Abstract
We assessed the influence of recreational physical activity in young healthy women on homocysteine, a potential risk factor for cardiovascular disease (CVD). Participants were 124 23-year-old normal-weight Italian recreational athletes (performing 8.7 ± 2.46 h week−1 exercise) and 116 controls. Median blood homocysteine, folate and lipid markers did not differ between athletes and controls. Elevated homocysteine levels at CVD risk ≥12.0 and ≥15.0 μmol l−1 were not different between groups. Continuous homocysteine was inversely related to folate (P < 0.001), positively associated with age (P = 0.009) and creatinine (P = 0.033), but not associated with hours of exercise, body mass index, and lipid markers. Women with folate depletion (<3.0 μg l−1) were 4.5-fold more likely to have homocysteine ≥15.0 μmol l−1. Recreational physical exercise does not adversely impact homocysteine levels among young women. Only low folate significantly increases the risk for hyperhomocysteinemia in young women.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In recent years, elevated plasma homocysteine (HCY) has emerged as a novel cardiovascular biomarker. Of interest, a large 24-year follow-up study performed in Swedish women demonstrated that elevated serum HCY at enrollment is an independent risk factor for myocardial infarction (Zylberstein et al. 2004). High levels of HCY have been related to endothelial damage, venous thrombosis, atherosclerosis, cardiovascular diseases (CVD), abnormal collagen cross-linking, oxidative stress, preterm birth, osteoporotic fracture and several disorders of the central nervous system (Herrmann et al. 2007; Kuo et al. 2005; Refsum et al. 2004; Scholl and Johnson 2000; Wald et al. 2006). However, the exact role of HCY in CVD is still debated, a recent study performed in middle-age white US women showed that the risk of incident cardiovascular events attributed to elevated HCY might be substantially lowered after adjustment for traditional risk factors and socioeconomic status (Zee et al. 2007). HCY is an amino acid derived by dietary methionine. HCY levels are normally maintained within a narrow range by the activity of the remethylation and transsulfuration pathways (Refsum et al. 2004). HCY is an attractive target for CVD prevention strategies, because its metabolism can be influenced by dietary habits and lifestyle factors, such as smoking, alcohol and coffee consumption (Refsum et al. 2004). There is evidence that HCY is inversely related to folate and vitamins B6 and B12 (Quinlivan et al. 2002; Refsum et al. 2004). However, folate more than vitamin B12 supplements may reduce HCY plasma levels and vascular risk (McNulty et al. 2008; Wang et al. 2007). Mainly, low folate and vitamin B12 levels impair the methylation that converts HCY into methionine. Although there is some evidence of beneficial effects of physical activity on reduction of plasma HCY concentrations, the results are not consistent throughout studies and the interpretation of pathways involved in exercise modulation of HCY is not conclusive (Herrmann et al. 2003; Okura et al. 2006; Unt et al. 2007).
It is well established that physical activity is a key component of good health and disease prevention (Pedersen and Saltin 2006). Increasing evidence suggests that physical activity reduces the risk of CVD, although the precise mechanisms are not well understood.
Some studies have directly compared the effects of physical activity on plasma HCY concentrations, and these studies have been mostly limited to men or over 45-year-old women (Herrmann et al. 2007; Mora et al. 2006; Unt et al. 2007; Zee et al. 2007; Zinellu et al. 2007). Few papers investigated factors modulating HCY concentrations in pre-menopausal women (Cauci et al. 2008; De Cree et al. 1999; Randeva et al. 2002), although knowledge of factors influencing HCY levels in young healthy females of reproductive age have potential implications for early prevention of venous thrombosis, CVD and adverse pregnancy outcomes (Refsum et al. 2004). In addition, HCY levels in young women are an important serum marker for clinicians to decide whether a woman can safely use hormonal contraception (Cauci et al. 2008). However, most studies examined lifestyle effects on HCY concentrations in post-menopausal women, because plasma HCY levels increase with natural menopause suggesting a close relationship between HCY levels and increased CVD risk in older age women (Ridker et al. 1999; Vanuzzo et al. 2007).
Recently, scientists have called for an effort to reduce the rate of CVD not only in the elderly population but in young adults as well (Lloyd-Jones and Tian 2006). To this end, the evaluation of factors modulating traditional and novel risk biomarkers for CVD in young women may prove useful.
So far studies on HCY in young recreational female athletes belonging to a homogeneous ethnic group are missing. To provide more information, we measured levels of plasma HCY and serum lipid markers, total cholesterol, low- and high-density lipoprotein (LDL and HDL) cholesterol, and triglycerides in young healthy white women. We sought to evaluate the association of recreational physical activity with novel and traditional CVD biomarkers.
Methods
Subjects
Healthy white Italian women (age range 18–35 years) were consecutively enrolled at Udine University campus, from June 2006 to November 2007. The study was conducted with the approval of the Ethics Committee of the Udine University Hospital. Written informed consent was obtained from all study participants. The methods used in this study were in accordance with the Helsinki Declaration of 1975 as revised in 1983.
Before entering the study, each woman was interviewed to determine whether she fulfilled the inclusion criteria: non-pregnant woman, without thyroid diseases, infections, chronic inflammatory diseases or major diseases such as diabetes and malignancies. The participants completed questionnaires on physical exercise activity, demographics, medical history, and lifestyle factors. Women taking supplements containing exclusively folate and/or vitamin B12 or who used any continuous and quantitatively relevant supplementation of folate and/or vitamin B12 were excluded. No woman was vegetarian. Most of the recreational athletes were enrolled at Sport Sciences, University of Udine. Athletes were performing more than 4 h week−1 of regular physical exercise including training and competition. Controls were women not practicing sport activities. Out of 263 women enrolled, 15 were not meeting inclusion criteria because of ongoing diseases, 8 did not completed questionnaires, thus 240 subjects were eligible for the study, of these 124 were recreational athletes and 116 were sedentary controls.
Self-reported body height and weight were obtained from the questionnaires and used to calculate body mass index (BMI, defined as weight in kilograms divided by the square of height in meters).
Measurements
Venous blood samples were drawn after overnight fasting from seated subjects in the morning as described (Casabellata et al. 2007). Women were required to avoid exercise 24 h prior to the blood donation (Di Santolo et al. 2008). Personnel executing the collection and measurement of samples were blinded to clinical, demographic, and habit data.
To determine HCY, whole venous blood was collected in tubes containing ethylenediamminotetraacetate (EDTA), immediately put on ice and centrifuged within 30 min, at 2,500g for 15 min at 8°C. HCY concentrations were measured by a fluorescence polarization immunoassay (AxSYM Homocysteine, Abbott Diagnostics, Chicago, IL, USA), with automated Abbott AxSYM system. Detection limit of the assay was ≤0.8 μmol l−1. Intra- and inter-assay imprecision CVs% were 2.3% (at 8.0 μmol l−1) and 3.2% (at 8.0 μmol l−1), respectively. Reference interval found by the manufacturer for the female population was 4.6−12.0 μmol l−1. In addition to this manufacturer’s upper threshold, we used the ≥15.0 μmol l−1 and ≥30.0 μmol l−1 cutpoints as high HCY levels at risk for CVD based on previous clinical studies (Refsum et al. 2004).
Serum folate concentrations (3.0–12.5 μg l−1 reference interval) were determined using a chemiluminescent microparticle folate binding protein immunoassay on Architect system (Architect Folate, Abbott Diagnostics). Detection limit of the assay was ≤0.8 μg l−1. Intra- and inter-assay imprecision CVs were 3.0% (at 3.0 μg l−1) and 5.4% (at 3.0 μg l−1), respectively.
Serum concentrations of creatinine were evaluated on Modular analyzer (Roche Diagnostics, Mannheim, Germany) by compensated alkaline picrate assay (Roche Diagnostics). Concentrations of triglycerides, total cholesterol, HDL cholesterol, albumin, and glucose were measured on Modular analyzer (Roche Diagnostics) by appropriate reagents (Roche Diagnostics). LDL cholesterol was calculated from total cholesterol, triglycerides, and HDL according to the Friedewald equation.
Statistical analysis
The Kolmogorov–Smirnov test was used to assess the normality of data distribution. Normally distributed variables were presented as mean and standard deviation (±SD). For skewed markers, median (25th to 75th percentile, interquartile, IQR) values were reported and non-parametric tests used. The t test or Mann–Whitney U test was used for comparison of continuous variables, as appropriate. The difference of proportions between athletes and controls was assessed by chi-square or Fisher’s exact test, as appropriate. Univariable odds ratios (ORs) and 95% confidence intervals (CIs) were evaluated for categorical variables. In addition, logistic regression was performed to evaluate the difference in HCY levels ≥12.0 or ≥15.0 μmol l−1 between recreational athletes and sedentary controls after adjustment for age, folate, creatinine, smoking, ≥2 coffee cups day−1, hormonal contraception and BMI. Bivariate relationships for continuous values of the biomarkers were evaluated by Spearman rank correlation coefficients (r s). All tests were two-sided. P values <0.050 were considered statistically significant. Statistical analysis was performed using the Statistical Package for Social Sciences (SPSS for Windows, version 11.0, SPSS Inc., Chicago, IL).
Results
A total of 240 subjects were examined, 124 were recreational athletes and 116 were sedentary controls. All study women had a middle-class socioeconomic status, nearly all (95%, 227/240) were in the normal-weight range (BMI ≥ 18 and ≤25 kg m−2). The mean age was 23.4 ± 4.53 years, and BMI was 20.8 ± 1.97 kg m−2. Sport disciplines practiced by recreational athletes are described in Table 1.
The comparison of demographic and lifestyle characteristics of the 124 recreational female athletes and 116 sedentary control women are described in Table 2. There were no significant differences between athletes and controls for most variables. Athletes had less frequently a university education level (P < 0.001), and were more frequently smokers (P = 0.001) compared to sedentary women. However, frequency of heavy smokers (≥10 cigarettes day−1) and coffee drinkers of ≥2 or ≥4 espresso coffee cups day−1 did not differ between athletes and sedentary women. Athletes tended to use more frequently nutritional supplements (not significant, P = 0.05). However, athletes and sedentary controls did not differ in alimentary habits with regard to meat, fish, vegetables, carbohydrate and alcohol consumption (data not shown).
Comparisons of continuous values of the blood markers measured in athletes and sedentary controls are illustrated in Table 3. Values of biomarkers did not differ significantly between the two groups, with the exception of creatinine.
The effects of physical exercise on categorical values of elevated homocysteine are shown in Table 4. Confirmatory with findings described in Table 3, physical exercise was not associated with high HCY levels ≥12.0 or ≥15.0 or ≥30.0 μmol l−1 as evaluated by crude and adjusted ORs.
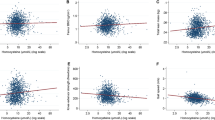
Table 5 shows two-tailed Spearman correlations of continuous concentrations of HCY with other biomarkers among the 240 study women. We found no association of HCY with BMI, hours of exercise per week and lipid markers. As expected (Refsum et al. 2004), HCY had an inverse correlation with folate (P < 0.001) and a positive association with age (P = 0.009) and creatinine (P = 0.033). We found no correlation of HCY with number of cigarette smoked, number of coffee cups and other lifestyle factors (data not shown).
Finally, we explored the frequency of elevated HCY levels in study women with deficient folate (<3.0 μg l−1) versus non-deficient folate (≥3.0 μg l−1), and folate below (<5.0 μg l−1) and above (≥5.0 μg l−1) the median folate value (Cauci et al. 2008) (Table 6). Only three women had HCY levels ≥30.0 μmol l−1, therefore this cutpoint was not further examined. Women with deficient folate were at increased risk of having HCY ≥12.0 and ≥15.0 μmol l−1 (2.5- and 4.5-fold, respectively). Notably, also women with folate in the normal interval, but <5.0 μg l−1 were 2.5-fold more likely to have elevated HCY ≥ 12.0 μmol l−1.
Discussion
Physical exercise is widely recommended as healthy behavior to reduce the risk of several diseases such as cardiovascular diseases, metabolic syndrome, insulin resistance, diabetes, osteoporosis, breast cancer and other clinical or subclinical disorders (Pedersen and Saltin 2006; Verloop et al. 2000). However, it is not yet established the exact mechanism by which exercise is beneficial against CVD and other diseases.
It is well documented that elevated HCY is a risk factor for CVD in women, although the exact role of HCY is still debated (Herrmann et al. 2007; Lippi et al. 2007; Ridker et al. 1999; Zee et al. 2007; Zylberstein et al. 2004). There is also an increasing evidence that physical activity is able to reduce the risk of CVD (Mora et al. 2007; Sesso et al. 2000). In addition, physical fitness has been suggested to down-modulate HCY in females but not in males (Kuo et al. 2005; Ruiz et al. 2007). Thus, an intriguing hypothesis is that one of the mechanisms by which physical exercise may reduce the risk of vascular thrombosis and CVD in women is the reduction of HCY. This hypothesis is of great interest for the female population because it has been observed that in elderly women when HCY levels raise the CVD risk increases.
However, data gathered up to now on the effects of physical activity on plasma HCY levels are contradictory. Some researches highlighted an exercise-induced fall in HCY concentrations (Randeva et al. 2002; Zinellu et al. 2007), but many reports provided evidence that physical exercise does not contribute to reduce HCY and/or that in some instances it would even produce a HCY increase (Borrione et al. 2008; De Cree et al. 1999; Herrmann et al. 2003; Konig et al. 2003; Okura et al. 2006). Intense endurance physical activity has the potential to worsen the HCY profile, likely as a consequence of folate consumption following exercise-induced metabolic demand (De Cree et al. 1999; Herrmann et al. 2003).
Women in fertile age are a population less investigated in regard to HCY levels than post-menopausal women, despite elevated pre- and peri-conceptional HCY levels are a cause of public health concern, because of the association with adverse pregnancy outcomes (such as spontaneous abortion, hypertensive illness, preeclampsia, placental abruption, intrauterine growth retardation (IUGR) and preterm delivery) and with newborn disorders (neural tube defects and low birth weight) (Ronnenberg et al. 2002; Scholl and Johnson 2000; Taparia et al. 2007). Additionally, in fertile age women hyperhomocysteinemia has been associated with amenorrhea, especially in female athletes (O’Donnell and De Souza 2004).
In general, there is poor attention to the health status of asymptomatic women performing physical activity at non-professional level. Recreational athletes are not invited to check and take care of their health status like elite athletes, despite recreational athletes account for a much larger proportion of the female population than elite athletes. To the best of our knowledge, our study was the largest cross-sectional investigation to assess the prevalence of elevated HCY in recreational young adult white female athletes with respect to matched sedentary women. It is to note that most of the women we enrolled never assessed their HCY, folate and lipid status prior to this study. We found that as much as 1 over 5 recreational athletes (23%) have HCY levels ≥12.0 μmol l−1, which are at risk for CVD, and nearly 1 over 10 (9%) have HCY levels ≥15.0 μmol l−1, which are at high risk for CVD. However, these rates of elevated HCY were not statistically different to those found in sedentary control women. Thus, we assessed that in young non-obese women the rate of elevated HCY cannot be reduced by recreational physical activity, on the other hand recreational sports do not worsen the HCY profile.
As expected, we found that HCY concentrations positively correlated with age, and inversely correlated to serum folate. Of interest, our study showed that serum folate levels below which young women are at risk of hyperhomocysteinemia are not only those below the reference range (<3.0 μg l−1), by definition considered “folate deficiency”, but also folate concentrations into the reference range but below the median value of 5.0 μg l−1.
A limitation of our study is that we did not assessed other vitamins of the B complex, however, it is demonstrated that vitamins B6 and B12 influence HCY concentrations with a lesser extent than folate (McNulty et al. 2008).
The positive correlation we found between HCY and creatinine is an association that has been extensively observed, although most studies were performed in middle-age or elderly populations. The reasons and significance of the positive correlation of HCY with creatinine remain still unclear (Elshorbagy et al. 2007). Inadequate folate status was associated with higher HCY levels independently of the creatinine levels. Interestingly, in our study trained women exhibited a marked elevation of creatinine. Although athletes and controls did not differ in BMI, increased creatinine could partly be due to larger muscle mass in athletes, since creatinine is produced from creatine, which is stored in muscles. Consequently, it is assumed that the increase of muscle mass rather than a subtle impairment of renal function makes serum creatinine to increase in athletes (Banfi et al. 2006). However, our observation that creatinine is positively associated to HCY, a venous thromboembolism risk factor, highlighted that the increase of creatinine in young female athletes could have potentially some adverse consequences; further studies are required to clarify this issue.
The strength of our study is that we examined an ethnically homogenous population of white Italian women, in a rather narrow interval of age (mean ± SD, 23.4 ± 4.53 years) and BMI (mean ± SD, 20.8 ± 1.97 kg m−2). On the other hand, as ethnic differences were noted for HCY and folate (Ganji and Kafai 2006; Okura et al. 2006; Scholl and Johnson 2000), our data could not take into account effects of physical training on adult healthy female populations belonging to other ethnic groups, and/or of older/younger age, and/or with lower/higher BMI. Another limitation is that our study was cross-sectional in design, and hence causal relationships cannot be inferred.
Research examining the impact of physical activity on blood homocysteine levels is equivocal, this could be partially due to a lack of control for confounding variables that affect homocysteine (Borrione et al. 2008; Herrmann et al. 2003). Duration, intensity, and mode of exercise could impact blood homocysteine levels differently, and may be dependent on individual fitness levels. Our results concur with those of Mora et al. who found that among a large cohort of mean 55-year-old US women HCY was only minimally correlated with either physical activity or BMI (Mora et al. 2006). However, we observed a lower frequency of elevated HCY levels than those found in a group of Italian winter elite athletes, comprising 59 males and 44 females, where plasma HCY levels ≥15.0 μmol l−1 were detected in 41% of males and in 21% of females (Borrione et al. 2007). The rate of hyperhomocysteinemia ≥15.0 μmol l−1 in female elite winter athletes was almost twice that we observed in recreational female athletes comprising mostly volleyball athletes. Another study by Borrione et al. (2008) comparing 23 female athletes (practising basketball, swimming and soccer) with 30 female blood donors found no difference in HCY concentrations, but a higher frequency of hyperhomocysteinemia ≥15.0 μmol l−1 in athletes than in controls by unadjusted analysis. Unfortunately, this study did not perform multivariate adjusted analyses for variables known to influence HCY such as age, creatinine and folate. In addition, rather surprisingly, Borrione et al. (2008) did not found an inverse correlation between HCY and folate. Further studies are necessary to assess whether the kind (aerobic vs. anaerobic) and/or intensity, and/or duration, and/or altitude of sport activity modulate HCY and whether there are striking gender differences among athletes.
A limitation of our study is that the majority of our female athletes were performing volleyball, an alternate aerobic–anaerobic discipline. However, volleyball is one of the recreational sports most frequently practiced by young women.
Overall, our study did not find negative effects of physical activity on blood HCY, folate and lipid status in healthy young non-obese women performing regular physical activity at recreational level, meaning that recreational physical activity should not be discouraged in young female athletes with elevated homocysteine or unfavorable lipid profile. However, we failed to demonstrate beneficial effects of physical exercise, on these basis, recreational physical activity should not be recommended to young sedentary women having elevated HCY with the aim to reduce hyperhomocysteinemia.
Of concern, we found that a consistent percent of healthy women in young age showed elevated HCY levels at CVD risk. We observed that the risk of elevated HCY is associated to a suboptimal folate level, which is not only folate below the reference range (<3.0 μg l−1), but also below the median value of folate (<5.0 μg l−1). Interestingly, we observed that in this population none of the studied lifestyle factors—physical activity, smoking, dietary habits, and coffee, tea, and alcohol consumption—was significantly associated with changes in HCY. In part, this finding could derive from the fact that only six of our study women smoked ≥10 cigarettes day−1 and five consumed ≥4 coffee cups day−1, which are levels of smoking and coffee consumption shown as able to increase HCY in women (de Bree et al. 2001). Overall, our results suggest that in young women HCY may not be reduced by lifestyle changes with the exception of a greater folate intake. In this respect, our finding confirm observations performed in a study conducted in male athletes (Rousseau et al. 2005).
Present findings highlight the importance of HCY and folate monitoring even in young normal-weight healthy women and supports the continuous effort to increase folate intake in fertile age women both for prevention of CVD and adverse pregnancy outcomes (McNulty et al. 2008; Scholl and Johnson 2000; Taparia et al. 2007).
Conclusion
Although the contribution of hyperhomocysteinemia in pathogenesis and risk assessment of cardiovascular disorders is still debated, raised levels of this sulfur-containing amino acid might exert an atherothrombotic effect, promoting also adverse pregnancy outcomes. To date, controversial evidence was provided on the association between physical activity and HCY levels in young women, who might be exposed to the risk of vascular events in the periconceptional period. The present study provides a clear evidence that recreational physical activity, which is traditionally associated with a lower risk of venous and arterial thrombosis, does not chronically influence a variety of cardiovascular risk factors (i.e. lipids and lipoproteins), nor it causes an increase in HCY level. This is a valuable support to the statement that a physically active lifestyle can be safely recommended in the young female population.
References
Banfi G, Del Fabbro M, Lippi G (2006) Relation between serum creatinine and body mass index in elite athletes of different sport disciplines. Br J Sports Med 40:675–678. doi:10.1136/bjsm.2006.026658
Borrione P, Pigozzi F, Massazza G, Schonhuber H, Viberti G, Paccotti P et al (2007) Hyperhomocysteinemia in winter elite athletes: a longitudinal study. J Endocrinol Invest 30:367–375
Borrione P, Rizzo M, Spaccamiglio A, Salvo RA, Dovio A, Termine A et al. (2008) Sport-related hyperhomocysteinemia: a putative marker of muscular demand to be noticed for cardiovascular risk. Br J Sports Med (in press)
Casabellata G, Di Santolo M, Banfi G, Stel G, Gonano F, Cauci S (2007) Evaluation of iron deficiency in young women in relation to oral contraceptive use. Contraception 76:200–207. doi:10.1016/j.contraception.2007.04.016
Cauci S, Di Santolo M, Culhane JF, Stel G, Gonano F, Guaschino S (2008) Effects of third-generation oral contraceptives on high-sensitivity C-reactive protein and homocysteine in young women. Obstet Gynecol 111:857–864
de Bree A, Verschuren WM, Blom HJ, Kromhout D (2001) Lifestyle factors and plasma homocysteine concentrations in a general population sample. Am J Epidemiol 154:150–154. doi:10.1093/aje/154.2.150
De Cree C, Malinow MR, van Kranenburg GP, Geurten PG, Longford NT, Keizer HA (1999) Influence of exercise and menstrual cycle phase on plasma homocyst(e)ine levels in young women—a prospective study. Scand J Med Sci Sports 9:272–278
Di Santolo M, Stel G, Banfi G, Gonano F, Cauci S (2008) Anemia and iron status in young fertile non-professional female athletes. Eur J Appl Physiol 102:703–709. doi:10.1007/s00421-007-0647-9
Elshorbagy AK, Oulhaj A, Konstantinova S, Nurk E, Ueland PM, Tell GS et al (2007) Plasma creatinine as a determinant of plasma total homocysteine concentrations in the Hordaland Homocysteine Study: use of statistical modeling to determine reference limits. Clin Biochem 40:1209–1218. doi:10.1016/j.clinbiochem.2007.07.014
Ganji V, Kafai MR (2006) Population reference values for plasma total homocysteine concentrations in US adults after the fortification of cereals with folic acid. Am J Clin Nutr 84:989–994
Herrmann M, Schorr H, Obeid R, Scharhag J, Urhausen A, Kindermann W et al (2003) Homocysteine increases during endurance exercise. Clin Chem Lab Med 41:1518–1524. doi:10.1515/CCLM.2003.233
Herrmann W, Herrmann M, Obeid R (2007) Hyperhomocysteinaemia: a critical review of old and new aspects. Curr Drug Metab 8:17–31. doi:10.2174/138920007779315008
Konig D, Bisse E, Deibert P, Muller HM, Wieland H, Berg A (2003) Influence of training volume and acute physical exercise on the homocysteine levels in endurance-trained men: interactions with plasma folate and vitamin B12. Ann Nutr Metab 47:114–118. doi:10.1159/000070032
Kuo HK, Yen CJ, Bean JF (2005) Levels of homocysteine are inversely associated with cardiovascular fitness in women, but not in men: data from the National Health and Nutrition Examination Survey 1999–2002. J Intern Med 258:328–335. doi:10.1111/j.1365-2796.2005.01546.x
Lippi G, Targher G, Franchini M, Guidi GC (2007) Has homocysteine shrunk? Clin Chem Lab Med 45:1419–1420. doi:10.1515/CCLM.2007.284
Lloyd-Jones DM, Tian L (2006) Predicting cardiovascular risk: so what do we do now? Arch Intern Med 166:1342–1344. doi:10.1001/archinte.166.13.1342
McNulty H, Pentieva K, Hoey L, Ward M (2008) Homocysteine, B-vitamins and CVD. Proc Nutr Soc 67:232–237. doi:10.1017/S0029665108007076
Mora S, Lee IM, Buring JE, Ridker PM (2006) Association of physical activity and body mass index with novel and traditional cardiovascular biomarkers in women. JAMA 295:1412–1419. doi:10.1001/jama.295.12.1412
Mora S, Cook N, Buring JE, Ridker PM, Lee IM (2007) Physical activity and reduced risk of cardiovascular events: potential mediating mechanisms. Circulation 116:2110–2118. doi:10.1161/CIRCULATIONAHA.107.729939
O’Donnell E, De Souza MJ (2004) The cardiovascular effects of chronic hypoestrogenism in amenorrhoeic athletes: a critical review. Sports Med 34:601–627. doi:10.2165/00007256-200434090-00004
Okura T, Rankinen T, Gagnon J, Lussier-Cacan S, Davignon J, Leon AS et al (2006) Effect of regular exercise on homocysteine concentrations: the HERITAGE Family Study. Eur J Appl Physiol 98:394–401. doi:10.1007/s00421-006-0294-6
Pedersen BK, Saltin B (2006) Evidence for prescribing exercise as therapy in chronic disease. Scand J Med Sci Sports 16(Suppl 1):3–63. doi:10.1111/j.1600-0838.2006.00520.x
Quinlivan EP, McPartlin J, McNulty H, Ward M, Strain JJ, Weir DG et al (2002) Importance of both folic acid and vitamin B12 in reduction of risk of vascular disease. Lancet 359:227–228. doi:10.1016/S0140-6736(02)07439-1
Randeva HS, Lewandowski KC, Drzewoski J, Brooke-Wavell K, O’Callaghan C, Czupryniak L et al (2002) Exercise decreases plasma total homocysteine in overweight young women with polycystic ovary syndrome. J Clin Endocrinol Metab 87:4496–4501. doi:10.1210/jc.2001-012056
Refsum H, Smith AD, Ueland PM, Nexo E, Clarke R, McPartlin J et al (2004) Facts and recommendations about total homocysteine determinations: an expert opinion. Clin Chem 50:3–32. doi:10.1373/clinchem.2003.021634
Ridker PM, Manson JE, Buring JE, Shih J, Matias M, Hennekens CH (1999) Homocysteine and risk of cardiovascular disease among postmenopausal women. JAMA 281:1817–1821. doi:10.1001/jama.281.19.1817
Ronnenberg AG, Goldman MB, Chen D, Aitken IW, Willett WC, Selhub J et al (2002) Preconception folate and vitamin B(6) status and clinical spontaneous abortion in Chinese women. Obstet Gynecol 100:107–113. doi:10.1016/S0029-7844(02)01978-6
Rousseau AS, Robin S, Roussel AM, Ducros V, Margaritis I (2005) Plasma homocysteine is related to folate intake but not training status. Nutr Metab Cardiovasc Dis 15:125–133. doi:10.1016/j.numecd.2005.02.002
Ruiz JR, Sola R, Gonzalez-Gross M, Ortega FB, Vicente-Rodriguez G, Garcia-Fuentes M et al (2007) Cardiovascular fitness is negatively associated with homocysteine levels in female adolescents. Arch Pediatr Adolesc Med 161:166–171. doi:10.1001/archpedi.161.2.166
Scholl TO, Johnson WG (2000) Folic acid: influence on the outcome of pregnancy. Am J Clin Nutr 71:1295S–1303S
Sesso HD, Paffenbarger RS Jr, Lee IM (2000) Physical activity and coronary heart disease in men: the Harvard Alumni Health Study. Circulation 102:975–980
Taparia S, Gelineau-van Waes J, Rosenquist TH, Finnell RH (2007) Importance of folate-homocysteine homeostasis during early embryonic development. Clin Chem Lab Med 45:1717–1727. doi:10.1515/CCLM.2007.345
Unt E, Zilmer K, Magi A, Kullisaar T, Kairane C, Zilmer M (2007) Homocysteine status in former top-level male athletes: possible effect of physical activity and physical fitness. Scand J Med Sci Sports 18:360–366
Vanuzzo D, Pilotto L, Lombardi R, Lazzerini G, Carluccio M, Diviacco S et al (2007) Both vitamin B6 and total homocysteine plasma levels predict long-term atherothrombotic events in healthy subjects. Eur Heart J 28:484–491. doi:10.1093/eurheartj/ehl470
Verloop J, Rookus MA, van der Kooy K, van Leeuwen FE (2000) Physical activity and breast cancer risk in women aged 20–54 years. J Natl Cancer Inst 92:128–135. doi:10.1093/jnci/92.2.128
Wald DS, Wald NJ, Morris JK, Law M (2006) Folic acid, homocysteine, and cardiovascular disease: judging causality in the face of inconclusive trial evidence. BMJ 333:1114–1117. doi:10.1136/bmj.39000.486701.68
Wang X, Qin X, Demirtas H, Li J, Mao G, Huo Y et al (2007) Efficacy of folic acid supplementation in stroke prevention: a meta-analysis. Lancet 369:1876–1882. doi:10.1016/S0140-6736(07)60854-X
Zee RY, Mora S, Cheng S, Erlich HA, Lindpaintner K, Rifai N et al (2007) Homocysteine, 5, 10-methylenetetrahydrofolate reductase 677C>T polymorphism, nutrient intake, and incident cardiovascular disease in 24, 968 initially healthy women. Clin Chem 53:845–851. doi:10.1373/clinchem.2006.083881
Zinellu A, Sotgia S, Caria MA, Tangianu F, Casu G, Deiana L et al (2007) Effect of acute exercise on low molecular weight thiols in plasma. Scand J Med Sci Sports 17:452–456
Zylberstein DE, Bengtsson C, Bjorkelund C, Landaas S, Sundh V, Thelle D et al (2004) Serum homocysteine in relation to mortality and morbidity from coronary heart disease: a 24-year follow-up of the population study of women in Gothenburg. Circulation 109:601–606. doi:10.1161/01.CIR.0000112581.96154.EA
Acknowledgments
Financial support was provided by University of Udine, Udine, Italy (research grants years 2006−2007). We thank Prof. Franco Quadrifoglio, Department Biomedical Sciences and Technologies, School of Medicine, University of Udine, for critical revision of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Di Santolo, M., Banfi, G., Stel, G. et al. Association of recreational physical activity with homocysteine, folate and lipid markers in young women. Eur J Appl Physiol 105, 111–118 (2009). https://doi.org/10.1007/s00421-008-0880-x
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00421-008-0880-x