FET and FET after GI (FETGI) (r = 0.31, P < 0.05 and r = 0.58, P < 0.0001, respectively). When the BHD data from the two groups was pooled versus the control data, the FVC values were also correlated with age but only in controls (Fig. 3).
Discussion
The principal findings of this study were the classically higher lung volumes (FVC and FEV1) in elite divers compared with the values in the less trained BHDs and controls. Dysanapsis and FET were increased after GI in the elite BHDs. These changes were associated with their best dynamic performance.
Pulmonary volumes
The greater lung volumes before GI were close to those found by previous authors in BHDs with similar BH experience (about +24%) (Lindholm and Nyren 2005; Overgaard et al. 2006; Seccombe et al. 2006; Tetzlaff et al. 2008) and in swimmers (Armour et al. 1993; Nygren-Bonnier et al. 2007a). Our novice BHDs had lower volumes than elite BHDs but higher than those of the controls (+9%) or the predicted values (+14%). Moreover, the greater lung volumes of the BHDs were correlated with MDBH, indicating a possible BH training effect on pulmonary volumes. Indeed, the above-normal lung volumes of our BHDs could be partly an adaptation to breath-hold diving. It has been suggested that these large lung volumes are the result of an increased number of alveoli or alveolar size due to training (Armour et al. 1993; Calder et al. 1987; Donnelly et al. 1995). However, the differences could be attributed to a selection of subjects with initially large lung volumes.
After GI, all groups had increased their FVC, but this was less so in controls (+4%) than in novices (+7%) and elite BHDs (+22%). Our results are close to the results of previous studies with BHDs (Table 5). It is interesting to note that when experienced subjects performed this technique, the increases were greater than with inexperienced subjects (Table 5). For example, the Δ changes were lower in healthy women and in the controls of our study (Nygren-Bonnier et al. 2007b). We chose to fix the number of gulps in each group, which could explain why the untrained subjects had lower added volumes with this technique even though they felt “full enough” like the BHDs. Although the increases and gains were lower in controls and healthy women (0.2 and 0.88 l, respectively, Table 5), these maneuvers always improved their ventilatory parameters. Indeed, GI training or GI maneuvers seem to enhance performance in trained BHDs but even more so in untrained subjects with “normal” lung volumes. The BHDs were probably able to increase VC in only ten GI because they increased their tidal volume during each GI. This ability to increase VC more than controls could also be partly explained by sensation (Loring et al. 2007; Whittaker and Irvin 2007) rather than the mechanics of the lung, chest wall, or respiratory muscles, and by long years of GI practice (>5 years). The correlation between FVC after GI (FVCGI) and their best dynamic performance, as well as the different slopes of the correlation between FVC and age for the control and BHD values before and after GI (−0.19 and −0.03, respectively; P < 0.01), accounted for this (Fig. 1). It has been found that the high lung volumes in BHDs after GI could be explained partly by the capacity to withstand greater transpulmonary pressures and volumes than those to which lungs would normally be exposed (Loring et al. 2007). Moreover, the GI maneuver has been associated with transient lung distension. Approximately one-third of the additional air is accommodated by air compression (Seccombe et al. 2006), thus the remainder is due to volume distension of the lungs. The pressure will reduce the amount of blood in the chest, which will give more space for air. Some studies suggested that respiratory muscle training and the subsequent increase in respiratory muscle force can increase lung volumes (Cordain et al. 1990; Doherty and Dimitriou 1997; Zinman and Gaultier 1986), whereas others found no change in lung volumes (Clanton et al. 1987; Wells et al. 2005). Indeed, maximal expiratory and inspiratory pressure did not change after any form of training (Nygren-Bonnier et al. 2007b; Tetzlaff et al. 2008). This was expected, as the GI maneuvers were aimed at stretching the chest wall alone (Eichinger et al. 2008; Nygren-Bonnier et al. 2007a, b). GI increases thoracic circumference. BHDs able to insufflate large volumes thus expand the chest significantly, giving them a barrel chest appearance. It is possible that they have increased joint mobility and stretch their respiratory muscles so they can increase the chest volume to whatever is anatomically possible (Eichinger et al. 2008; Whittaker and Irvin 2007).
Expiratory flow rates
It is generally assumed that divers have large lung volumes with proportionately greater increases in vital capacity than in FEV1, which lowers the FEV1/FVC ratio (Lemaitre et al. 2002, 2006). In our study, the FVC of the elite BHDs was higher than that of the novice BHDs and controls, but with proportionately greater increases in FVC than in FEV1, which decreased the FEV1/FVC ratio. Thus, FEV1/FVC was diminished in the elite BHDs compared with novice BHDs and controls both before and after GI. Only one other study reported diminished FEV1/FVC in BHDs (Tetzlaff et al. 2008). However, our mean value and that of Tetzlaff et al. were still within the normal range even after GI. In patients with respiratory disease, a low FEV1/FVC, even when FEV1 is within the normal range, predicts morbidity and mortality (Mannino et al. 2003). For healthy subjects, low FEV1/FVC with FEV1 within the normal range is probably due to “dysanaptic” or unequal growth of the airways and lung parenchyma (Green et al. 1974). These differences may have an embryologic basis reflecting disproportionate but physiologically normal growth of the airways and parenchyma within the lung. The FEF25–75%/FVC ratio has been used as a non-invasive measure of dysanapsis (Parker et al. 2003) and is associated with airways sensitivity and reactivity to metacholine (Parker et al. 2003). Individuals with a low FEF25–75%/FVC ratio will have small airways size relative to lung size and may be more likely to develop expiratory flow limitation than subjects with a higher ratio. In our study, FEF25–75%/FVC was low because the subjects had normal FEF25–75% but increased FVC before and after GI. The FEF25–75%/FVC ratio was “normal” compared with the predicted values and the other groups, but decreased more after GI, reflecting “artificial” dysanapsis. Thus, the higher lung volumes and normal expiratory flow rates in our elite BHDs indicated small airways size relative to their lung size. This “artificial” dysanapsis was correlated with the best dynamic performance of the BHDs, indicating a possible training effect. FET has gained new interest in the joint recommendations of the American Thoracic Society and the European Respiratory Society for the assessment of spirometry. Mean FET is about 11 s in a non-selected adult population and about 10 s in healthy non-smokers (Kainu et al. 2008). The FETs were shorter for our subjects than those of Kainu et al. (2008), which could be partially explained by the younger age of our subjects. The prolonged FET observed after GI in our divers, especially the elite BHDs (+43%), and in the controls was concurrent with changes in the pulmonary mechanics, such as “dysanapsis”. The negative correlation of FET with FEV1/FVC may indicate that physiological airflow limitation tends to prolong FET.
Conclusion
This study evaluated the acute ventilatory effects of GI in BHDs and revealed increased FVC and FEV1 before and also after GI. These ventilatory changes could be explained by the combination of several factors, particularly the capacity to withstand greater transpulmonary pressures and volumes than those to which lungs are normally exposed. These changes could be important to ensure the best static and dynamic performance. Artificial dysanapsis could be created by GI in elite BHDs. Long term-effect remains to be examined.
References
Andersson J, Schagatay E (1998) Effects of lung volume and involuntary breathing movements on the human diving response. Eur J Appl Physiol Occup Physiol 77:19–24
Armour J, Donnelly PM, Bye PT (1993) The large lungs of elite swimmers: an increased alveolar number? Eur Respir J 6:237–247
Astrand PO, Eriksson BO, Nylander I, Engstroem L, Karlberg P, Saltin B, Thoren C (1963) Girl swimmers. With special reference to respiratory and circulatory adaptation and gynaecological and psychiatric aspects. Acta Paediatr 43(Suppl 147):141–175
Bach JR, Alba AS, Bodofsky E, Curran FJ, Schultheiss M (1987) Glossopharyngeal breathing and noninvasive aids in the management of post-polio respiratory insufficiency. Birth Defects Orig Artic Ser 23:99–113
Calder IM, Sweetnham K, Chan KK, Williams MM (1987) Relation of alveolar size to forced vital capacity in professional divers. Br J Ind Med 44:467–469
Clanton TL, Dixon GF, Drake J, Gadek JE (1987) Effects of swim training on lung volumes and inspiratory muscle conditioning. J Appl Physiol 62:39–46
Collier CR, Dail CW, Affeldt JE (1956) Mechanics of glossopharyngeal breathing. J Appl Physiol 8:580–584
Cordain L, Tucker A, Moon D, Stager JM (1990) Lung volumes and maximal respiratory pressures in collegiate swimmers and runners. Res Q Exerc Sport 61:70–74
Dail CW, Affeldt JE, Collier CR (1955) Clinical aspects of glossopharyngeal breathing; report of use by one hundred postpoliomyelitic patients. J Am Med Assoc 158:445–449
Doherty M, Dimitriou L (1997) Comparison of lung volume in Greek swimmers, land based athletes, and sedentary controls using allometric scaling. Br J Sports Med 31:337–341
Donnelly PM, Grunstein RR, Peat JK, Woolcock AJ, Bye PT (1995) Large lungs and growth hormone: an increased alveolar number? Eur Respir J 8:938–947
Durnin JV, Womersley J (1974) Body fat assessed from total body density and its estimation from skinfold thickness: measurements on 481 men and women aged from 16 to 72 years. Br J Nutr 32:77–97
Eichinger M, Walterspacher S, Scholz T, Tetzlaff K, Rocker K, Muth CM, Puderbach M, Kauczor HU, Sorichter S (2008) Lung hyperinflation: foe or friend? Eur Respir J 32:1113–1116
Green M, Mead J, Turner JM (1974) Variability of maximum expiratory flow-volume curves. J Appl Physiol 37:67–74
Kainu A, Lindqvist A, Sarna S, Sovijarvi A (2008) Spirometric and anthropometric determinants of forced expiratory time in a general population. Clin Physiol Funct Imaging 28:38–42
Lemaitre F, Bedu M, Coudert J (2002) Pulmonary function of recreational divers: a cross sectional study. Int J Sports Med 23:273–278
Lemaitre F, Tourny-Chollet C, Lemouton MC (2006) Ventilatory function in experienced recreational scuba divers: Evidence of small airways disease? Int J Sports Med 27:875–879
Lindholm P, Nyren S (2005) Studies on inspiratory and expiratory glossopharyngeal breathing in breath-hold divers employing magnetic resonance imaging and spirometry. Eur J Appl Physiol 94:646–651
Loring SH, O’Donnell CR, Butler JP, Lindholm P, Jacobson F, Ferrigno M (2007) Transpulmonary pressures and lung mechanics with glossopharyngeal insufflation and exsufflation beyond normal lung volumes in competitive breath-hold divers. J Appl Physiol 102:841–846
Mannino DM, Buist AS, Petty TL, Enright PL, Redd SC (2003) Lung function and mortality in the United States: data from the First National Health and Nutrition Examination Survey follow up study. Thorax 58:388–393
Martin TR, Castile RG, Fredberg JJ, Wohl ME, Mead J (1987) Airway size is related to sex but not lung size in normal adults. J Appl Physiol 63:2042–2047
Muth CM, Ehrmann U, Radermacher P (2005) Physiological and clinical aspects of apnea diving. Clin Chest Med 26:381–394
Nygren-Bonnier M, Gullstrand L, Klefbeck B, Lindholm P (2007a) Effects of glossopharyngeal pistoning for lung insufflation in elite swimmers. Med Sci Sports Exerc 39:836–841
Nygren-Bonnier M, Lindholm P, Markstrom A, Skedinger M, Mattsson E, Klefbeck B (2007b) Effects of glossopharyngeal pistoning for lung insufflation on vital capacity in healthy women. Am J Phys Med Rehabil 86:290–294
Nygren-Bonnier M, Wahman K, Lindholm P, Markstrom A, Westgren N, Klefbeck B (2009) Glossopharyngeal pistoning for lung insufflation in patients with cervical spinal cord injury. Spinal Cord 47:418–422
Overgaard K, Friis S, Pedersen RB, Lykkeboe G (2006) Influence of lung volume, glossopharyngeal inhalation and P(ET) O2 and P(ET) CO2 on apnea performance in trained breath-hold divers. Eur J Appl Physiol 97:158–164
Parker AL, Abu-Hijleh M, McCool FD (2003) Ratio between forced expiratory flow between 25% and 75% of vital capacity and FVC is a determinant of airway reactivity and sensitivity to methacholine. Chest 124:63–69
Pellegrino R, Viegi G, Brusasco V, Crapo RO, Burgos F, Casaburi R, Coates A, van der Grinten CP, Gustafsson P, Hankinson J, Jensen R, Johnson DC, MacIntyre N, McKay R, Miller MR, Navajas D, Pedersen OF, Wanger J (2005) Interpretative strategies for lung function tests. Eur Respir J 26:948–968
Potkin R, Cheng V, Seigel R (2007) Effects of glossopharyngeal insufflation on cardiac function: an echocardiographic study in elite breath-hold divers. J Appl Physiol 103:823–827
Quanjer PH, Tammeling GJ, Cotes JE, Pedersen OF, Peslin R, Yernault JC (1993) Lung volumes and forced ventilatory flows. Report Working Party Standardization of Lung Function Tests, European Community for Steel and Coal. Official Statement of the European Respiratory Society. Eur Respir J Suppl 16:5–40
Simpson G, Ferns J, Murat S (2003) Pulmonary effects of ‘lung packing’ by buccal pumping in an elite breath-hold diver. SPUMS J 33:122–126
Seccombe LM, Rogers PG, Mai N, Wong CK, Kritharides L, Jenkins CR (2006) Features of glossopharyngeal breathing in breath-hold divers. J Appl Physiol 101:799–801
Tetzlaff K, Scholz T, Walterspacher S, Muth CM, Metzger J, Roecker K, Sorichter S (2008) Characteristics of the respiratory mechanical and muscle function of competitive breath-hold divers. Eur J Appl Physiol 103:469–475
Wells GD, Plyley M, Thomas S, Goodman L, Duffin J (2005) Effects of concurrent inspiratory and expiratory muscle training on respiratory and exercise performance in competitive swimmers. Eur J Appl Physiol 94:527–540
Whittaker LA, Irvin CG (2007) Going to extremes of lung volume. J Appl Physiol 102:831–833
Zinman R, Gaultier C (1986) Maximal static pressures and lung volumes in young female swimmers. Respir Physiol 64:229–239
Acknowledgments
Special thanks are given to the BHDs for their cooperation. We also thank Cathy Carmeni for help in preparing the manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Susan Ward.
Rights and permissions
About this article
Cite this article
Lemaître, F., Clua, E., Andréani, B. et al. Ventilatory function in breath-hold divers: effect of glossopharyngeal insufflation. Eur J Appl Physiol 108, 741–747 (2010). https://doi.org/10.1007/s00421-009-1277-1
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00421-009-1277-1