Abstract
Teraspanin transmembrane protein, Perp (P53 apoptosis effector related to PMP22), which is found in the plasma membrane as a component of the desmosome, is reported to be involved in the morphogenesis of the epithelium and the enamel formation of the incisor. However, its expression pattern and signaling regulation during molar development have not been elucidated in detail. We have examined the precise expression patterns of Perp in developing lower molars and employed the knock-down of Perp by antisense oligodeoxynucleotide treatment during in vitro organ cultivation at embryonic day 13 to define the precise developmental function of Perp. Perp was expressed mainly in the dental lamina and stellate reticulum regions at the bud and cap stages. After Perp knock-down, the tooth germ showed disruption of the dental lamina and stellate reticulum with altered apoptosis and proliferation. The changed expression levels of related signaling molecules from the enamel knot and desmosome were evaluated by real-time quantitative polymerase chain reaction. A renal capsule transplantation method was employed to examine the effects of Perp knock-down on molar crown development. Ultrastructural observations revealed that enamel was deposited more densely in an irregular pattern in the cusp region, and that dentin was hypo-mineralized after Perp knock-down at the cap stage. Thus, Perp might play important roles in the formation and integration of stellate reticulum, dental lamina structure and enamel formation through signaling interactions with the enamel knot and desmosome-related signaling molecules at the cap stage of lower molar development.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Teeth develop from the ectoderm and the underlying ectomesenchyme, and their morphogenesis is regulated by interactions between the epithelium and the underlying mesenchyme establishing the primary dental lamina (DL) in the future tooth rows (Thesleff 2003), which also mark the initial morphological signs of tooth formation called the placode. The placode invaginates the mesenchyme forming a bud that further progresses to produce bell-shaped structures with epithelial folding (Tummers and Thesleff 2009). At the cap and bell stages, a group of star-shaped cells, which are connected to one another by desmosomes, develop between the outer and inner enamel epithelium in a region called the stellate reticulum (SR), which expands mainly by an increase in the intercellular fluid containing glycosaminoglycans, alkaline phosphatase, and small amounts of RNA and glycogen (Nancy 2012). Based on these morphological and biochemical features, the SR seems to act as a cushion against physical forces that might change the conformation of the developing dentino-enamel junction giving rise to morphological changes (Nancy 2012). At the late bell stage, the inner dental epithelium (IDE) differentiates into ameloblasts, and the dental papilla cells differentiate into odontoblasts, the two cell types forming the enamel and dentin, respectively (Thesleff 2003).
During these distinct morphological alterations during tooth development, important signaling regulation occurs through epithelial-mesenchymal interactions mediated by multiple growth factors and transcription factors (Thesleff and Mikkola 2002; Tummers and Thesleff 2009). The epithelial-mesenchymal interactions are mediated by the conserved signal pathways, which include transforming growth factor-β, BMP (bone morphogenetic protein), FGF (fibroblast growth factor), hedgehog, Eda (ectodysplasin), and WNT (Tummers and Thesleff 2009). During the bud-to-cap stage transition of tooth development, the enamel knot (EK) signaling center appears in the epithelium; this center contains non-dividing cells that stimulate the proliferation of nearby epithelial and mesenchymal cells and that determine the secondary EK positions for the sites of future cusps (Jernvall and Thesleff 2000). The various signaling molecules expressed in the EK include sonic hedgehog (Shh), Bmp-2, Bmp-4, Bmp-7, Fgf4, Wnt10, and Fgf9, which make the EK an organizing center for positional information for tooth morphogenesis and for the regulation of the growth of tooth cusps (Vaahtokari et al. 1996; Kettunen and Thesleff 1998; Kratochwil et al. 2002).
Perp (P53 apoptosis effector related to PMP22) is a transmembrane protein (21.4 kDa) that is believed to have tetraspanin characteristics (Attardi et al. 2000). The protein is present in the plasma membrane and has been reported to be a component of desmosomes (DS) of stratified epithelia (Ihrie et al. 2005). A recent study, however, has reported that it is present in all kinds of epithelia (Franke et al. 2013). Perp is thought to have important roles in the morphogenesis of skin and related ectodermal structures, including hair and teeth (Ihrie et al. 2005; Senoo et al. 2007). Moreover, it is a direct target of P63 (Ihrie et al. 2005), a master regulator of epithelial development. Studies on Perp knockout mice have shown that a significant downregulation of the genes involved in amelogenesis occurs and that the enamel structure is affected (Jheon et al. 2011). Furthermore, reports are available regarding the putative role of DS on the development of molars and incisors and their localization on the enamel organ (EO) and EO-derived cells (Fausser et al. 1998; Kieffer-Combeau et al. 2001; Cam et al. 2002) and the displacement of ameloblasts from the stratum intermedium and compromised DS structure in knockout mice (Jheon et al. 2011). However, these reports have not elucidated the detailed signaling regulations of Perp in molar development.
In this study, we have examined the detailed expression pattern and developmental roles of Perp during lower molar development, which is regulated mainly by EK signaling molecules at the cap stage. For these evaluations, antisense oligodeoxynucleotide (AS-ODN) treatment has been used as a knock-down method in a range of in vitro organ culture systems.
Materials and methods
All experiments were performed according to the guidelines of the Kyungpook National University, School of Dentistry, Intramural Animal Use and Care Committee.
Animals
Adult ICR mice were housed in a temperature-controlled room (22 °C) under artificial illumination (lights on from 05:00 to 17:00), at 55 % relative humidity, with access to food and water ad libitum. The mouse embryos were obtained from time-mated pregnant mice. The day on which a vaginal plug was confirmed was designated as embryonic day 0 (E0). Embryos at E12.5, E13.0, E13.5, E14.5, and E16.5 were used. Post natal mice at day 2 and 6 were also used in our study.
In situ hybridization
In situ hybridization on whole mounts and sections was performed as described previously (Sohn et al. 2012) by using standard protocols. For both types of hybridization, digoxigenin-labeled RNA probes were pre-warmed to 80 °C and hybridized overnight at 62 °C. After whole mount in situ hybridization, 20-μm-thick frontal frozen sections were prepared to examine the detailed expression pattern of Perp.
In vitro organ cultivation and renal capsule transplantation
The molar tooth buds of embryonic mice were micro-dissected from the lower jaw at E13 under stereo microscope in phosphate buffered saline (PBS). The tooth buds were cultured in DMEM (HyClone, Logan, Utah, USA; cat. no. SH30243.01), supplemented with 10 % fetal bovine serum (Hyclone) and antibiotics, by means of a modified Trowell's culture method for a designated period as described by Kim et al. (2009). The cultivated tooth germs were transplanted into the renal subcapsular layer of adult male mice as described previously (Cai et al. 2007). After 3 weeks, the host mice were killed, and the kidneys were dissected to obtain the calcified teeth.
AS-ODN treatment
AS-ODNs for Perp were designed and used during in vitro organ culture (Sohn et al. 2012). The ODNs were designed as AS-ODN Perp 5’-ATGCTGCGCTGCGGCCTGGC-3’, and sense (S)-ODN 5’-GCCAGGCCGCAGCGCAGCAT-3'. These ODNs were purchased from GENOTECH (Korea). They were added to the culture medium at a final concentration of 1 μM.
Three-dimensional reconstruction
All serial sections after in vitro cultivation of tooth organs were photographed by using a DM2500 microscope (Leica, Germany). The tooth organs were reconstructed for each specimen. “Reconstruct” software was used to reconstruct three-dimensional images. Serial frontal sections with a thickness of 7 μm including the lower molar regions were reconstructed (Sohn et al. 2014). The images were aligned both automatically and manually by using the software.
Histology and immunohistochemistry
The sections were stained routinely with hematoxylin and eosin and examined by optical microscopy. The specimens were fixed overnight in 4 % paraformaldehyde (PFA) in PBS at 4 °C, embedded in paraffin wax by using conventional methods, and then cut at a thickness of 7 μm. The primary antibodies used were against Perp (Abcam, Cambridge, UK; cat. no. ab48032) and Ki67 (Neo Markers, Calif., USA; cat. no. RM-9106). The secondary antibodies used were biotinylated goat anti-rabbit or anti-mouse IgG. The binding of the primary antibody to the sections was visualized by using a diaminobenzidine tetrahydrochloride (DAB) reagent kit (Zymed, Calif., USA; cat. no. 00–2014).
TUNEL assay
A TUNEL (terminal deoxynucleotidyl-transferase-mediated dUTP nick-end labeling) assay was performed as described previously (Sohn et al. 2012) by using an in situ cell apoptosis detection kit (Trevigen, Md., USA) according to the manufacturer's instructions. DAB was used as a substrate solution to detect the sites of in situ apoptosis under a light microscope. At least 10 specimens were examined in each experiment.
Real-time quantitative polymerase chain reaction
Total RNA was isolated from the cultured tooth germ by using an RNeasy Micro Kit (QIAGEN, Germany; cat. no. 205111). For cDNA synthesis, reverse transcription was performed by using the Omniscript RT Kit (QIAGEN, Germany; cat. no. 205111) with 2 μg total RNA. Real-time quantitative polymerase chain reaction (RT-qPCR) was performed by using the Applied Biosystems 7500/7500 fast real-time PCR system (USA) with the SYBR Green PCR master mix (Applied Biosystems, USA). The results of RT-qPCR for each sample were normalized to hypoxanthine phosphoribosyl transferase. The results were expressed as the normalized ratios. Table 1 lists the primer sequences used in this study. The data were expressed as means±SD. The mean expression levels were compared between the experimental and control groups by using Student’s t-test.
Statistical analysis
All experiments were performed a minimum of three times. Two-sample t-tests (equal variance) were used to analyze the significance of the differences. P-values of <0.05 were considered significant.
Scanning electron microscopy and energy dispersive X-ray analysis
Ultrastructural observations of the enamel surface of teeth cultivated within the kidney capsule were performed as described previously (Sohn et al. 2011). Briefly, after being fixed, washed, and acid-etched, the samples were dehydrated, mounted on stubs, coated with platinum, and examined by scanning microscopy (SEM; JEOL JSM-6700 F Field emission SEM, Japan). The same instrument, but in the position-tagged spectrometry (PTS) mode, was used to study the elemental distribution of enamel surface.
Ground sections of calcified teeth
Ground sections of the teeth were prepared as described previously (Kwon et al. 2014). Briefly, the teeth were stained with Villanueva stain for 3 days, dehydrated, infiltrated with a mixture of propylene oxide and resin at various concentrations, and embedded in resin at 60 °C for 5 days. Sections were prepared by using a diamond saw (Accutom 50, Struer, Ballerup, Denmark) and ground (Rotopol-35, Struer), after which they were mounted on slides in historesin mounting media (Leica, Germany).
Results
Expression and localization patterns of Perp in developing lower molars
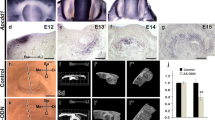
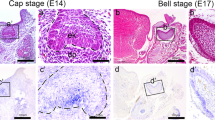
In order to understand the developmental function of Perp in molar morphogenesis, the expression pattern of Perp was examined by using in situ hybridization methods. From E12.5 to E16.5, the specific expression pattern of Perp was observed in the tooth-forming tissue (Fig. 1a-c). After whole mount in situ hybridization, frozen frontal sections were prepared (Fig. 1d-e). At E12.5, Perp was expressed in the epithelial thickening (Fig. 1d). At E13.5, strong and broad epithelial expression was examined in the DL and oral epithelium (OE), respectively (Fig. 1e). At E14.5, the epithelial expression pattern of Perp was expressed distinctly in the DL and SR (Fig. 1f). However, the expression of Perp was not detected in the EK, outer dental epithelium (ODE), IDE, or the mesenchyme (Fig. 1f). The in situ hybridization on sections revealed similar results to those of the cryo-sections of the whole mounts examined by in situ hybridization (data not shown). At E16.5, Perp was expressed in the DL and SI (Fig. 1g-i), but not the SR (Fig. 1f). The localization of Perp was examined by using immunohistochemistry at E16.5 and on postnatal day 2 (PN2) and PN6 in order to understand its developmental roles during later prenatal and postnatal stages including differentiation, matrix secretion, and mineralization (Fig. S1). At E16.5, Perp was localized in the DL, SR, and SI (Fig. S1a-d). At PN2 and PN6, strong positive localization was observed in the DL and OE (Fig. S1e-l). In particular, a similar localization pattern of Perp to that at E16 was found in SR and SI, except for the ameloblasts at PN2 and PN6 (Fig. S1e-l).
a–c Whole mount in situ hybridization with digoxigenin-labeled Perp mRNA probes at embryonic day 12.5 (E12.5) to E14.5. d–f Frozen sections obtained after whole mount in situ hybridization. Expression is detected in the oral epithelium and epithelial thickening at E12.5 (a, d). At E13.5, Perp expression is detected in the molar-forming region (b, e). Strong expression of Perp is seen in the dental lamina (DL) and oral epithelium (OE) and is not observed in the enamel knot (EK) and mesenchyme (e). At E14.5, epithelial Perp expression is detected in the OE, DL, and stellate reticulum (SR) and is not detected in the EK, inner dental epithelium (IDE), or outer dental epithelium (ODE; c, f). In situ hybridization of sections at E16.5 shows the Perp expression in DL and stratum intermedium (SI; g–i). Dotted lines demarcate the section levels (a–c) and margin of the SI with the ameloblast (i). Bars 500 μm (a–c), 100 μm (d–g), 50 μm (h, i)
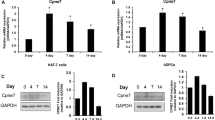
Evaluation of Perp knock-down by using in vitro organ cultivation
The AS-ODN method was used as a loss-of-function study to determine the precise developmental function of Perp in molar morphogenesis during in vitro molar organ cultures at E13, an ideal developmental stage for evaluating the developmental roles of Perp through interactions with EK signaling molecules (Tucker and Sharpe 2004). The knock-down of Perp by AS-ODN treatment for 1 day showed, by using RT-qPCR, an almost 65 % decrease in the levels of expression compared with those of the control (Fig. 2a). At E13, the 2-day cultivated specimens showed similar morphological changes to those of the E14.5 tooth germ (Fig. 2b, c). The epithelium of the cultured AS-ODN tooth showed a uniform opaque region of EK (Fig. 2h) compared with the alternating transparent and opaque region of SR and EK in the control (Fig. 2b). Moreover, the bucco-lingual diameter was increased, and the mesial end was more rounded after AS-ODN treatment (Fig. 2h) compared with the control (Fig. 2b). Hematoxylin and eosin staining of the frontal section of cultivated teeth showed that the DL and SR structures were disrupted in the AS-ODN specimen (Fig. 2k) compared to the control (Fig. 2e). In the AS-ODN tooth, the cells in the DL were arranged at denser intervals (Fig. 2i, arrow) with an increased width of DL structure. The structure of SR was disrupted in the epithelial region close to the EK (Fig. 2k, l, boxed area). In order to understand the altered morphology of the developing tooth after AS-ODN treatment, the tooth germ was reconstructed from frontal serial sections (Fig. 2d, g, j, m). Three-dimensional reconstruction of AS-ODN-treated specimens showed that the tooth organ was much wider at the bucco-lingual side, but the DL and SR structures were disrupted (Fig. 2j, m) when compared with those of the control (Fig. 2d, g).
aDownregulated Perp expression after antisense oligodeoxynucleotide (AS-ODN) treatment. **P < 0.01. b–m Morphological alterations of the molar tooth after AS-ODN treatment during in vitro cultivation (b–g control; h-m AS-ODN). E13 embryonic tooth after 2-day cultivation by a modified Trowell’s culture method shows alternating opaque and transparent regions with a dome-shaped mesial end (b), whereas the AS-ODN-treated tooth organ shows a uniform opaque region with a rounded mesial end (h). Hematoxylin and eosin staining of the control and AS-ODN reveals morphological changes in the SR and DL structures (c, e, f, i, k, l). DL is wider and shorter containing compactly arranged cells in AS-ODN (between arrows in i) compared with control (c). SR is poorly developed (k, l) and disrupted in the cervical region compared with control (e, f). Serial frontal sections and three-dimensional reconstruction (3D-Recon) of a molar at E13 + 2-day culture shows short and thick DL and compressed SR (g, j) in AS-ODN when compared with control (d, g). Dotted lines indicate the section levels (Epi, gray epithelium, light blue SR, arrows DL). Bars 100 μm (a–c, f–i, l), 50 μm (d, j), 10 μm (e, k)
Altered cellular physiology after knock-down of Perp
A TUNEL assay and immunostaining of Ki67 were employed to evaluate the effects of the knock-down of Perp on cellular physiology, including proliferation and apoptosis. Histological examination revealed altered localization patterns of apoptotic cells and Ki67-positive cells, particularly in DL and SR after AS-ODN treatment (Fig. 3a, d, f, h). Following 2 days of cultivation at E13, the control and AS-ODN specimens showed the mesenchymal and epithelial localization of apoptotic cells (Fig. 3a, d). In particular, the AS-ODN-treated specimen revealed an increased localization of apoptotic cells in the DL and SR (Fig. 3e) but the localization of apoptotic cells in the EK remained unaltered (Fig. 3a, d). In the designated area of 50 μm2 of DL and SR, the number of TUNEL positive cells in AS-ODN (15.2 ± 2.3; Fig. 3e) was higher than that of the control (6.4 ± 2.1; Fig. 3b). Ki67 immunostaining in the cultivated tissue sections showed that Ki67-positive mesenchymal cells in the dental papilla were identical between the control and AS-ODN specimens (Fig. 3f, h). We found that proliferation was decreased in the designated area of 50 μm2 in the DL and SR of the AS-ODN specimens (18.0 ± 2.6; Fig. 3i) compared with the control (28.8 ± 1.5; Fig. 3g).
Altered cellular events including apoptosis and cell proliferation after knock-down of Perp. TUNEL assay shows increased number of apoptotic cells in the DL and SR (d, e) after Perp knock-down compared with the control (a, b). Ki67 immunostaining reveals a decreased number of Ki67-positive cells in DL and SR after knock-down of Perp (h, i) compared with the control (f, g). Graphs showing the number of TUNEL- and Ki67-positive cells (c) in the designated 50 μm2 area (dotted square) of DL and SR (c, e, g, i). Dotted lines demarcate the margin of the basal layer. **P < 0.01. Bars 100 μm (a, d, f, h), 50 μm (b, e, g, i)
Altered expression patterns of signaling molecules after knock-down of Perp
RT-qPCR was employed after knock-down of Perp at E13 followed by 1 day in vitro cultivation to examine the altered expression patterns of well-known signaling molecules expressed in EK at the cap stage in molar development. After the knock-down of the Perp, we examined the expression pattern of DS-related molecules (i.e., Dsc1, Dsc3, Dsg1, Dsg3, Dsp, Pkg, Pkp3), because the localization of DS in the enamel organ has previously been described, and because Perp is a component of DS (Fausser et al. 1998; Ihrie et al. 2005), and of EK signaling molecules (Bmp2, Bmp4, Fgf4, Shh) expressed at the cap stage (Tummers and Thesleff 2009; for an explanation of all these abbreviations, see Fig. 4). The EK signaling genes, Bmp2 and Bmp4, showed increased expression patterns (Fig. 4a), whereas Fgf4 and Shh showed decreased expression patterns after AS-ODN treatment (Fig. 4a). The altered expression patterns were confirmed by in situ hybridization on sections (Fig. 4c-j). As a control, 2-day cultivated teeth at E13 showed similar expression patterns of Bmp2, Bmp4, Fgf4 and Shh to those at E14.5 (Fig. 4c-f). After the knock-down of Perp, specimens examined by in situ hybridization exhibited similar results to those obtained in RT-qPCR experiments (Fig. 4g-j). Increased expression of Bmp2 and Bmp4 after the knock-down of Perp was seen in the epithelium and mesenchyme, respectively (Fig. 4c, d, g, h). Moreover, Fgf4 and Shh expression was markedly decreased after the knock-down of Perp (Fig. 4e, f, i, j). The DS-related signaling molecules were evaluated in order to define the detailed function of Perp in mouse molar morphogenesis via DS. Interestingly, all DS-related molecules, such as Dsc1, Dsc3, Dsg1, Dsg3, Dsp, Pkg, and Pkp3, examined in this study showed a dramatic decrease in their expression levels (Fig. 4b).
Altered expression pattern of the EK- and DS-related molecules. Bone morphogenetic protein 2 (Bmp2) and Bmp4 show increased expression levels, whereas fibroblast growth factor 4 (Fgf4) and sonic hedgehog (Shh) reveal decreased expression levels (a). All DS-related molecules selected in this study showed decreased expression levels (b) after knock-down of Perp. *P < 0.05, **P < 0.01. In situ hybridization on sections shows the altered expression pattern of EK signaling genes Bmp2, Bmp4, Fgf4, and Shh in control (c–f) and AS-ODN (g–j) specimens. Dotted lines demarcate the margin of basal layer (DS desmosome, EK enamel knot, Dsc desmocollin, Dsg desmogelin, Dsp desmoplakin, Pkg plakoglobin, Pkp plakophilin, Hprt hypoxanthine phosphoribosyl transferase). Bars 50 μm (c-j)
Alterations of crown size and shape in calcified teeth
To examine the changes in tooth morphology after the treatment of AS-ODN at the cap stage, in vitro organ cultivation was performed at E13 for 2 days, and cultivated specimens were transplanted into the sub-capsular layer of the kidney for 3 weeks. After 3 weeks, the calcified teeth obtained were examined for morphological alterations. The control mimicked the morphology of in vivo teeth at PN10. Compared with the control, the AS-ODN teeth showed an altered crown and cusp morphology. The cusps were blunt, and the inter-cusp groove was shallower than that in the control (Fig. 5a, c). The cusp and crown height of the metaconid was measured to evaluate the detailed alteration pattern of the tooth crown structure. These measurements showed that the height of the crown (AS-ODN: 0.62 ± 0.02 mm; control: 0.86 ± 0.02 mm) and cusp heights (AS-ODN: 0.22 ± 0.01 mm; control: 0.27 ± 0.01 mm) were lower than those of the control (Fig. 5a, c, e). The length of the root was also evaluated comparatively. The results showed that the majority of teeth of mice following AS-ODN treatment had a longer root than those of the control (data not shown).
Calcified teeth that had been taken at E13, cultured for 2 days, and then transplanted into the kidney capsule for 21 days. a, c Buccal view of the control and AS-ODN teeth showing crown and cusp height. The crown and cusp height are shorter in the AS-ODN tooth (c) compared with the control (a). b, d Occlusal view of the control and AS-ODN teeth showing cusp positions. Asterisks indicate the apex of the metaconid cusp. Dotted curves indicate the cement-enamel junction. Bars 500 μm (a, c), 200 μm (b,d). e Graph showing the comparative cusp and crown length of the control and AS-ODN teeth at the metaconid cusp region. **P < 0.01
Evaluation of calcified teeth by SEM and ground section
SEM was employed after kidney transplantation in order to elucidate the developmental function of Perp in molar tooth crown formation including amelogenesis. The structure of enamel in the cervical part of the crown was examined by SEM (Fig. 6a, c) after acid etching for 7 s; we found that the AS-ODN formed small enamel prisms with irregular and collapsed interprismatic enamel structures (Fig. 6d) compared with the stereotypical normal structure of the control showing a type 1 etching pattern (Nancy 2012; Fig. 6b). The dissolution of apatite crystals from the enamel prisms in the AS-ODN teeth was less pronounced. In addition, in order to examine the level of calcification in the renal capsule calcified teeth, ground sections were prepared following Villanueva staining of the control and AS-ODN teeth. The ground sections showed no distinct dentino-enamel junction (black arrows, Fig. 6h) in the AS-ODN compared with the control teeth (Fig. 6f). The dentinal tubules along the cusp region showed purple staining indicating hypo-mineralization in AS-ODN teeth (Fig. 6g) compared with that in the control (Fig. 6e). Similarly, the coronal enamel in AS-ODN was more uniform and compact (Fig. 6g) than in the same area of the control (Fig. 6e, Fig. S2).
Ultra-structural images of enamel and ground sections of calcified teeth that had been taken at E13, cultured for 2 days, and then transplanted into the kidney capsule for 3 weeks. Altered enamel structures with compact and irregularly arranged inter-rod structures with small enamel spaces can be seen in AS-ODN tooth (d) compared with the stereotypical enamel structure with large enamel spaces in control tooth (b). The ground section shows hypo-mineralized dentin and indistinct dentino-enamel junction (DEJ, black arrows; g, h) compared with the control (e, f). The enamel deposition is compact in AS-ODN (g) compared with the control (e). Bars 50 μm (a, c, e–h), 5 μm (b, d)
Discussion
Expression pattern of Perp in DL and SR of developing tooth
In this study, we mainly focused on the stage-specific function of Perp expressed in the SR and DL at the cap stage of molar development. In addition, we examined the further effects of Perp at the cap stage until tooth crown formation, such as the effects on enamel and dentin, in order to define the developmental function of SR and DL. Whole mount in situ hybridization and the frontal sections revealed the specific expression patterns of Perp in the OE, DL, and SR (Fig. 1). However, no Perp expression was observed in EK, ODE, and IDE. This expression, which was restricted only to the DL and SR, suggests that the genes expressed in these regions interact with the underlying ectomesenchyme and EK signaling molecules leading to normal morphogenesis of the tooth at the cap stage. Jheon et al. (2011) have detected Perp in the dental epithelia of the upper molar at E14.5 and E16.5 including the IDE, SI, SR, and ODE. However, they have not shown the sequential expression patterns of Perp in the developing lower molars, which are suitable organs for examining the signaling regulation in tooth development. The broad localization of Perp in the E14.5 upper molar is similar to the expression patterns of Perp in SR, DL, and OE of the developing lower molars (see Fig. 1 of Jheon et al. 2011). At E16.5, the strong expression of Perp has been observed in SI, but the tissue loses the expression of Perp in SR (Fig. 1) at which time the localization of Perp predominates, further reinforcing our hypothesis that the Perp expression at cap stage interacts with EK signaling molecules for the normal morphogenesis of the tooth. The uniform localization pattern of Perp in DL, SR, and SI at E16.5, PN2, and PN6 suggests that Perp is an indispensable component for cellular integrity and vitality in molar development during differentiation, matrix secretion, and mineralization.
Perp has been reported to be a component of DS. The expression of Perp in SR and DL of the developing molar shows similar patterns to the DS- and hemidesmosome-related molecules such as Dsp, Dsg, Pkg, and E-cadherin (Ihrie et al. 2005; Fausser et al. 1998; Cam et al. 2002; Jheon et al. 2011. Based on the synexpression patterns of the DS-related genes at the cap stage, Perp has been hypothesized as being involved in the structural formation of SR and DL through DS signaling. However, the developmental function of SR and DL together with their underlying mechanisms in molar morphogenesis requires further study. The molecular dissection of Perp, which shows specific and restricted expression in SR and DL, would improve the understanding of the precise roles of SR and DL in molar development.
Perp controls the morphology of the developing tooth
This study was performed to elucidate the precise roles of Perp in molar development at the cap stage and the subsequent effects of Perp in molar morphogenesis. More than 60 % of Perp was knocked down by using AS-ODN against Perp during in vitro organ cultivation for 1 day, as confirmed by RT-qPCR (Fig. 2a); the knock-down of the gene to this extent alters the signaling regulation of the developing organ, thus allowing the morphological alterations to be visualized. The transient knock-down of the gene by AS-ODN during in vitro organ cultivation has been used because it provides better insight into the developmental function of the genes in a tissue- and developmental-stage-specific manner. When the morphology was examined after two days of cultivation at E13, the bucco-lingual diameter was increased after AS-ODN treatment (Fig. 2h). In three dimensions, the control was found to be more voluminous than that in the AS-ODN teeth (Fig. 2d, j). The SR was compressed in AS-ODN teeth compared with that of the control (Fig. 2g, m). During the initiation of tooth development, the single-layered epithelium changes to a multi-layered tooth bud (Tucker and Sharpe 2004). As described previously, DS are present in the stratified epithelia as adhesive molecules, and Perp is required for normal desmosomal assembly (Beaudry et al. 2010). At the differentiation stages, SR, which comprises a group of star-shaped cells arranged loosely and connected to one another and to the SI by DS, is visible with large extracellular spaces (Nancy 2012). These results and previous reports suggest that the knock-down of Perp makes the SR cell more fragile, and it bonds weakly to other SR cells. After treatment with AS-ODN, the structures of DL and SR are disrupted, and the IDE becomes broader than that of the control (Fig. 2k). These results are also similar to that of Perp-null mice, which display blisters in their epithelia and the features of ectodermal dysplasia syndromes (Ihrie et al. 2006). Therefore, the disruption of SR after the knock-down of Perp during in vitro cultivation might alter the morphogenesis of the molar in the subsequent developmental phases.
Perp controls cell dynamics through EK and DS signaling
Apoptosis and proliferation have been previously established to play important roles in the proper morphogenesis of the normal tooth (Jernvall et al. 1998). We have examined the altered cellular events that would lead to morphological changes in the tooth after the knock-down of Perp. The immunolocalization of Ki67-positive cells showed their decreased numbers in the DL and the SR regions of the AS-ODN treated specimen compared with the controls (Fig. 3c, g, i), whereas the number of apoptotic cells in the DL of AS-ODN is significantly increased above that in the control (Fig. 3b, c, e). Based on these results, Perp seems to be required for the stratification of DL and SR through the stable assembly of DS. The knock-down of Perp in the developing tooth might affect the proper assembly of DS, which in turn affects the morphogenesis of the teeth through altered cellular events. In addition, these altered cellular events can be explained by the cap-stage-specific signaling regulation that is modulated by EK signaling molecules including Bmps, Fgfs, and Shh (Tummers and Thesleff 2009). We employed the RT-qPCR method after the knock-down of Perp to examine the altered expression patterns of EK signaling molecules expressed at the cap stage in molar development. EK expresses paracrine signaling molecules, including Shh and several members of the Bmp, Fgf, and Wnt families (Vaahtokari et al. 1996; Jernvall and Thesleff 2000), at the cap stage of the developing molar. After the knock-down of Perp, the altered expression patterns of EK signaling molecules indicate that Bmp2 and Bmp4 are upregulated, and that Fgf4 and Shh are downregulated (Fig. 4a). These results would explain the lower number of Ki67-positive cells in the AS-ODN-treated specimens, because the downregulation of Fgf4 would affect the mitogenic potential of the surrounding cells, as reported previously (Sasaki et al. 2005). The upregulation of apoptosis in the enamel organ in the present study might be attributable to the upregulation of Bmp2 and Bmp4 after AS-ODN treatment. Previous studies have suggested that Bmp4 in the mesenchyme induces p21 (cyclin dependent kinase inhibitor) and regulates apoptosis (Jernvall et al. 1998). Similarly, the role of Bmp2 in apoptosis during limb development has been described (Guha et al. 2002). On the other hand, the over-expression of Bmp4 represses Shh expression in the developing tooth (Zhang et al. 2000). These reports suggest that an increase in apoptosis and a decrease in proliferation are related to the altered expressions of Bmp2, Bmp4, Fgf4, and Shh after AS-ODN treatment.
In the present study, the knock-down of Perp decreases the expression of DS molecules (Fig. 4b) needed for cellular integrity (Ihrie et al. 2005); these molecules have been reported to be localized in the DL and SR during the cap stage (Fausser et al. 1998). The antero-posterior stabilization of the enamel organ depends on DS, and the molecular component of DS is also involved in signal transduction (Kieffer-Combeau et al. 2001). The postnatal viability of newborn mice requires Perp because this molecule is needed for normal desmosomal assembly (Ihrie et al. 2005) and for the normal proliferation of epithelial tissues (Senoo et al. 2007). These results also suggest that the increased early apoptosis in DL and SR at the cap stage after AS-ODN treatment results from the disruption of DS and its related signaling regulation (Baratella et al. 1999). The altered cell physiology including cell proliferation and apoptosis via the EK and DS signaling molecules after Perp knock-down suggest that the developing tooth cusp and crown morphology are regulated by DL and SR via Perp at the cap stage.
Perp expressed in SR and DL is required for tooth crown morphogenesis
The morphology of the cusp in the kidney-capsule-calcified teeth was changed in the AS-ODN teeth compared with the control (Fig. 5a, c). Occlusally, the cusps were formed within a small area compared with the control; this might be related to a change in the number of cusps and their morphology, and has been reported previously (Cai et al. 2007). The crown and cusp heights of AS-ODN were shorter than those of the control (Fig. 5a, c, e) when the metaconid cusp region was measured, thereby revealing the obvious and distinctive differences between the control and experimental groups. This suggests that Perp signaling at the cap stage is involved in tooth crown and cusp morphogenesis through the structural formation of DL and SR. Crown and cusp morphology is preserved by the correct interplay of these signaling molecules, including the proper structural intactness of SR through the DS (Fausser et al. 1998; Nancy 2012). These morphological alterations in molar crown and cusp formation have been attributed to the altered expression of EK and DS signaling molecules during the cellular events (Figs. 3, 4). In particular, Dsc3 and Dsg3 are associated positionally with cell proliferation, differentiation, and keratinization (Legan et al. 1994). Similarly, the tissue-specific knockout of Shh in the developing tooth results in small, abnormally shaped teeth (Dassule et al. 2000). The downregulation of Shh produces growth defects producing very little outer enamel epithelium leading to disrupted crown morphogenesis (Dassule et al. 2000; Ishida et al. 2011). In addition, the downregulation of Shh with decreased proliferation has been observed, which might contribute to the shortening of the cusp and crown height.
SEM of the enamel of the control and AS-ODN teeth (Fig. 6a-d) also showed that the enamel structure was different in the AS-ODN-treated teeth than the control, when images were taken after a phosphoric acid treatment for 7 s. Jheon et al. (2011) have reported a difference in the enamel structures of the Perp null first mandibular molar at PN2. In the present study, the control showed normal and regular patterned enamel structures made from interprism enamel, but the AS-ODN-treated specimens showed compacted and irregular structures of the enamel surface made by interprism enamel (Fig. 6b, d). These results might be attributable to the altered orientation of the enamel prisms and interprisms, synthesized by ameloblasts (Lyngstadaas et al. 1998; Jheon et al. 2011). Such structural variations in the formation of the interprism enamel suggest that the AS-ODN-treated teeth are mineralized compactly in the cusp region; this might result from the hypo-mineralization of the dentin around the cusp region and might facilitate the transport of minerals from the dental pulp, apart from the enamel organ, and the role of Bmp2 and Bmp4 in odontoblast differentiation and enamel formation, as reported previously (Gluhak-Heinrich et al. 2010; Yang et al. 2012; Feng et al. 2011; Fig. 6g). In addition, the change in calcification has been additionally supported by energy dispersive X-ray analysis (Fig. S2), which implies that the higher rate of calcification on the designated surface of enamel in the AS-ODN-treated specimens would not have resulted from sound enamel formation but from the irregularity of enamel rod arrangement. The knock-down of Perp does not affect ameloblast differentiation but disrupts the patterned arrangement of ameloblasts, which is regulated by SR or SI. This result is also similar to that from the resin sections, which have shown the denser arrangement of enamel rod in the AS-ODN-treated specimen (Fig. 6h). Further, the dentino-enamel junctional structures in the present study are different from those of the control after the knock-down of Perp (Fig. 6h); this is probably related to the formation of enamel prisms and interprisms in the same plane, rather than at angles to each other (Lyngstadaas et al. 1998).
The validation and functional analysis of DL- and SR-specific-expressing genes has shown that Perp plays crucial roles in proper molar structural formation, including amelogenesis and dentinogenesis via the morphogenesis of DL and SR by interaction with EK- and DS-related signaling molecules (Fig. 7). Overall, this is the first study to reveal the detailed function of SR and DL at the cap stage in molar development through the regulation of Perp to produce patterned enamel and dentin structures.
Representation of putative developmental functions of Perp in mice molar morphogenesis. Perp inhibits the level of programmed cell death through Bmp signaling and enhances proliferation through Shh, Fgf4, and DS-related signaling. These interactions ensure the proper morphogenesis of SR and DL leading to molar crown development with patterned enamel and dentin formation (OE oral epithelium, DL dental lamina, SR stellate reticulum, DS desmosome, EK enamel knot)
References
Attardi LD, Reczek EE, Cosmas C, Demicco EG, McCurrach ME, Lowe SW, Jacks T (2000) PERP, an apoptosis-associated target of p53, is a novel member of the PMP-22/gas3 family. Genes Dev 14:704–718
Baratella L, Arana-Chavez VE, Katchburian E (1999) Apoptosis in the early involuting stellate reticulum of rat molar tooth germs. Anat Embryol 200:49–54
Beaudry VG, Ihrie RA, Jacobs SBR, Nguyen B, Pathak N, Park E, Attardi LD (2010) Loss of the desmosomal component PERP impairs wound healing in vivo. Dermatol Res Pract 2010:759731
Cai J, Cho SW, Kim JY, Lee MJ, Cha YG, Jung HS (2007) Patterning the size and number of tooth and its cusps. Dev Biol 304:499–507
Cam Y, Fausser JL, Vonesch JL, Peterkova R, Peterka M, Halaskova M, Lesot H (2002) Asymmetrical morphogenesis and medio-lateral positioning of molars during mouse development. Eur J Oral Sci 110:35–43
Dassule HR, Lewis P, Bei M, Maas R, McMahon AP (2000) Sonic hedgehog regulates growth and morphogenesis of the tooth. Development 127:4775–4785
Fausser JL, Schlepp O, Aberdam D, Meneguzzi G, Ruch JV, Lesot H (1998) Localization of antigens associated with adherens junctions, desmosomes, and hemidesmosomes during murine molar morphogenesis. Differentiation 63:1–11
Feng J, Yang G, Yuan G, Gluhak-Heinrich J, Yang W, Wang L, Chen Z, Schulze McDaniel J, Donly KJ, Harris SE, Macdougall M, Chen S (2011) Abnormalities in the enamel in Bmp2-deficient mice. Cells Tissues Organs 194:216–221
Ferone G, Mollo MR, Thomason HA, Antonini D, Zhou H, Ambrosio R, De Rosa L, Salvatore D, Getsios S, van Bokhoven H, Dixon J, Missero C (2013) p63 control of desmosome gene expression and adhesion is compromised in AEC syndrome. Hum Mol Genet 22:531–543
Franke WW, Heid H, Zimbelmann R, Kuhn C, Winter-Simanowski SW, Dörflinger Y, Grund C, Rickelt S (2013) Transmembrane protein PERP is a component of tessellate junctions and of other junctional and non-junctional plasma membrane regions in diverse epithelial and epithelium-derived cells. Cell Tissue Res 353:99–115
Gluhak-Heinrich J, Guo D, Yang W, Harris MA, Lichtler A, Kream B, Zhang J, Feng JQ, Smith LC, Dechow P, Harris SE (2010) New roles and mechanism of action of BMP4 in postnatal tooth cytodifferentiation. Bone 46:1533–1545
Guha U, Gomes WA, Kobayashi T, Pestell RG, Kessler JA (2002) In vivo evidence that Bmp signaling is necessary for apoptosis in the mouse limb. Dev Biol 249:108–120
Ihrie RA, Marques MR, Nguyen BT, Horner JS, Papazoglu C, Bronson RT, Mills AA, Attardi LD (2005) PERP is a p63-regulated gene essential for epithelial integrity. Cell 120:843–856
Ihrie RA, Bronson RT, Attardi LD (2006) Adult mice lacking the p53/p63 target gene Perp are not predisposed to spontaneous tumorigenesis but features of ectodermal dysplasia syndromes. Cell Death Differ 13:1614–1618
Ishida K, Murofushi M, Nakao K, Morita R, Ogawa M, Tsuji T (2011) The regulation of tooth morphogenesis is associated with epithelial cell proliferation and the expression of Sonic hedgehog through epithelial-mesenchymal interactions. Biochem Biophys Res Commun 405:455–461
Jernvall J, Thesleff I (2000) Reiterative signaling and patterning in mammalian tooth morphogenesis. Mech Dev 92:19–29
Jernvall J, Abert T, Kettunen P, Keranen S, Thesleff I (1998) The life history of embryonic signaling center: BMP-4 induces p21 and is associated with apoptosis in the mouse tooth enamel knot. Development 125:161–169
Jheon AH, Mostowfil P, Snead ML, Ihrie RA, Sone E, Pramparo Attardi LD, Klein OD (2011) PERP regulates enamel formation via effects on cell–cell adhesion and gene expression. J Cell Sci 124:745–754
Kettunen P, Thesleff I (1998) Expression and function of FGFs-4, −8, and −9 suggest functional redundancy and repetitive use as epithelial signals during tooth morphogenesis. Dev Dyn 211:256–268
Kieffer-Combeau S, Meyer JM, Lesot H (2001) Cell-matrix interactions and cell-cell junctions during epithelial histo-morphogenesis in the developing mouse incisor. Int J Dev Biol 45:733–742
Kim JY, Lee MJ, Cho KW, Lee JM, Kim YJ, Kim JY, Jung HI, Cho JY, Cho SW, Jung HS (2009) Shh and ROCK1 modulate the dynamic epithelial morphogenesis in circumvallate papilla development. Dev Biol 325:273–280
Kratochwil K, Galceran J, Tontsch S, Roth W, Grosschedl R (2002) FGF4, a direct target of LEF1 and Wnt signaling, can rescue the arrest of tooth organogenesis in Lef1−/− mice. Genes Dev 16:3173–3185
Kwon TG, Lee CO, Park JW, Choi SY, Rijal G, Shin HI (2014) Osteonecrosis associated with dental implants in patients undergoing bisphosphonate treatment. Clin Oral Implants Res 25:632–640
Legan PK, Yue KK, Chidgey MA, Holton JL, Wilkinson RW, Garrod DR (1994) The bovine desmocollin family: a new gene and expression patterns reflecting epithelial cell proliferation and differentiation. J Cell Biol 126:507–518
Lyngstadaas SP, Moinichen CB, Risnes S (1998) Crown morphology, enamel distribution and enamel structure in mouse molars. Anat Rec 250:268–280
Nancy A (2012) Development of tooth and its supporting tissues. Ten Cate’s oral histology, 8th edn. Elsevier, Mosby, pp 80–81
Sasaki T, Ito Y, Xu X, Han J, Bringas P Jr, Maeda T, Slavkin HC, Grosschedl R, Yang C (2005) Lef1 is a critical epithelial survival factor during tooth morphogenesis. Dev Biol 278:130–143
Senoo M, Pinto F, Crum CP, McKeon F (2007) p63 is essential for the proliferative potential of stem cells in stratified epithelia. Cell 129:523–536
Sohn WJ, Yamamoto H, Shin HI, Ryoo ZY, Lee S, Bae YC, Jung HS, Kim JY (2011) Importance of region-specific epithelial rearrangements in mouse rugae development. Cell Tissue Res 344:271–177
Sohn WJ, Kim HS, Gwon GJ, Chae YM, An CH, Park HD, Jung HS, Ryoo ZY, Lee S, Kim JY (2012) Rgs19 regulates mouse palatal fusion by modulating cell proliferation and apoptosis in the MEE. Mech Dev 129:244–254
Sohn WJ, Choi MA, Yamamoto H, Lee S, Jung JK, Jin MU, An CH, Jung HS, Suh JY, Shin HI, Kim JY (2014) Contribution of mesenchymal proliferation in tooth root morphogenesis. J Dent Res 93:78–83
Stewart GA, Lowrey JA, Wakelin SJ, Fitch PA, Lindey S, Dallman MJ, Lamb JR, Howie SEM (2002) Sonic hedgehog signaling modulates activation of and cytokine production by human peripheral CD4+ T cells. J Immunol 169:5451–5457
Thesleff I (2003) Epithelial-mesenchymal signalling regulating tooth morphogenesis. J Cell Sci 116:1647–1648
Thesleff I, Mikkola M (2002) The role of growth factors in tooth development. Int Rev Cytol 217:93–135
Tucker A, Sharpe P (2004) The cutting edge of mammalian development; how the embryo makes teeth. Nat Rev Genet 5:499–508
Tummers M, Thesleff I (2009) The importance of signal pathway modulation in all aspect of tooth development. J Exp Zool B Mol Dev Evol 312B:309–319
Vaahtokari A, Åbert T, Jernvall J, Keränen S, Thesleff I (1996) The enamel knot as a signaling center in the developing mouse tooth. Mech Dev 54:39–43
Yang W, Harris MA, Cui Y, Mishina Y, Harris SE, Gluhak-Heinrich J (2012) Bmp2 is required for odontoblast differentiation and pulp vasculogenesis. J Dent Res 91:58–64
Zhang Y, Zhang Z, Zhao X, Yu X, Hu Y, Geronimo B, Fromm SH, Chen YP (2000) A new function of BMP4: dual role for BMP4 in regulation of Sonic hedgehog expression in the mouse tooth germ. Development 127:1431–1443
Author information
Authors and Affiliations
Corresponding author
Additional information
This study was supported by a National Research Foundation of Korea (NRF) grant funded by the Korean Government (MSIP; no. 2008–0062282).
Electronic supplementary material
Below is the link to the electronic supplementary material.
Figure S1
Immunolocalization of Perp. At E16.5, Perp is localized in the DL, SR and SI (a). Higher magnifications of images showing Perp localization in DL (b), SR (c) and SI (d). Similar localization pattern of Perp is observed in the OE, DL, SR and SI except the AB at PN2 and PN6 (e-l). OE; Oral Epithelium, DL; Dental Lamina, SR; Stellate Reticulum, SI; Stratum Intermedium, AB; Ameloblast. Scale bars 100 μm (a, e, i), 50 μm (b-c, f-g, j-k), 10 μm (d, h, l) (GIF 384 kb)
Figure S2
EDX-analysis (EDXA) of kidney capsule calcified control (a-c) and AS-ODN (d-f) teeth. The EDXA mapping images (b-c, e-f) for calcium and phosphorus distribution are based on SEM micrographs (a, d). The table shows the calcium and phosphorus distribution in the control and AS-ODN teeth. Ca; Calcium, P; Phosphorus (GIF 241 kb)
Rights and permissions
About this article
Cite this article
Neupane, S., Sohn, WJ., Rijal, G. et al. Developmental regulations of Perp in mice molar morphogenesis. Cell Tissue Res 358, 109–121 (2014). https://doi.org/10.1007/s00441-014-1908-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00441-014-1908-7