Abstract
Adenomatosis polyposis coli downregulated 1 (APCDD1), a negative regulator of Wnt signaling, was examined to understand detailed mechanisms underlying Wnt signaling tooth development. In situ hybridization showed that Apcdd1 was expressed in the condensed mesenchyme at the bud stage, and in the inner enamel epithelium (IEE), including enamel knot (EK) at the cap stage. In vitro organ cultivation by using Apcdd1 antisense oligodeoxynucleotides was performed at E13.5 for 2 days to define the developmental functions of APCDD1 during tooth development. Analysis of histogenesis and cellular events such as cell adhesion, proliferation, apoptosis and epithelial rearrangement after Apcdd1 knockdown showed altered morphogenesis of the tooth germ with decreased cell proliferation and altered localization of cell adhesion molecules. Actin filament staining and 1,1′-dioctadecyl-3,3,3′,3′-tetramethylindocarbocyanine perchlorate (DiI) labeling of IEE cells showed that Apcdd1 knockdown enhanced epithelial rearrangement in the IEE and EK. To understand the precise signaling regulations of Apcdd1, we evaluated the altered expression patterns of signaling molecules, related with Wnt and enamel knot signalings using RT-qPCR. Tooth germs at cap stage were transplanted into the kidney capsules and were allowed to develop into calcified teeth for 3 weeks. Apcdd1 knockdown increased the number of ectopic cusps on the mesial side of the tooth. Our results suggested that APCDD1 modulates the gene expression of Wnt- and EK-related signaling molecules at the cap stage of tooth development, and is involved in tooth cusp patterning by modulating the epithelial rearrangement in the IEE.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Tooth development is a well-known model system to understand the developmental signaling regulations with the specific morphological changes in epithelium from thickening to bud, cap and bell stages (Thesleff 2003). During the mouse molar development, the primary enamel knot (PEK) signaling center, which controls tooth morphogenesis via the regulation of cell proliferation in surrounding tissues, is formed at the cap stage (Thesleff 2003). Tooth development occurs through epithelial rearrangement, which involves controlled proliferation, selected apoptosis and specific migration of cells in the inner enamel epithelium (IEE) (Williams-Masson et al. 1998; Cobourne and Sharpe 2005; Obara and Lesot 2007; Sohn et al. 2011). Epithelial rearrangement of IEE cells facilitates the formation and positioning of secondary EKs (SEKs) that determine cusp-forming sites (Coin et al. 1999; Cobourne and Sharpe 2005; Obara and Lesot 2007). During the late bell stage, IEE cells differentiate into ameloblasts, which secrete amelogenin, and undergo mineralization starting from the cusp tips (Jernvall and Thesleff 2012). Formation of enamel organ architecture through epithelial rearrangement, which involves cell adhesion, cell polarization and proliferation, is important for proper tooth morphogenesis and thus mastication. Epithelial rearrangement is modulated by cadherin molecules through actin cytoskeleton and ROCK (Palacios et al. 1995; Halbleib and Nelson 2006; Obara and Lesot 2007; Martinez-Rico et al. 2009; Otsu et al. 2011). In this study, we examined the effect of Apcdd1 knockdown on epithelial cell rearrangement by assessing functional molecules such as E-cadherin, P-cadherin and ROCK2 and formation of actin filaments.
Tooth development is regulated by multiple growth and transcription factors such as bone morphogenetic protein (BMP), ectodysplasin, fibroblast growth factor (FGF), hedgehog (Hh), transforming growth factor-beta and wingless (Wnt) (Tummers and Thesleff 2009; Pispa and Thesleff 2003; Biggs and Mikkola 2014; Thesleff and Mikkola 2002). Although various studies have provided abundant information on genes involved in tooth development, detailed molecular mechanisms underlying tooth morphogenesis are not completely understood. Wnt signaling plays important roles in crown formation and tooth number determination. Overexpression of Wnt or activation of β-catenin in the oral epithelium results in the fusion of tooth germs (Pispa et al. 2004). Mutations in genes encoding Wnt inhibitors such as ectodin and APC result in the formation of supernumerary teeth (Kassai et al. 2005; Wang et al. 2009). Overexpression of Wnt inhibitor Dkk1 in epithelial cells results in tooth agenesis (Liu et al. 2008). Therefore, identification of factors involved in the fine-tuning of Wnt signaling is important to understand precise mechanisms underlying tooth morphogenesis, especially crown morphogenesis.
Adenomatosis polyposis coli downregulated 1 (APCDD1) is a membrane-bound glycoprotein conserved during vertebrate evolution that regulates important biological processes controlled by Wnt signaling (Jukkola et al. 2004; Shimomura et al. 2010; Tsai et al. 2014). Abundant expression of Apcdd1 is observed in human and mouse hair follicles (Jukkola et al. 2004; Shimomura et al. 2010), and a point mutation in Apcdd1 causes hereditary hypotrichosis simplex (Shimomura et al. 2010). Studies on cancer and hair development have reported that APCDD1 is a direct target of Wnt/β-catenin in colon cancer cells (Takahashi et al. 2002) and that APCDD1 inhibits Wnt/β-catenin signaling in cultured cells, by possibly preventing the binding of FZD2 to Wnt (Shimomura et al. 2010). However, detailed functional evaluation of APCDD1 in tooth development has not been performed to date. In this study, we investigated in detail the functions of APCDD1 in tissue- and stage-specific regulation of Wnt signaling during tooth development. The results of this study will help in understanding the novel regulation of Wnt signaling in a range of organ development.
Materials and methods
Animals
All experiments involving animals were performed according to the guidelines of the Kyungpook National University, School of Dentistry, Intramural Animal Use and Care Committee. Mouse embryos were obtained from time-mated pregnant mice kept in an optimal environment. The day on which a vaginal plug was confirmed was designated as embryonic day 0 (E0). Embryos at stages E12–E15 were used.
In situ hybridization
Whole-mount and section in situ hybridizations were performed at 68 °C by using digoxigenin (DIG)-labeled RNA probes using standard protocols, as described previously (Neupane et al. 2014).
In vitro organ cultivation and renal capsule transplantation
Embryonic molar tooth buds of mice were cultivated for 2 days and were transplanted into the kidney capsule, as described previously (Neupane et al. 2014). During in vitro organ culture, antisense oligodeoxynucleotides (AS-ODNs) against Apcdd1 were added to the medium at a final concentration of 1 μM. Sequences of ODNs are as follows: AS-ODN 5′-CACTGTGACTCCTTGAAAGCC-3′ and sense (S)-ODN 5′-GGCTTTCAAGGAGTCACAGTG-3′ (GENOTECH, Korea).
Three-dimensional reconstruction
Serial sections obtained after in vitro cultivation of tooth organs were photographed using DM2500 microscope (Leica, Germany). Tooth organs were reconstructed using ‘Voloom 2.3’ software (Micro Dimensions, Germany).
Histology and immunohistochemistry
Histological analysis and immunostaining were performed as described previously (Sohn et al. 2014). Immunostaining was performed using anti-Ki67 (RM-9106; Neo Markers, CA, USA), anti-P-cadherin (NBP1 59222; Novus Biologicals, CO, USA), anti-E-cadherin (AF748; R&D Systems, USA), anti-ROCK2 (bs-10173R; Bioss Antibodies, MA, USA) and anti-β-catenin (8814 and 9562; Cell Signaling Technology, MA, USA) primary antibodies and biotinylated goat anti-rabbit or anti-mouse secondary antibodies. Immunocomplexes were visualized using a diaminobenzidine tetrahydrochloride reagent kit (00-2014; Zymed Laboratories, CA, USA).
TUNEL assay
TUNEL assay was performed as described previously (Sohn et al. 2014) by using an in situ cell apoptosis detection kit (Trevigen, MD, USA), according to the manufacturer’s instructions.
Phalloidin staining
Phalloidin staining was performed as described previously (Sohn et al. 2011). Briefly, frozen sections were washed with PBS and were permeabilized with 0.1 % Triton X-100 in PBS. The sections were then incubated with phalloidin–fluorescein isothiocyanate (p5282; Sigma, MO, USA) at room temperature for 1 h and were visualized using a fluorescence microscope (MZ-FL16FA; Leica, Germany).
Slice cultivation and DiI labeling
Slice cultivation and 1,1′-dioctadecyl-3,3,3′,3′-tetramethylindocarbocyanine perchlorate (DiI) labeling were performed as described previously (Cho et al. 2007) with a slight modification. Briefly, mandibles were dissected from E13.5 embryos and were embedded in 2 % low-melting agarose (V2111; Promega, Madison, WI, USA). Next, 200-μm frontal slice sections were prepared using a Vibratome (Technical Products International Inc., St. Louis MO, USA). Fluorescent carbocyanine dye DiI (C-7001; Life Technologies, OR, USA) was microinjected into the exposed IEE of the tooth organ in the slice section of the mandible. The sections were then covered with a cover glass and were incubated for 24 h.
Quantitative PCR
Quantitative PCR (qPCR) was performed as described previously (Neupane et al. 2014). The results of qPCR for each sample were normalized to Hprt and were expressed as normalized ratios. Table 1 lists the primer sequences used for performing qPCR. Data are expressed as the mean ± standard deviation (SD). Mean expression levels were compared between experimental and control groups by using Student’s t test. P values of <0.05 were considered significant.
Results
Expression pattern of Apcdd1 in the developing lower molars
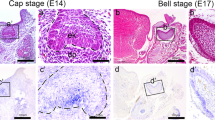
Distinct Apcdd1 expression patterns were observed in the tooth-forming tissue at stages E12–E15 (Fig. 1a–g). During epithelial invagination at E12, Apcdd1 was expressed in the invaginated dental epithelium (Fig. 1a, d). At E13, Apcdd1 was expressed in the condensed mesenchyme (Fig. 1b, e). At the cap stage E14, Apcdd1 was expressed distinctly in the EK and IEE (Fig. 1c, f). However, Apcdd1 expression was not detected in the outer enamel epithelium (OEE) and mesenchyme at E14 (Fig. 1f). Similar expression pattern was observed at E15 (Fig. 1g). The expression pattern of Apcdd1 was confirmed using frozen frontal sections after whole-mount in situ hybridization (data not shown). These results suggested that APCDD1, a negative regulator of Wnt signaling, would involve in the cap stage of tooth development.
In situ hybridization and in vitro organ cultivation. Whole-mount in situ hybridization by using DIG-labeled Apcdd1 mRNA probes (a–c) (E12–E14). Frozen sections obtained after whole-mount in situ hybridization (d). Apcdd1 expression is detected in the invaginated epithelium at E12 (d). Section in situ hybridization showing Apcdd1 expression in the condensed mesenchyme at E13 and in the EK and IEE at E14 (e–f). Apcdd1 expression at E15 is similar to that at E14 (g). AS-ODN-treated embryonic tooth cultured at E13.5 for 2 days by using modified Trowell’s culture method showing a larger tooth structure (k) than the control tooth (h). Downregulated Apcdd1 expression after AS-ODN treatment (j). Frontal section and 3D reconstruction showing altered EK and IEE structures in the AS-ODN-treated specimen (l–l″) compared to the control specimen (i–i″). The dotted lines indicate sectioning position (h, k); **p < 0.01. Bu, buccal; Di, distal; Li, lingual; Me, mesial. Scale bars 500 μm (b–c), 200 μm (a, h and k) and 50 μm (d–g)
Knockdown of Apcdd1 during in vitro organ cultivation alters cellular events
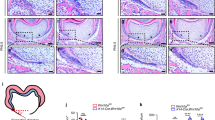
Prior to epithelial Apcdd1 expression, we treated the tooth organs cultivated at E13.5 with AS-ODNs to knockdown Apcdd1 in order to understand the role of APCDD1 in tooth development. Morphological changes in control specimen cultivated at E13.5 for 2 days were similar to those in tooth germs at E14.5 (Fig. 1h). Results of qPCR showed that knockdown of Apcdd1 by treatment with AS-ODNs for 1 day decreased Apcdd1 expression by approximately 45 % compared with that in the control specimen (Fig. 1j). Buccolingual diameter was increased in the AS-ODN-treated specimen (Fig. 1k, S1a). Hematoxylin and eosin (H&E) staining of the frontal sections showed larger cell structures in the EK and IEE of the AS-ODN-treated specimen (Fig. 2b–b″) than in the EK and IEE of the control specimen (Fig. 2a–a″). Ki67 immunostaining and TUNEL assay were performed to evaluate the effects of Apcdd1 knockdown on cellular physiology including proliferation and apoptosis (Fig. 2c–f). The number of Ki67-positive cells, particularly in the IEE, cervical loop and dental papilla, was decreased after Apcdd1 knockdown (Fig. 2d–d″, S1c) compared with that in the control specimen (Fig. 2c–c″, S1c). In addition, Ki67 immunostaining showed an enlarged non-proliferative region in the EK of the AS-ODN-treated specimen (Fig. 2d′). The number of apoptotic cells was higher in the EK of the AS-ODN-treated specimen (Fig. 2f–f″, S1b) than in the EK of the control specimen (Fig. 2e–e″, S1b).
Histogenesis and cellular events. H&E staining showing improperly formed and disoriented cells in the EK and IEE of the AS-ODN-treated specimen (b–b″) compared with those in the in the EK and IEE of the control specimen (a–a″). Ki67 immunostaining showing altered localization of Ki67-positive cells in the IEE after Apcdd1 knockdown (d–d″) compared with those in the IEE of the control specimen (c–c″). Results of TUNEL assay showing more number of apoptotic cells in the EK after Apcdd1 knockdown (f–f′) than that in the EK of the control specimen (e–e′). The dotted lines indicate the epithelium of tooth organ (a′–f′). Bu buccal, *EK enamel knot, IEE inner enamel epithelium, Li lingual, OEE outer enamel epithelium. Scale bars 100 μm (a–f) and 50 μm (a′–a″, b′–b″, c′–c″, d′–d″, e′, f′)
Altered localization patterns of epithelial cell adhesion- and rearrangement-related factors
Immunostaining of P-cadherin, E-cadherin, ROCK2, β-catenin and actin filaments was performed to evaluate the effects of Apcdd1 knockdown on cellular physiology including cell adhesion and epithelial rearrangement (Fig. 3a–l). Histological analysis showed decreased localization of adhesion molecules P- and E-cadherins in the IEE of the AS-ODN-treated specimen (Fig. 3b–b″, d–d″) compared with that in the IEE of the control specimen (Fig. 3a–a″, c–c″), which mimicked the in vivo localization pattern in the E15 control specimen (data not shown). In contrast, actin filament staining was more intense in the IEE of the AS-ODN-treated specimen (Fig. 3f–f″) than in the IEE of the control specimen (Fig. 3e–e″). Similarly, localization of both total and active β-catenin (Fig. 3h–h″, j–j″) and ROCK2 (Fig. 3l–l″) was more robust in the EK and IEE of the AS-ODN-treated specimen than in the EK and IEE of the control specimen (Fig. 3g–g″, i–i″, k–k″). Altered localization of factors involved in epithelial cell adhesion and rearrangement clearly suggested that APCDD1 played an important role in the formation of actin filaments through ROCK2 and β-catenin to form a proper epithelial structure during tooth development.
Localization pattern of P-cadherin, E-cadherin, β-catenin, phalloidin and ROCK2. Adhesion markers P- and E-cadherins are localized weakly in the IEE of the AS-ODN-treated specimen (b–b″, d–d″) compared with that in the control specimen (a–a″, c–c″). Frontal sections showing intense phalloidin staining in the IEE and EK of the AS-ODN-treated specimen (f′–f″) compared with that in the control specimen (e′–e″). Strong localization of β-catenin (total and active form) in the IEE and EK of the AS-ODN-treated specimen (h–h″, j–j″) compared with that in the control specimen (g–g″, i–i″). Staining intensity of ROCK2 is stronger in the AS-ODN-treated specimen (l–l″) than in the control (k–k″). Bu buccal, EK enamel knot, IEE inner enamel epithelium, Li lingual, OEE outer enamel epithelium. The dotted lines indicate the epithelium of the tooth (a–l). Scale bars 100 µm (a–l) and 50 µm (a′–a″, b′–b″, c′–c″, d′–d″, e′–e″, f′–f″, g′–g″, h′–h″, i′–i″, j′–j″, k′–k″ and l′–l″)
Altered expression of EK- and Wnt-related signaling molecules
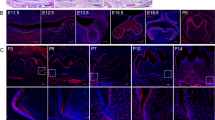
We examined the altered expression of Wnt signaling- and EK-related molecules by performing qPCR and in situ hybridization (Fig. 4). Knockdown of Apcdd1 downregulated the expression of Wnt-related factors, downregulated the expression of β-catenin and cyclin D1 (Ccnd1) (Fig. 4a, S2), and upregulated the expression of EK-related signaling factors such as Bmp2, Bmp4, Bmp7, Fgf4, Lef1 and Shh (Fig. 4b). Expression of Fgf4, a distinct marker of SEK formation, was examined by performing whole-mount in situ hybridization (Fig. 4c, d). AS-ODN-treated tooth germs cultivated at E13.5 for 4 days showed increased number of Fgf4 expression spots (Fig. 4d) compared with control germs cultivated at E13.5 for 4 days (Fig. 4c). Control frontal sections showed distinct Fgf4 expression spots in the SEKs (Fig. 4c′), whereas AS-ODN-treated frontal sections showed multiple spots in the IEE, including SEKs (Fig. 4d′). The IEE cells were labeled with DiI at E13.5, to examine the cell rearrangement pattern in the IEE, which would be involved in formation of secondary enamel knots during slice in vitro organ cultivation as was performed previously (Cho et al. 2007; Sohn et al. 2011). After 24-h incubation, we observed that DiI-labeled cells in the AS-ODN-treated specimen migrated faster from the point of injection to the EK and cervical loop region in AS-ODN-treated specimen (Fig. 4f–f′) than DiI-labeled cells in the control specimen (Fig. 4e–e′).
Altered expression patterns of signaling molecules and epithelial rearrangement patterning. Expression of Wnt signaling-related molecules is downregulated (a), whereas that of EK-related molecules is upregulated (b). Whole-mount in situ hybridization of specimens cultivated at E13.5 for 4 days by using Fgf4 probes (c, d). Fgf4 expression pattern is broader in the AS-ODN-treated specimen (d) than in the control specimen (c). Results of whole-mount in situ hybridization showing Fgf4 expression in the IEE of the AS-ODN-treated specimen (d′) and restricted Fgf4 expression in the SEK of the control specimen (c′). Altered migration of DiI-labeled cells in the AS-ODN-treated specimen (f–f′) compared with that in the control specimen (e–e′) after 24-h cultivation. The insets show merged figures. The dotted lines indicate the epithelium of tooth organ (c′–d′) and the level of sectioning position (c, d); *p < 0.05 and **p < 0.01. Bu buccal, Di distal, EK enamel knot, Li lingual, Me mesial, SEK secondary enamel knot. Scale bars 200 μm (c, d), 100 μm (e–f) and 50 μm (c′, d′)
Altered morphology of calcified teeth after renal capsule transplantation
To determine the effect of Apcdd1 knockdown at the cap stage on tooth development, we performed renal capsule transplantation and examined morphological alterations in the calcified teeth (Fig. 5). AS-ODN-treated and control tooth germs cultivated at E13.5 for 2 days were transplanted into the subcapsular layer of the kidney for 3 weeks and were harvested to examine their morphological features. Control teeth showed normal morphology of PN10 in vivo (Fig. 5a–a″), while AS-ODN-treated teeth showed irregular crown and cusp morphology (Fig. 5b–b″). In addition, most AS-ODN-treated teeth showed increased number of cusps (+1, 57 % [n = 8/14] or +2, 36 % [n = 5/14] or +3, 7 % [n = 1/14]) in the mesial side (Fig. 5b–b″; Table 2).
Calcified teeth in the kidney capsule at 21 days after transplantation of tooth germs cultivated at E13.5 for 2 days. Buccal view of control and AS-ODN-treated teeth (a, b). Lateral and occlusal views showing increased number of cusps with irregular crown morphology in the AS-ODN-treated specimen (b′–b″) compared with that in the control specimen (a′–a″). Scale bars 500 μm (a, b) and 200 μm (a′, a″, b′, b″)
Discussion
In mice, Apcdd1 is abundantly expressed in the hair follicles, in the nervous and vascular systems and during inner ear formation (Jukkola et al. 2004; Shimomura et al. 2010). The signaling pathways involved in the ectodermal organogenesis share similar signaling pathways, including the Wnt pathway (Biggs and Mikkola 2014; Tsai et al. 2014). Therefore, we hypothesized that APCDD1 could also be involved in tooth crown morphogenesis by fine-tuning the Wnt signaling pathway. We found that Apcdd1 was expressed in the invaginated epithelium at E12, and in the condensed mesenchyme at E13 and EK and IEE at E14 and E15, respectively, in the developing molar tooth in mice (Fig. 1a–d). This oscillating expression of Apcdd1 in the epithelium and mesenchyme was similar to that of integrin V, suggesting that APCDD1 modulated cellular physiology such as cell adhesion and migration (Rallis et al. 2010; Salmivirta et al. 1996).
Knockdown of Apcdd1 by treatment with AS-ODNs during in vitro organ cultivation induced structural alterations in the IEE and EK, which increased the EK area. The increased EK area showed altered cellular events, including increased apoptosis (Fig. 2d, S1b), whereas the IEE showed decreased cell proliferation (Fig. 2f, S1c, S1d). The changes in the epithelial structure of the EK and IEE after the treatment with AS-ODN showed weak localization of P-cadherin and E-cadherin, which were reported to co-localize with Apcdd1 (Shimomura et al. 2010). Previous studies have shown strong localization patterns of P- and E-cadherins in the cap stage of molar development in mice (Obara and Lesot 2004). Our results suggested that Apcdd1 knockdown compromised cell adhesion in the IEE by decreasing the localization of E- and P-cadherins. Strong localizations of β-catenin (total and active) in the IEE after knockdown of Apcdd1 knockdown confirmed that APCDD1 inhibited Wnt signaling as reported previously (Takahashi et al. 2002; Shimomura et al. 2010). The increase in protein level may be because of the accumulation of β-catenin in the cytoplasm of IEE cells after Apcdd1 knockdown, as observed in a previous study (Shimomura et al. 2010). Meanwhile, decreased mRNA expression may be because of the immediate interruption of APCDD1 signaling in all tooth-forming tissues, including epithelial and mesenchymal cells, after Apcdd1 knockdown. Strong localization of ROCK2 in the IEE after Apcdd1 knockdown suggested altered actin filament formation during epithelial rearrangement in the IEE (Fig. 3l). These results suggested that Apcdd1 knockdown at the cap stage altered epithelial rearrangement through the Wnt signaling, thus altering the structure of EK and IEE and subsequently crown morphogenesis.
Epithelial rearrangement is a normal mechanism in the IEE and EK, which triggers the formation of SEKs (Obara and Lesot 2007). Gene expression patterns of SEKs are similar to those of PEK, with Fgf4 and Slit1 expression being the marker of both PEK and SEKs (Cho et al. 2007; Jernvall and Thesleff 2000; Loes et al. 2001). Intense actin filament staining in lingual IEE cells of the AS-ODN-treated specimen suggested that epithelial rearrangement preceded the cessation of Fgf4 expression in PEK (Figs. 4c–d, 5); this was further confirmed by performing whole-mount in situ hybridization. However, the number of proliferative cells in the IEE was low, suggesting that structural alteration in the IEE occurred because of cell migration rather than cell proliferation. This finding was confirmed by Fgf4 expression in the entire IEE of the AS-ODN-treated specimen (Fig. 4d). After Apcdd1 knockdown, Fgf4 expression pattern in these specimens at the cap stage deviated from the normal pattern to a linear pattern, suggesting that patterned IEE formation was important for crown morphogenesis and for determining the number of cusps (Figs. 5, 6, S3).
Schematic representation of altered Fgf4 expression and cuspal patterning after Apcdd1 knockdown. Fgf4 is expressed during the bud to cap stages and produces specific spots in the cusp-forming regions as tooth development progresses to the bell stage (a–c). Apcdd1 knockdown linearizes the expression pattern of Fgf4 in the mesial region of the tooth germ (d), thus increasing the number of cusps on the mesial side (f), compared with that in the control specimen (e). Black spots indicate Fgf4 expression (a–d), and circles indicate the position of cusps in the calcified teeth (e, f). Asterisk indicates extra cusps in the AS-ODN-treated teeth (f). Bu buccal, Di distal, Li lingual, Me mesial
EK determines the number of cusps as well as the shape of an individual tooth (Jernvall et al. 1994, Vaahtokari et al. 1996). PEK develops on the buccal side during the bud to cap stages of tooth development, and SEK forms during epithelial folding and marks the cusp-forming sites (Cho et al. 2007; Cobourne and Sharpe 2005). Apcdd1 knockdown increased apoptosis of PEK cells (Fig. 2f) and resulted in the faster migration and proliferation of DiI-labeled IEE cells (Fig. 4f) compared with that in the control specimen (Fig. 4e). Therefore, we hypothesize that some PEK cells remain in the SEK and migrate to initiate the formation of new SEKs, as reported earlier (Coin et al. 1999). Particularly, epithelial rearrangement in the PEK and IEE, which was affected by Apcdd1 knockdown, was responsible for the formation of more lingual SEKs. These cellular events would facilitate the formation of excessive SEKs, thus increasing the number of mesial cusps in the calcified tooth (Fig. 5). Our results showed that Wnt signaling is important for cusp formation, which is consistent with that reported previously (Liu et al. 2008; Wang et al. 2009; Jarvinen et al. 2006). This elucidation through the in vitro organ cultivation system suggests that precise spatiotemporal expression of specific genes is important for the formation of functional structures in different organs.
In summary, our data indicated that APCDD1 acts as a negative regulator of Wnt signaling for proper organogenesis and morphogenesis of molar teeth and tightly controls cellular rearrangement during patterned IEE formation. APCDD1 regulates the localization of P- and E-cadherins in the IEE during SEK formation to achieve proper tooth cusp development and to maintain proper epithelial structure and actin filament formation. In addition, fine-tuning of Wnt signaling by APCDD1 would determine proper cusp patterning through the optimal expression of β-catenin in the IEE at the cap stage of tooth development.
References
Biggs LC, Mikkola ML (2014) Early inductive events in ectodermal appendage morphogenesis. Semin Cell Dev Biol 25–26:11–21
Cho SW, Lee HA, Cai J, Lee MJ, Kim JY, Ohshima H, Jung HS (2007) The primary enamel knot determines the position of the first buccal cusp in developing mice molars. Differentiation 75:441–451
Cobourne MT, Sharpe PT (2005) Sonic hedgehog signaling and developing tooth. Curr Top Dev Biol 65:255–287
Coin R, Lesot H, Vonesch JL, Haikel Y, Ruch JV (1999) Aspects of cell proliferation kinetics of the inner dental epithelium during mouse molar and incisor morphogenesis: a reappraisal of the role of the enamel knot area. Int J Dev Biol 43:261–267
Halbleib JM, Nelson WJ (2006) Cadherins in development: cell adhesion, sorting, and tissue morphogenesis. Genes Dev 20:3199–3214
Jarvinen E, Salazar-Ciudad I, Birchmeier W, Taketo MM, Jernvall J, Thesleff I (2006) Continuous tooth generation in mouse is induced by activated epithelial Wnt/beta-catenin signaling. Proc Natl Acad Sci USA 103:18627–18632
Jernvall J, Thesleff I (2000) Reiterative signaling and patterning in mammalian tooth morphogenesis. Mech Dev 92:19–29
Jernvall J, Thesleff I (2012) Tooth shape formation and tooth renewal: evolving with the same signals. Development 139:3487–3497
Jernvall J, Kettunen P, Karavanova I, Martin LB, Thesleff I (1994) Evidence for the role of the enamel knot as a control center in mammalian tooth cusp formation: non-dividing cells express growth stimulating Fgf-4 gene. Int J Dev Biol 38(3):463–469
Jukkola T, Sinjushina N, Partanen J (2004) Drapc1 expression during mouse embryonic development. Gene Expr Patterns 4:755–762
Kassai Y, Munne P, Hotta Y, Penttila E, Kavanagh K, Ohbayashi N, Takada S, Thesleff I, Jernvall J, Itoh N (2005) Regulation of mammalian tooth cusp patterning by ectodin. Science 309:2067–2070
Liu F, Chu EY, Watt B, Zhang Y, Gallant NM, Andl T, Yang SH, Lu MM, Piccolo S, Schmidt-Ullrich R, Taketo MM, Morrisey EE, Atit R, Dlugosz AA, Miller SE (2008) Wnt/beta catenin signaling directs multiple stage of tooth morphogenesis. Dev Biol 313:210–224
Loes S, Luukko K, Kvinnsland IH, Kettunen P (2001) Slit1 is specifically expressed in the primary and secondary enamel knots during molar tooth cusp formation. Mech Dev 107:155–157
Martinez-Rico C, Pincet F, Thiery JP, Dufour S (2009) Integrins stimulate E-cadherin mediated intercellular adhesion by regulating Src-kinase activation and actomyocin contractility. J Cell Sci 123:712–722
Neupane S, Sohn WJ, Rijal G, Lee YJ, Lee S, Yamamoto H, An CH, Cho SW, Lee Y, Shin HI, Kwon TY, Kim JY (2014) Developmental regulations of Perp in mice molar morphogenesis. Cell Tissue Res 358(1):109–121
Obara N, Lesot H (2004) Subcellular localization of beta-catenin and cadherin expression in the cap-stage enamel organ of the mouse molar. Histochem Cell Biol 121:352–358
Obara N, Lesot H (2007) Asymmetrical growth, differential cell proliferation and dynamic cell rearrangement underlie epithelial morphogenesis in mouse molar. Cell Tissue Res 330:461–473
Otsu K, Kishigami R, Fujiwara N, Ishizeki K, Harada H (2011) Functional role of rho-kinase in ameloblast differentiation. J Cell Physiol 226:2527–2534
Palacios J, Benito N, Berraquero R, Pizarro A, Cano A, Gamallo C (1995) Differential spatiotemporal expression of E- and P-cadherin during mouse tooth development. Int J Dev Biol 39:363–666
Pispa J, Thesleff I (2003) Mechanisms of ectodermal organogenesis. Dev Biol 262(2):195–205
Pispa J, Mustonen T, Mikkola ML, Kangas AT, Koppinen P, Lukinmaa PL, Jernvall J, Thesleff I (2004) Tooth patterning and enamel formation can be manipulated by misexpression of TNF receptor Edar. Dev Dyn 231:432–440
Rallis C, Pinchin SM, Ish-Horowicz D (2010) Cell-autonomous integrin control of Wnt and Notch signaling during somitogenesis. Development 137:3591–3601
Salmivirta K, Gullberg D, Hirsch E, Altruda F, Ekblom P (1996) Integrin subunit expression associated with epithelial-mesenchymal interactions during murine tooth development. Dev Dyn 205(2):104–113
Shimomura Y, Agalliu D, Vonica A, Luria V, Wajid M, Baumer A, Belli S, Petukhova L, Schinzel A, Brivanlou AH, Barres BA, Christiano AM (2010) APCDD1 is a novel Wnt inhibitor mutated in hereditary hypotrichosis simplex. Nature 464:1043–1047
Sohn WJ, Yamamoto H, Shin HI, Ryoo ZY, Lee S, Bae YC, Jung HS, Kim JY (2011) Importance of region specific epithelial rearrangements in mouse rugae development. Cell Tissue Res 344:271–277
Sohn WJ, Choi MA, Yamamato H, Lee S, Lee Y, Jung JK, Jin MU, An CH, Jung HS, Shin HI, Kim JY (2014) Contribution of mesenchymal proliferation in tooth root morphogenesis. J Dent Res 93(1):78–83
Stewart GA, Lowrey JA, Wakelin SJ, Fitch PA, Lindey S, Dallman MJ, Lamb JR, Howie SE (2002) Sonic hedgehog signaling modulates activation of and cytokine production by human peripheral CD4+ T cells. J Immunol 169(10):5451–5457
Takahashi M, Fujita M, Furukawa Y, Hamamoto R, Shimokawa T, Miwa N, Oqawa M, Nakamura Y (2002) Isolation of a novel human gene, APCDD1, as a direct target of the b-catenin/T-cell factor 4 complex with probable involvement in colorectal carcinogenesis. Cancer Res 62:5651–5656
Thesleff I (2003) Epithelial-mesenchymal signalling regulating tooth morphogenesis. J Cell Sci 116:1647–1648
Thesleff I, Mikkola M (2002) The role of growth factors in tooth development. Int Rev Cytol 217:93–135
Tsai SY, Sennett R, Rezza A, Clavel C, Grisanti L, Zemla R, Najam S, Rendl M (2014) Wnt/β-catenin signaling in dermal condensates is required for hair follicle formation. Dev Biol 385(2):179–188
Tummers M, Thesleff I (2009) The importance of signal pathway modulation in all aspect of tooth development. J Exp Zool B Mol Dev Evol 312B(4):309–319
Vaahtokari A, Åbert T, Jernvall J, Keränen S, Thesleff I (1996) The enamel knot as a signaling center in the developing mouse tooth. Mech Dev 54:39–43
Wang XP, O’Connell DJ, Lund JJ, Saadi I, KuraguchiM Turbe-Doan A, Cavallesco R, Kim H, Park PJ, Harada H, Kucherlapati R, Maas RL (2009) Apc inhibition of Wnt signaling regulates supernumerary tooth formation during embryogenesis and throughout adulthood. Development 136:1939–1949
Williams-Masson EM, Heid PJ, Lavin CA, Hardin J (1998) The cellular mechanism of epithelial rearrangement during morphogenesis of Caenorhabditis elegans dorsal hypodermis. Dev Biol 204(1):263–276
Acknowledgments
This study was supported by a National Research Foundation of Korea (NRF) Grant funded by the Korean Government (MSIP; No. 2008–0062282).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Figure 1
Graphical presentation of buccolingual diameter (a), number of TUNEL-positive cells in the EK (b) and number of Ki67-positive cells in the IEE (c). The scheme for counting IEE cells (d). The shaded area indicates the region from which Ki67-positive cells were counted (d); **p < 0.01. EK, enamel knot; IEE, inner enamel epithelium (TIFF 4687 kb)
Supplementary Figure 2
Altered expression of Wnt signaling-related signaling molecules in tooth germ cultivated at E13 for 2 days (TIFF 769 kb)
Supplementary Figure 3
Whole-mount in situ hybridization of Fgf4 after in vitro cultivation at E15.5 for 3 days. The AS-ODN-treated specimen showing increased number of Fgf4 expression spots on the mesial and distal parts of the tooth germ compared with that in the control specimen (a, b). The arrows indicate increased Fgf4 expression. Bu, buccal; Di, distal; Li, lingual; Me, mesial. Scale bars: 200 μm (TIFF 590 kb)
Rights and permissions
About this article
Cite this article
Neupane, S., Sohn, WJ., Gwon, GJ. et al. The role of APCDD1 in epithelial rearrangement in tooth morphogenesis. Histochem Cell Biol 144, 377–387 (2015). https://doi.org/10.1007/s00418-015-1345-z
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00418-015-1345-z