Abstract
We analyzed the subcellular distribution of β-catenin in the cap-stage enamel organ and compared it with the expression of E- and P-cadherin by using confocal laser microscopy. The amounts of the molecules in the cytoplasm and the nucleus showed regional variations in the enamel organ, whereas cell surface-associated β-catenin was ubiquitous. In both the enamel knot and the inner dental epithelium, β-catenin was detected in the cytoplasm and in the nucleus. However, the amount of nuclear β-catenin was apparently higher in the enamel knot than in the inner dental epithelium. P-cadherin also gave a stronger signal in the enamel knot than in other parts of the enamel organ. In the stellate reticulum, where E-cadherin was preferentially expressed, as well as in the cervical loop and outer dental epithelium, β-catenin was localized in the cytoplasm but not in the nucleus. The nuclear localization of β-catenin in the enamel knot suggests a specific activation of the canonical Wnt signaling pathway. A coincident upregulation of P-cadherin was observed in this area. Altogether, these observations suggest the possibility of a linkage between cell adhesion and Wnt signaling in the enamel knot.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Beta-catenin is a multifunctional molecule, which participates in both Wnt signaling and cell–cell adhesion (Ozawa et al. 1989; Willert and Nusse 1998; Hecht and Kemler 2000). Wnt proteins are a family of secreted signaling molecules that have been shown to control a variety of developmental processes during embryogenesis and organogenesis. Many Wnt and related genes including their Frizzled receptors have been investigated in both vertebrate and invertebrate systems (Cadigan and Nusse 1997). In vertebrates, the Wnt proteins activate at least three different pathways, i.e., the Wnt/Ca2+ pathway, the planar cell polarity pathway, and the β-catenin activation-dependent canonical Wnt pathway (Huelsken and Birchmeier 2001; van Es et al. 2003).
Beta-catenin is a component of the molecular assembly required for cell–cell adhesion mediated by classical cadherins (Ozawa et al. 1989). It directly interacts with the cytoplasmic domain of cadherins as well as with α-catenin, consequently linking cadherins to the actin filament network (Nagafuchi 2001). In cells not exposed to Wnt signaling, β-catenin free from cadherin is phosphorylated through an association with a multiprotein complex containing casein kinase 1 (CK1), glycogen synthase kinase-3β (GSK3β), tumor suppressor adenomatous polypopsi coli (APC), and axin. This phosphorylated β-catenin is then degraded by the ubiquitin-proteasome pathway (Aberle et al. 1997) so that the β-catenin level remains low in these cells. Alternatively, GSK3β can be inactivated in the presence of a Wnt signal and does not phosphorylate β-catenin anymore, resulting in an increase in the cytoplasmic pool of β-catenin. Under this condition, β-catenin enters the nucleus where it interacts with the TCF/LEF family of DNA-binding proteins to act as a transcriptional activator of Wnt target genes (Behrens et al. 1996; Huber et al. 1996; Molenaar et al. 1996; Young et al. 1998). Thus, β-catenin can be located in three different sites within a cell: in association with the plasma membrane, in the cytoplasm, and in the nucleus. The different functions of β-catenin in particular cells and tissues are closely related to its subcellular localization and its being phosphorylated or not (van Noort et al. 2002).
Classical cadherins are possible regulators of the β-catenin/LEF-1 signaling pathway. Overexpression of cadherins prevents the transcriptional activity of β-catenin (Heasman et al. 1994; Fagotto et al. 1996; Sadot et al. 1998; Orsulic et al. 1999), and the tumor suppressor activity of E-cadherin results from an inhibition of the β-catenin/TCF signaling pathway (Gottardi et al. 2001). Conversely, it is also possible for β-catenin/LEF-1 to regulate E-cadherin transcription (Jamora et al. 2003).
In the developing tooth germ, three classical cadherins, E-, P-, and N-cadherin, are expressed in the epithelial compartment, i.e., the enamel organ (Palacios et al. 1995; Obara et al. 1998; Heymann et al. 2002). During early stages of tooth development (i.e., before amelogenesis), the expression patterns of E- and P-cadherins within the enamel organ change spatiotemporally in a complementary fashion. Similar observations were made in developing hair follicles (Hirai et al. 1989), where the Wnt signaling pathway was shown to participate in morphogenetic processes (Fuchs et al. 2001, Millar 2002). However, the exact significance of this differential expression of cadherins during development still remains unknown.
Wnt and related genes are expressed during mouse tooth development, in specific spatiotemporal patterns (Dassule and McMahon 1998; Sarkar and Sharpe 1999; Nadiri et al. 2004). Complementary data have indicated that several Wnt signaling pathways participate in both epithelial–mesenchymal and epithelial–epithelial interactions during tooth organogenesis. Ectopically expressed Dickkop 1, a potent diffusible inhibitor of the canonical Wnt pathway, blocked molar and incisor tooth development before the bud stage (Andl et al. 2002). Furthermore, the addition of an excess of Mfrzb1, which appears to function as a Wnt antagonist, resulted in retarded tooth bud formation and buds of smaller size (Sarkar and Sharpe 2000). Wnt 7b seems to play a role in maintaining the boundaries between oral and dental ectodermal cells (Sarkar et al. 2000). Furthermore, Wnt 6 induces ectodysplasin (Eda) in the dental epithelium (Laurikkala et al. 2001), and Wnt 10b induces expression of the Lef-1 gene in the dental mesenchyme (Dassule and McMahon 1998). LEF-1 is expressed in the dental epithelium as well as in the mesenchyme (van Genderen et al. 1994; Kratochwil et al. 1996). It is essential for tooth development, as such development is arrested at the bud stage in Lef-1 −/− mice (van Genderen et al. 1994). The role of LEF-1 during odontogenesis is dependent on its interaction with β-catenin (Kratochwil et al. 2002). LEF-1 would thus act as an effector of the Wnt/β-catenin signaling pathway in the tooth germs. One of the direct targets of LEF-1 and Wnt signaling in developing tooth germs is the Fgf-4 gene, which is exclusively expressed in the enamel knots and can rescue the arrest of tooth development in Lef-1 −/− mice (Jernvall et al. 1994; van Genderen et al. 1994).
Immunohistochemical studies have shown that β-catenin is expressed ubiquitously in the tooth germs except for a late stage of amelogenesis (Fausser et al. 1998; Sorkin et al. 2000) and that the non-phosphorylated “active” form of β-catenin shows a localized accumulation in the epithelium at the late bud stage (van Noort et al. 2002). However, the subcellular localization of β-catenin that probably reflects the different functional states of this molecule has never been investigated during tooth development. In this study, we investigated the subcellular localization of β-catenin in the enamel organ at the early cap stage, when the epithelial cells are differentiating into several compartments with different morphologies and fates (Lesot et al. 2002). In addition, we stained E- or P-cadherin on the same sections to directly compare their localization with that of β-catenin. β-catenin showed a preferential nuclear localization in the cells of the enamel knot when its expression in them was compared with that in the inner dental epithelium cells. This nuclear localization also coincided with a strong expression of P-cadherin.
Materials and methods
Materials
Embryos were obtained by mating ICR mice. The morning of the appearance of the vaginal plug was designated as day 0 of embryonic development, and embryos at day 14 were used. Embryos were obtained from pregnant females anesthetized with chloroform and killed by cervical dislocation. Tissues including tooth germs were immediately dissected and fixed in ice-cold periodate-lysine-2% paraformaldehyde (PLP) solution (McLean and Nakane 1974) for 6 h at 4°C. The fixed specimens were washed three times, 30 min each time, in 0.1 M sodium phosphate buffer containing 50 mM NH4Cl at 4°C, and these washings were followed by an overnight wash at 4°C in the same buffer containing 5% sucrose. The specimens were then immersed for 6 h at 4°C in phosphate-buffered saline (PBS) containing 20% sucrose, embedded in Tissue-Tek OCT compound (Sakura Finetek Europe, Zoeterwoude, The Netherlands), and frozen in liquid nitrogen. The frozen tissues were stored at −80°C.
Antibodies
Monoclonal rat antibodies to mouse E-cadherin (ECCD-2) and mouse P-cadherin (PCD-1) were purchased from Takara Biomedicals (Kyoto, Japan) and used at a concentration of 10 μg/ml. Rabbit anti-human β-catenin (Biosource Europe, Nivelles, Belgium) was used at a concentration of 20 μg/ml. For control staining, specific antibodies were replaced by purified rat and rabbit immunoglobulins, using the same concentrations as with the corresponding specific antibodies. Highly cross-absorbed secondary antibodies, Alexa 488-conjugated goat anti-rat IgG (H+L) and Alexa 568-conjugated goat anti-rabbit IgG (H+L) were purchased from Molecular Probes (Eugene, OR, USA) and used at a dilution of 1:500 (anti-rat and anti-rabbit IgG).
Immunohistochemistry
Frozen sections were cut at 8 μm thickness with a JUNG CM 3000 cryostat (Leica Microsystems, Germany), picked up on silane-coated slide glasses, and air-dried for 30 min at room temperature. All staining procedures except for the incubation with the primary antibodies were performed at room temperature. The frozen sections were rehydrated for 10 min in PBS containing 0.1% Triton X-100 (PBST) and incubated with normal goat serum (diluted 1:10 in PBST) for 10 min to block nonspecific staining. The sections were then incubated overnight at 4°C with a mixture of primary antibodies diluted in TBS containing 1% bovine serum albumin and 0.1% Triton X-100, washed with PBST three times for 5 min each time, and incubated with a mixture of secondary antibodies for 2 h. After a brief wash with PBS, the sections were reacted with DAPI (Molecular Probes Europe, Leiden, The Netherlands) at a concentration of 300 nM in PBS for 5 min, briefly washed again, and mounted with aqueous mounting medium Permafluor (Immunotech, Marseille, France).
Observation and processing of images
The stained sections were examined under a confocal laser scanning microscope, a Leica TCS SP1 equipped with a UV laser (Leica Microsystems). Digital images for each of three channels (blue, green, and red) were sequentially acquired at every 0.1628 μm distance, and three adjacent images were piled to make virtual 0.4884-μm sections for each channel. When it was necessary to combine images of two or three different colors, the separately piled images were merged. All the image processing procedures were performed with Photoshop 7.0 (Adobe Systems).
Results
Expression of cadherins and β-catenin in tooth germs
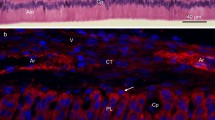
At embryonic day 14, both the upper and lower first molars were at the cap stage (Figs. 1A, 2L). Within each embryo, the development of the first molar appeared to be more advanced in the upper jaw than in the lower one. Both E- and P-cadherin were expressed in the enamel organ but not in the mesenchyme (Fig. 1B, C), whereas β-catenin was expressed in both tissues (Fig. 1C). The intensities of the labelings for the two cadherins varied throughout the enamel organ, and their patterns seemed to be complementary. The staining for P-cadherin was more intense than the one for E-cadherin in the inner and the outer dental epithelium and also in the enamel knot (Fig. 1C). Conversely, the staining for E-cadherin was stronger in the prospective stellate reticulum (Fig. 1B). To explore further relationships between β-catenin and E- or P-cadherin expression, we used confocal microscopy to study the subcellular localization of β-catenin together with the expression of both cadherins.
Expression of cadherins and β-catenin in a lower first molar at ED14. A Schematic representation of the epithelial compartments: the inner and outer dental epithelium (ide and ode, respectively), the enamel knot (ek), and the stellate reticulum (sr). B Immunofluorescence localization of E-cadherin. Double immunostaining for P-cadherin (C) and β-catenin (D). Scale bars 100 μm
Confocal images (A–K) and a scheme (L) of an upper first molar at the early cap stage after triple staining with DAPI, anti-β-catenin, and anti-P-cadherin antibodies. The developmental stage of this tooth germ is slightly more advanced than that in Fig. 1. A–F A part of the enamel knot area. G–K An area including both the enamel knot (ek) and inner dental epithelium (ide). The positions of A–F and G–K are indicated on the scheme (L) by the gray squares a and g, respectively. The prospective border between the enamel knot and the inner dental epithelium as well as the position of the basement membrane is indicated by a white line in G. A, G Localization of nuclei visualized with DAPI. B, H Localization of β-catenin. C, I Localization of P-cadherin. D, J Images combining nuclei and β-catenin; D corresponds to the merged images in A and B, and J, to those in G and H. The pink signal corresponds to β-catenin present in the nucleus. E, K Images combining β-catenin and P-cadherin; E corresponds to the merged images in B and C, and K, to those in H and I. The yellow signal represents the colocalization of β-catenin and P-cadherin at the cell surface. F The image combines all three elements shown in A, B, and C. Arrowheads indicate apoptosis-related structures that include membrane antigen P-cadherin and nuclear elements but are devoid of β-catenin. Scale bars 10 μm
Enamel knot
In the enamel knot, β-catenin was not only associated with the cell surface but also was present in both nuclear and cytoplasmic compartments (Fig. 2B, D–F, H, J, K). The intensity of the nucleus-associated β-catenin varied among the cells of the enamel knot (Fig. 2D, F). The β-catenin present in both the cytoplasm and nucleus was more abundant in the enamel knot cells than in those cells in other areas of the enamel organ (compare Fig. 2H with Figs. 3B, F and 4C; compare Fig. 2J with Figs. 3C, H and 4D; compare Fig. 2K with Fig. 3D, I). P-cadherin expression was stronger in the enamel knot than in the adjacent inner dental epithelium (Fig. 2C, I). The boundary between the areas with strong or moderate levels of P-cadherin matched well with the margin of the enamel knot (Fig. 2I, K). The same boundary delimited areas with a high or low content of cytoplasmic and nuclear β-catenin (compare Fig. 2H, J with I, K).
Confocal images (A–I) from a section triple-stained with DAPI, anti-β-catenin, and anti-P-cadherin antibodies and a scheme (J) indicating the positions of the images in the tooth germ. A–D The area indicated by rectangle a in J corresponds to the cervical loop. E–I The area indicated by square e in J includes the outer dental epithelium (ode) and the stellate reticulum (sr). A The nuclei were visualized with DAPI (blue), whereas P-cadherin was visualized in green. B, F Localization of β-catenin. C, H Images combining nuclei and β-catenin. D, I Images combining β-catenin and P-cadherin. E Localization of the nuclei after DAPI staining. G Localization of P-cadherin. Scale bars 10 μm
Confocal images of the stellate reticulum (A–E) and scheme (F) indicating the position of the images (gray square). A Image combining nuclei (blue) and P-cadherin (green). B–E Images after triple staining with DAPI, anti-β-catenin, and anti-E-cadherin antibodies. B Image combining nuclei (blue) and E-cadherin (green). C Localization of β-catenin. D Image combining nuclei and β-catenin. E Image combining β-catenin and E-cadherin. In the section shown in B–E, a few cells with nuclear localization of β-catenin were found in the left upper part of the images (arrowheads), indicating that they belong the enamel knot (ek in F). Scale bars 10 μm
Inner dental epithelium
In the inner dental epithelium, the intensity of the staining for β-catenin was weaker than in the enamel knot. The presence of β-catenin in the nuclei was evident despite a low intensity (Fig. 2H, J, K). However, these cells showed a more intense staining for β-catenin in the cytoplasm especially in association with the cell surface delineated by P-cadherin (Fig. 2H, J, K).
Cervical loop and outer dental epithelium
In both the cervical loop and the outer dental epithelium, the localization of β-catenin was apparent in the cytoplasm but not in the nuclei (Fig. 3B–D, F, H, I). The presence of β-catenin associated with the cell surface was also evident in these areas (Fig. 3A, B, D).
Stellate reticulum
At a higher magnification, P-cadherin in the cells of the stellate reticulum appeared to be localized only in a restricted portion of the cell surface (Figs. 3G, 4A), whereas E-cadherin was abundant and localized over the entire cell surface (Fig. 4B). However, this might rather have resulted from a difference in the relative amounts of the two antigens than that in their localization. Cell–cell contacts in the stellate reticulum were restricted to specific parts of the cell surface (Fig. 4B–E), where classical cadherins could be concentrated as a constituent of adherens junctions. Therefore, it is possible that P-cadherin was detectable only in such places, while E-cadherin could be detected in the remaining part of the cell surface as well. The staining intensity for β-catenin was high at the site of cell–cell adhesions, where that for E-cadherin was also strong (Fig. 4B, C, E), although weak staining for β-catenin was also visible in the inner part of the cytoplasm. When detected, β-catenin gave only faint positive staining in the nuclei of the stellate reticulum cells (Fig. 4C–E). The boundary between the stellate reticulum and the enamel knot was unclear at this developmental stage (Figs. 1B, C, 4A, B), whereas the former was easily distinguished from the outer dental epithelium due to differences in cell morphology (Fig. 3E–I). The intensity of the staining for β-catenin associated with the nucleus was apparently higher in the cells of the enamel knot than in the differentiating stellate reticulum cells (Fig. 4C–E).
Discussion
At the cap stage, an intense staining for β-catenin was observed at the surface of all cells of the enamel organ, suggesting that this molecule served as a component of cell–cell adhesion mediated by classical cadherins everywhere. On the other hand, the accumulation of β-catenin in the cytoplasm and the nuclei was not uniform within the enamel organ. Since the canonical Wnt signaling pathway regulates the stability of β-catenin in the cytoplasm, the differences in the subcellular distribution of β-catenin observed in the enamel organ might be due, at least in part, to differential activity of the canonical Wnt pathway. The combination of Wnt and receptor molecules with or without co-receptors appears to initially determine which Wnt pathway is activated in a specific cell (Pinson et al. 2000; van Es et al. 2003). Seven Wnt genes and a Frizzled receptor as well as two Frizzled-related genes that encode secreted forms of Frizzleds are known to be expressed in the mouse tooth germ (Dassule and McMahon 1998; Sarkar and Sharpe 1999). Among these seven Wnt genes, six are differentially expressed in the enamel organ (Wnt 3, 4, 6, 7b, 10a, and 10b), whereas Wnt 5a is expressed in the mesenchyme. More than two Wnt genes are thus expressed in each area of the enamel organ, and the combination of the Wnt genes expressed in a given area is different from that in another one. Furthermore, the localization of the Wnt proteins could be very different from that of the corresponding gene expression, as was observed for Wnt 10b (Nadiri et al. 2004). Although the Wnt gene product(s) that activates the canonical pathway in the tooth germ has not been identified yet, the combinatorial expression of several Wnt genes might account for a spatially distinct activation of Wnt signaling pathways within the enamel organ.
LEF-1 is strongly expressed in the enamel knot (Keränen et al. 1998; Laurikkala et al. 2001) and is known to serve as a nuclear mediator of the canonical Wnt pathway in association with β-catenin (Behrens et al. 1996). Thus, the extensive nuclear accumulation of β-catenin in the enamel knot strongly suggests that these two molecules bind to each other and control the expression of Wnt target genes there. Recently, a gene targeting strategy showed that the function of LEF-1 in tooth formation requires the interaction of this protein with β-catenin (Kratochwil et al. 2002). The extensive nuclear accumulation of β-catenin in the enamel knot as observed here thus supports the above functional mechanism. Furthermore, the level of β-catenin in the nuclei was obviously higher in the enamel knot than in any other areas in the enamel organ, suggesting that the enamel knot is the place where the activation of the Wnt/β-catenin pathway is the highest. Outside of the enamel knot, the level of β-catenin in the cytoplasm and the nucleus was much lower or almost undetectable, while a considerable amount of the molecule was localized in association with the plasma membrane. This finding suggests a weak activity or silence of the canonical Wnt pathway outside the enamel knot.
A weak staining for P-cadherin was observed in the stellate reticulum, although previous investigations reported that the inner part of the enamel organ at the cap stage was negative for P-cadherin (Palacios et al. 1995; Obara et al. 1998). Since the same primary antibody was used for all three studies, the discrepancy probably resulted from an improved sensitivity when using secondary antibodies conjugated with different fluorescent reagents and from the higher resolution of the confocal laser scanning microscope when compared with that attained by conventional fluorescence microscopy. Actually, P-cadherin in the stellate reticulum was detected only in a limited part of the cell surface (Figs. 3G, 4A). On the other hand, the difference in the staining intensity between the outer and the inner cells in the enamel organ became more evident at later developmental stages (data not shown). Therefore, the pattern of P-cadherin expression observed in the present study is in line with the previous ones in that it is complementary to the expression of E-cadherin.
The nuclear localization of β-catenin and the strong expression of P-cadherin observed in the cells of the enamel knot are intriguing when compared with the poorer accumulation of β-catenin in the nucleus, the weaker expression of P-cadherin, and the stronger expression of E-cadherin observed in other parts of the enamel organ. P-cadherin is transiently expressed in many tissues (Nose and Takeichi 1986) and has been thought to play a role in the segregation of cell layers in concert with other cadherins. However, a recent analysis of cadherin-mediated cell adhesion revealed that, in the combination of E- and P-cadherin, the strength of adhesions mediated by either homo- (E-E or P-P) or heterocadherins (E-P) was similar (Duguay et al. 2003). Thus it is unlikely that the cadherin switch in the enamel organ directly participates in the segregation of different groups of the epithelial cells. On the other hand, the coincident upregulation of P-cadherin and downregulation of E-cadherin, as observed in the enamel organ, is similar to what has been described in the epithelial bud of the developing hair follicle (Hirai et al. 1989). Interestingly, the repression of E-cadherin expression by β-catenin and LEF-1 was recently reported to occur in the developing hair follicle, providing an evidence for links between Wnt signaling and cell adhesion (Jamora et al. 2003). Our results imply the existence of a linkage between cell adhesion and signaling in the developing tooth. Therefore, searching the evidence for such a linkage between Wnt signaling and the expression of E- and P-cadherins during tooth development could be a clue to understanding the general significance of the complementary expression of the two cadherins during epithelial histomorphogenesis.
References
Aberle H, Bauer A, Stappert J, Kispert A, Kemler R (1997) β-catenin is a target for the ubiquitin-proteasome pathway. EMBO J 16:3797–3804
Andl T, Reddy ST, Gaddapara T, Millar SE (2002) WNT signals are required for the initiation of hair follicle development. Dev Cell 2:643–653
Behrens J, von Kries JP, Kühl M, Bruhn L, Wedlich D, Grosschedl R, Birchmeier W (1996) Functional interaction of β-catenin with the transcription factor LEF-1. Nature 382:638–642
Cadigan KM, Nusse R (1997) Wnt signaling: a common theme in animal development. Genes Dev 11:3286–3305
Dassule HR, McMahon AP (1998) Analysis of epithelial–mesenchymal interactions in the initial morphogenesis of the mammalian tooth. Dev Biol 202:215–227
Duguay D, Foty RA, Steinberg MS (2003) Cadherin-mediated cell adhesion and tissue segregation: qualitative and quantitative determinants. Dev Biol 253:309–323
Fagotto F, Funayama N, Glück U, Gumbiner BM (1996) Binding to cadherins antagonizes the signaling activity of β-catenin during axis formation in Xenopus. J Cell Biol 132:1105–1114
Fausser JL, Schlepp O, Aberdam D, Meneguzzi G, Ruch JV, Lesot H (1998) Localization of antigens associated with adherens junctions, desmosomes, and hemidesmosomes during murine molar morphogenesis. Differentiation 63:1–11
Fuchs E, Merrill BJ, Jamora C, DasGupta R (2001) At the roots of a never-ending cycle. Dev Cell 1:13–25
Gottardi CJ, Wong E, Gumbiner BM (2001) E-cadherin suppresses cellular transformation by inhibiting β-catenin signaling in an adhesion-independent manner. J Cell Biol 153:1049–1060
Heasman J, Crawford A, Goldstone K, Garner-Hamrick P, Gumbiner B, McCrea P, Kintner C, Noro CY, Wylie C (1994) Overexpression of cadherins and underexpression of β-catenin inhibit dorsal mesoderm induction in early Xenopus embryos. Cell 79:791–803
Hecht A, Kemler R (2000) Curbing the nuclear activities of β-catenin. Control over Wnt target gene expression. EMBO Rep 1:24–28
Heymann R, About I, Lendahl U, Franquin JC, Obrink B, Mitsiadis TA (2002) E- and N-cadherin distribution in developing and functional human teeth under normal and pathological conditions. Am J Pathol 160:2123–2133
Hirai Y, Nose A, Kobayashi S, Takeichi M (1989) Expression and role of E- and P-cadherin adhesion molecules in embryonic histogenesis. II. Skin morphogenesis. Development 105:271–277
Huber O, Korn R, McLaughlin J, Ohsugi M, Herrmann BG, Kemler R (1996) Nuclear localization of β-catenin by interaction with transcription factor LEF-1. Mech Dev 59:3–10
Huelsken J, Birchmeier W (2001) New aspects of Wnt signaling pathways in higher vertebrates. Curr Opin Genet Dev 11:547–553
Jamora C, DasGupta R, Kocieniewski P, Fuchs E (2003) Links between signal transduction, transcription and adhesion in epithelial bud development. Nature 422:317–322
Jernvall J, Kettunen P, Karavanova I, Martin LB, Thesleff I (1994) Evidence for the role of the enamel knot as a control center in mammalian tooth cusp formation: non-dividing cells express growth stimulating Fgf-4 gene. Int J Dev Biol 38:463–469
Keränen SV, Åberg T, Kettunen P, Thesleff I, Jernvall J (1998) Association of developmental regulatory genes with the development of different molar tooth shapes in two species of rodents. Dev Genes Evol 208:477–486
Kratochwil K, Dull M, Fariñas I, Galceran J, Grosschedl R (1996) Lef1 expression is activated by BMP-4 and regulates inductive tissue interactions in tooth and hair development. Genes Dev 10:1382–1394
Kratochwil K, Galceran J, Tontsch S, Roth W, Grosschedl R (2002) FGF4, a direct target of LEF1 and Wnt signaling, can rescue the arrest of tooth organogenesis in Lef1(−/−) mice. Genes Dev 16:3173–3185
Laurikkala J, Mikkola M, Mustonen T, Åberg T, Koppinen P, Pispa J, Nieminen P, Galceran J, Grosschedl R, Thesleff I (2001) TNF signaling via the ligand-receptor pair ectodysplasin and edar controls the function of epithelial signaling centers and is regulated by Wnt and activin during tooth organogenesis. Dev Biol 229:443–455
Lesot H, Kieffer-Combeau S, Fausser JL, Meyer JM, Perrin-Schmitt F, Peterkova R, Peterka M, Ruch JV (2002) Cell–cell and cell–matrix interactions during initial enamel organ histomorphogenesis in the mouse. Connect Tissue Res 43:191–200
McLean IW, Nakane P (1974) Periodate-lysine-paraformaldehyde fixative. A new fixative for immunoelectron microscope. J Histochem Cytochem 22:1077–1083
Millar SE (2002) Molecular mechanisms regulating hair follicle development. J Invest Dermatol 18:216–225
Molenaar M, van de Wetering M, Oosterwegel M, Peterson-Maduro J, Godsave S, Korinek V, Roose J, Destrée O, Clevers H (1996) XTcf-3 transcription factor mediates β-catenin-induced axis formation in Xenopus embryos. Cell 86:391–399
Nadiri A, Kuchler-Bopp S, Haikel Y, Lesot H (2004) Immunolocalization of BMP-2/-4, FGF-4, and WNT10b in the developing mouse first molar. J Histochem Cytochem 52:103–112
Nagafuchi A (2001) Molecular architecture of adherens junctions. Curr Opin Cell Biol 13:600–603
Nose A, Takeichi M (1986) A novel cadherin cell adhesion molecule: its expression patterns associated with implantation and organogenesis of mouse embryos. J Cell Biol 103:2649–2658
Obara N, Suzuki Y, Nagai Y, Takeda M (1998) Expression of E- and P-cadherin during tooth morphogenesis and cytodifferentiation of ameloblasts. Anat Embryol 197:469–475
Orsulic S, Huber O, Aberle H, Arnold S, Kemler R (1999) E-cadherin binding prevents β-catenin nuclear localization and β-catenin/LEF-1-mediated transactivation. J Cell Sci 112:1237–1245
Ozawa M, Baribault H, Kemler R (1989) The cytoplasmic domain of the cell adhesion molecule uvomorulin associates with three independent proteins structurally related in different species. EMBO J 8:1711–1717
Palacios J, Benito N, Berpaquero R, Pizarro A, Cano A, Gamallo C (1995) Differential spatiotemporal expression of E- and P-cadherin during mouse tooth development. Int J Dev Biol 39:663–666
Pinson KI, Brennan J, Monkley S, Avery BJ, Skarnes WC (2000) An LDL-receptor-related protein mediates Wnt signalling in mice. Nature 407:535–538
Sadot E, Simcha I, Shtutman M, Ben-Ze’ev A, Geiger B (1998) Inhibition of β-catenin-mediated transactivation by cadherin derivatives. Proc Natl Acad Sci U S A 95:15339–15344
Sarkar L, Sharpe PT (1999) Expression of Wnt signalling pathway genes during tooth development. Mech Dev 85:197–200
Sarkar L, Sharpe PT (2000) Inhibition of Wnt signaling by exogenous Mfrzb1 protein affects molar tooth size. J Dent Res 79:920–925
Sarkar L, Cobourne M, Naylor S, Smalley M, Dale T, Sharpe PT (2000) Wnt/Shh interactions regulate ectodermal boundary formation during mammalian tooth development. Proc Natl Acad Sci U S A 97:4520–4524
Sorkin BC, Wang MY, Dobeck JM, Albergo KL, Skobe Z (2000) The cadherin–catenin complex is expressed alternately with the adenomatous polyposis coli protein during rat incisor amelogenesis. J Histochem Cytochem 48:397–406
van Es JH, Barker N, Clevers H (2003) You Wnt some, you lose some: oncogenes in the Wnt signaling pathway. Curr Opin Genet Dev 13:28–33
van Genderen C, Okamura RM, Fariñas I, Quo RG, Parslow TG, Bruhn L, Grosschedl R (1994) Development of several organs that require inductive epithelial–mesenchymal interactions is impaired in LEF-1-deficient mice. Genes Dev 8:2691–2703
van Noort M, Meeldijk J, van der Zee R, Destree O, Clevers H (2002) Wnt signaling controls the phosphorylation status of β-catenin. J Biol Chem 277:17901–17905
Willert K, Nusse R (1998) β-catenin: a key mediator of Wnt signaling. Curr Opin Genet Dev 8:95–102
Young CS, Kitamura M, Hardy S, Kitajewski J (1998) Wnt-1 induces growth, cytosolic beta-catenin, and Tcf/Lef transcriptional activation in Rat-1 fibroblasts. Mol Cell Biol 18:2474–2485
Acknowledgements
The authors wish to thank J.L. Vonesch and the team of the imaging center of the IGBMC (Illkirch) for their help.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Obara, N., Lesot, H. Subcellular localization of β-catenin and cadherin expression in the cap-stage enamel organ of the mouse molar. Histochem Cell Biol 121, 351–358 (2004). https://doi.org/10.1007/s00418-004-0637-5
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00418-004-0637-5