Abstract
In tooth development matrix metalloproteinases (MMPs) are under the control of several regulatory mechanisms including the upregulation of expression by inducers and downregulation by inhibitors. The aim of the present study was to monitor the occurrence and distribution pattern of the extracellular matrix metalloproteinase inducer (EMMPRIN), the metalloproteinases MMP-2 and MT1-MMP and caveolin-1 during the cap and bell stage of rat molar tooth germs by means of immunocytochemistry. Strong EMMPRIN immunoreactivity was detected on the cell membranes of ameloblasts and cells of the stratum intermedium in the bell stage of the enamel organ. Differentiating odontoblasts exhibited intense EMMPRIN immunoreactivity, especially at their distal ends. Caveolin-1 immunoreactivity was evident in cells of the internal enamel epithelium and in ameloblasts. Double immunofluorescence studies revealed a focal co-localization between caveolin-1 and EMMPRIN in ameloblastic cells. Finally, western blotting experiments demonstrated the expression of EMMPRIN and caveolin-1 in dental epithelial cells (HAT-7 cells). A substantial part of EMMPRIN was detected in the detergent-insoluble caveolin-1-containing low-density raft membrane fraction of HAT-7 cells suggesting a partial localization within lipid rafts. The differentiation-dependent co-expression of MMPs with EMMPRIN in the enamel organ and in odontoblasts indicates that EMMPRIN takes part in the induction of proteolytic enzymes in the rat tooth germ. The localization of EMMPRIN in membrane rafts provides a basis for further investigations on the role of caveolin-1 in EMMPRIN-mediated signal transduction cascades in ameloblasts.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Teeth are formed by the ectoderm-derived oral epithelium and neural crest-derived ectomesenchymal cells. First sign in the tooth development is a thickening of the oral epithelium, which invaginates in the underlying mesenchyme and forms the bud, cap and bell of the tooth germ. At the bell stage, the enamel organ components include preameloblasts and their progenitors, the internal enamel epithelium, the stratum intermedium, and the stellate reticulum epithelial cells of the outer enamel epithelium. In the late bell stage, ectomesenchymal cells of the dental papilla differentiate into polarized odontoblasts and secrete the dentin matrix, whereas ameloblasts derived from the internal enamel epithelium synthesize the enamel matrix (for review see Thesleff and Aberg 1999).
Throughout odontogenesis, an intense remodeling of the extracellular matrix facilitates the migration of cells and the mesenchymal condensation (Chin and Werb 1997). Furthermore, the specific cleavage of tooth-specific matrix proteins in the predentin and in the enamel matrix is involved in the regulation of the mineralization process of dental hard tissues (Simmer and Hu 2002; Fanchon et al. 2004). It is therefore evident that matrix metalloproteinases (MMPs) take part in the remodeling of the ECM concomitant with the tooth development.
MMPs can be subdivided into two groups, soluble and membrane-type MMPs (MT-MMPs). The soluble MMPs are expressed as inactive pro-enzymes that become activated in the extracellular environment. MT-MMPs are intracellularly activated and anchored in the plasma membrane. MT-MMPs were identified as activators of soluble MMPs and were also shown to be able to degrade extracellular matrix proteins (Hamacher et al. 2004).
In addition to the proteolytic activation of pro-MMPs or to the inhibitory effects by endogenous inhibitors, MMPs are regulated transcriptionally by cytokines or growth factors (Tsuruda et al. 2004). Several studies for MMP inducing factors in tumor cells lead to the identification of EMMPRIN/CD147/basigin, a highly glycosylated transmembrane protein (Ellis et al. 1989; Kataoka et al. 1993). The term EMMPRIN reflects its Extracellular Matrix Metalloproteinase Inducer activity. Depending on the cell system, EMMPRIN can stimulate the production of MMP-1, MMP-2, MMP-3 and MT1-MMP (MMP-14) (Gabison et al. 2005). Furthermore, the EMMPRIN stimulation of MMP synthesis is dependent on N-glycosylation of its extracellular domain. Recent data have shown that the less glycosylated (LG) EMMPRIN is associated with caveolin-1 on the surface of multiple cell types resulting in reduced EMMPRIN clustering on the cell surface and therefore, diminished MMP expression (Tang and Hemler 2004).
The presence of EMMPRIN in non-tumoral tissues suggests a role in physiological processes, which may be associated with increased production of MMPs (Toole 2003). Findings on the occurrence of EMMPRIN in dental development are rare (Kumamoto and Ooya 2006).
The aims of the present immunocytochemical and biochemical study were as follows: (1) to examine the distribution pattern of EMMPRIN in the developing tooth germ, especially in cells responsible for odontogenesis (odontoblasts) and amelogenesis (ameloblasts), (2) to investigate the co-expression of EMMPRIN with MMPs and (3) to study, whether EMMPRIN colocalizes with caveolin in the tooth germ as well as in cultured cells of the ameloblastic lineage.
Material and methods
Antibodies for the detection of EMMPRIN, caveolin, MMP-2, MT1-MMP and cytokeratin 14 in immunocytochemical staining and western blotting experiments
Monoclonal mouse caveolin-1 antibody and polyclonal rabbit anti-caveolin-(1–3) antiserum were obtained from Transduction Laboratories (distributed by BD Biosciences, Heidelberg, Germany). The antiserum against CD147/EMMPRIN was purchased from Santa Cruz (Santa Cruz, Biotechnology, Inc., Santa Cruz, CA). The antibodies against MMP-2 and MT1-MMP were obtained from Chemicon (Chemicon Europe, Hampshire, UK). The polyclonal rabbit anti-cytokeratin(CK) 14 antiserum was a gift by Dr. B. Lane (Dundee, UK).
Immunocytochemistry of tissue samples
Total heads of fetal (E18; n = 2) and newborn male and female Wistar rats (n = 6) were fixed in 4% buffered formalin for 5 h at room temperature (RT), washed, dehydrated, and embedded in paraffin. Sections of 5 μm were cut and mounted on silane-coated glas slides. The sections were dewaxed, and irradiated with microwaves in 0.01 M sodium citrate buffer (pH 6.0), 2 × 5 min at 850 W. After washing in phosphate-buffered saline (PBS; pH 7.4), the sections were treated with 0.3% hydrogen peroxide for 30 min, and then incubated for 1 h at 37°C with the primary antibodies: polyclonal goat anti-CD147 (EMMPRIN) (Santa Cruz 1:2,000); monoclonal mouse anti-MT1-MMP (Chemicon 1:1,200); monoclonal mouse anti-MMP-2 (Chemicon 1:200); monoclonal mouse anti-caveolin-1 (BD 1:400); polyclonal rabbit anti-caveolin (BD 1:800); polyclonal rabbit anti-CK14 antiserum (Dr. B. Lane, 1:1,600). The antibodies were detected with biotinylated secondary antibodies, followed by the incubation with streptavidin/biotin–peroxidase complex (Vectastain Elite, Vector; Burlingame, CA). The peroxidase activity was visualized with 3-3′-diaminobenzidine/H2O2.
For double-immunofluorescence experiments, sections were incubated with the polyclonal goat anti-CD147 (1:200) overnight at 4°C, washed with PBS and incubated with Alexa Fluor® 555 donkey anti-goat IgG (1:200) (Molecular Probes; Eugene, OR, USA) for 0.5 h at 37°C, and washed again in PBS. Then, the sections were incubated with the polyclonal rabbit anti-caveolin-1 antiserum (1:40) overnight at 4°C. Finally, the sections were incubated with Alexa Fluor® 488 donkey anti-rabbit IgG (1:200) (Molecular Probes) for 1 h at 37°C and washed in PBS.
In another double-label experiment, sections were incubated with the polyclonal goat anti-CD147 (1:200) overnight at 4°C, washed with PBS and incubated with Alexa Fluor® 555 donkey anti-goat IgG (1:200). Then, sections were incubated with the monoclonal mouse anti-MMP-2 (1:50) (Chemicon) overnight at 4°C. After further washings, sections were incubated with Alexa Fluor® 488 donkey anti-mouse IgG (1:200) (Molecular Probes) for 1 h at 37°C.
Finally, sections were mounted in PBS-glycerol (1:9) containing 2.5% 1,4-diazabicyclo (2.2.2) octane (DABCO; Sigma, Germany) to prevent fading.
For controls, the primary antibodies were replaced with either non-specific goat or rabbit immunoglobulins or irrelevant hybridoma supernatant.
Immunocytochemistry of HAT-7 cells
For the immunocytochemical detection of CK14, cells were embedded in fibrin and fixed with 4% buffered formaline (for detailed description see Koslowski et al. 2004). Briefly, the culture medium was removed and cells were washed with PBS, scraped off and collected by centrifugation. Cells were carefully mixed with fibrin glue (Tissuecoll Duo S; Immuno, Heidelberg, Germany) and fibrin was coagulated with thrombin solution (Immuno). After fixation with neutral-buffered formaline (1 h, 4°C) and washing with PBS for 30 min, cells were embedded in paraffin. Sections of 5 μm were mounted on silane-coated slides. After washing in phosphate-buffered saline (PBS pH 7.4), the sections were treated with 0.3% hydrogen peroxide for 30 min, incubated with respective normal serum and then incubated for 1 h at 37°C with the polyclonal rabbit anti-CK14 antiserum (1:3,200; Dr. B. Lane, Dundee, UK). The antiserum was detected with biotinylated secondary antibody, followed by incubation with streptavidin/biotin-peroxidase complex (Vectastain Elite, Vector; Burlingame, CA). The peroxidase activity was visualized with 3-3′-diaminobenzidine/H2O2.
Double-immunofluorescence of caveolin-1 and EMMPRIN was carried out in the same way as described above.
Preparations were examined and photographed in a Nikon microscope (Optiphot-2; Nikon Corporation, Japan). Fluorescence microscopic studies were performed using an Olympus BX60 microscope (Olympus, Hamburg, Germany) or in a Leica confocal laser scanning microscope (TCS 4D; Leica, Bensheim, Germany).
Cell culture of HAT-7 cells
HAT-7 cells originate from a dental epithelial cell line originating from the apical bud of a rat incisor (A detailed description of the preparation procedure and of cell characteristics is given by Kawano et al. 2004).
Cells were cultured in Dulbecco’s Modified Eagle’s medium (DMEM)/Ham’s F12 supplemented with 10% fetal bovine serum, penicillin (100 Units/ml) and streptomycin (100 Units/ml). All cultures were maintained in humidified atmosphere of 5% CO2 at 37°C.
Triton X-100 solubility
Confluent cells of a T75 flask were washed twice with ice-cold PBS, pH 7.2, 500 μl MBS (25 mM Mes, pH 6.5, 150 mM NaCl) containing 1% Triton X-100 plus protease inhibitors. After 30 min incubation on ice, the soluble fraction was collected. The remaining Triton X-100 insoluble fraction was dissolved by adding 500 μl of 1% SDS to the T75 flask and passed through a 26-gauge needle 10 times in order to lower its viscosity. Equal volumes of the Triton X-100 soluble and insoluble fractions were separated by SDS-PAGE and subjected to Western blot analysis as described above.
SDS-PAGE and western blot analysis
Samples were loaded onto 12% SDS polyacrylamide gels and separated according to Laemmli (Laemmli 1970). The separated proteins were transferred to a PVDF-membrane. After blocking in PBS-T (137 mM NaCl, 2.7 mM KCl, 6.7 mM Na2HPO4·2H2O, 1.5 mM KH2PO4, 0.1% Tween20; pH 7.2–7.5) containing 5% nonfat dry milk for 1 h at RT or overnight at 4°C the membrane was incubated with monoclonal mouse anti-caveolin-1 (clone 2297; BD Biosciences, Pharmingen, USA; 1:1,000), polyclonal goat anti-CD147 (G-19; Santa Cruz Biotechnology Heidelberg, Germany; 1:500), monoclonal mouse anti-T1α (1:2,000, a gift by Dr. Mary Williams, Boston, MA, USA), monoclonal mouse anti-human transferrin receptor (TfR) (Clone H68.4, Zymed Laboratories INC., South San Franscisco, US; 1:500), polyclonal rabbit anti-protein disulfide isomerase (PDI) (StressGen Biotechnologies Corp., Victoria, Canada; 1:750) and polyclonal rabbit anti-β-coatomer protein (β-Cop) (Ongogene Research Products, Boston, US; 1:750) for 2 h at RT or overnight at 4°C. Next, the membrane was washed three times for 10 min and reacted with secondary HRP conjugated antibodies (ECL anti-mouse IgG, Amersham Biosciences, Buckinghamshire, UK, 1:4,000; goat anti-rabbit IgG, Bio-Rad Laboratories, Hercules, CA; 1:2,000, or rabbit anti-Syrian-hamster IgG, Jackson ImmunoResearch Lab., distributed by Dianova, Hamburg, Germany, 1:10,000) for 1 h at RT. The chemiluminescent signal was generated by using ECL™ western blot analysis detection reagents (Amersham Biosciences, Uppsala, Sweden) and detected by Image Reader LAS-3000 (Fujifilm).
Preparation of detergent-insoluble membrane fractions
Preparation of detergent-insoluble membrane fractions was carried out as described previously (Iwabuchi et al. 1998) with minor modifications. Confluent HAT-7 cells of six T75 flask were washed twice with ice-cold PBS, pH 7.2 and then scraped in 1 ml of ice-cold MBS (25 mM Mes, pH 6.5, 150 mM NaCl) containing 1% Triton X-100 plus protease inhibitors. After 30 min the lysate was centrifuged at 2,000×g for 10 min. The supernatant was removed from the pellet and stored on ice. The pellet was resuspended in ice-cold MBS (25 mM Mes, pH 6.5, 150 mM NaCl) containing protease inhibitors. The cells were passed through a tight fitting dounce homogeniser 10 times and mixed with an equal volume of 80% sucrose (prepared in MBS lacking Triton X-100) and transferred to an ultracentrifuge tube. 1.4 ml 35% sucrose and 0.8 ml 5% sucrose in MBS (25 mM Mes, pH 6.5, 150 mM NaCl) containing protease inhibitors were bedded on the top of the lysate. The gradient was centifuged at 200,000×g for 20 h in a MLS 50 rotor (Beckman Coulter, Palo Alto, CA). Fourteen 300 μl fractions were collected, beginning from the top of the tube. Aliquots (20 μl) of each fraction were subjected to SDS-PAGE and western blot analysis. Experiments were performed at 4°C.
Results
In the cap stage, the enamel organ is concave on the surface in relation to the dental papilla. The bell stage of the molar tooth germ represents sequences of early dentinogenesis and early amelogenesis in the same specimen. The four layers of the enamel organ (i.e., the internal enamel epithelium, the stratum intermedium, the stellate reticulum, and the external enamel epithelium) enclose the undifferentiated and condensed mesenchymal cells of the dental papilla. In the area of the future tooth cusp, cells of the adjacent surface of the dental papilla begin to differentiate to form the odontoblast layer. In the bell stage of the enamel organ, cells of the internal epithelium differentiate into the highly spezialized ameloblasts. Stratum intermedium cells were located at the proximal end of the ameloblasts and formed two or three cell layers. Table 1 summarises the distribution pattern of EMMPRIN, MMPs and caveolin-1 in different cell types in tooth germs of newborn rats (bell stage of the enamel organ).
Developmental distribution of EMMPRIN, MMP-2, MT1-MMP and caveolin-1 in the tooth germ-immunocytochemical detection
EMMPRIN
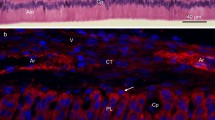
Occurrence of cells that were positive after immunoreaction (IR) with EMMPRIN antiserum varies during ameloblast development (Fig. 1a, b). In the cup stage of the enamel organ, cells of the internal enamel epithelium expressed moderate immunoreactivity for EMMPRIN (Fig. 1a). In the bell stage of the enamel organ (Fig. 1b), the column-shaped preameloblasts and ameloblasts expressed a strong granular reaction pattern. IR was localized in the basolateral membranes and in the apical region of ameloblasts, the Tomes’ process. In addition, we found EMMPRIN in the contact region between ameloblasts and stratum intermedium cells and in the stratum intermedium proper.
Photomicrographs demonstrate immunoreactivity (IR) for EMMPRIN, MT1 MMP, MMP-2 and caveolin-1 in paraffin sections of rat tooth germ tissue. a Cells of the inner enamel epithelium (cap stage of the enamel organ) show intense EMMPRIN IR (Alexa-coupled). b Ameloblasts as well as odontoblasts (bell stage of the enamel organ) exhibite strong EMMPRIN IR. Note the IR in the borderline between ameloblasts and the stratum intermedium. Mesenchymal cells of the dental papilla are only weakly immunoreactive. c MT1-MMP IR is present in the apical region of cells of the inner enamel epithelium (bell stage). d MMP-2 IR is present in the cell membrane of cells in the inner enamel epithelium (bell stage). Double-label experiments demonstrate only weak co-localization (yellow-colored areas) between MMP-2 (FITC-coupled) and EMMPRIN (Alexa-coupled). e Intense MMP-2 IR is seen in the apical and basal region of ameloblasts and in the apical region of odontoblasts. Note the positive IR pattern of ameloblasts and odontoblasts correspondent to the EMMPRIN expression in b. f, g Caveolin-1 IR is detected in cells of the enamel organ (bell stage). Cells of the inner enamel epithelium (f) and ameloblasts (g) show intense IR in their lateral cell membrane (asterisks blood vessel). Double-label experiments of caveolin-1 (FITC coupled) and EMMPRIN (Alexa-coupled) reveal colocalization of both antigens in the yellow-colored areas of the cell membrane (g). Scale bar: a 100 μm, b–f 50 μm, g 25 μm. Abbreviations: Am ameloblast; DL dental lamina; EEE external enamel epithelium; IEE internal enamel epithelium; EO enamel organ; Od odontoblast; SI stratum intermedium; SR stellate reticulum
In contrast, the stellate reticulum and cells of the external enamel epithelium expressed only weak immunoreactivity for EMMPRIN.
In the dental papilla, there was only weak EMMPRIN immunoreactivity in some mesenchymal cells. In contrast, the odontoblastic cell layer exhibited a strong membranous EMMPRIN-IR, particularly at the basolateral cell membranes and in the odontoblastic processes (Tomes fibres).
MT1-MMP, MMP-2
The IR pattern of MT1-MMP and MMP-2 in the enamel organ correlated with the distribution of EMMPRIN (Fig. 1c–e). Immunoreactivities for MT1-MMP (Fig. 1c) and MMP-2 (Fig. 1d, e) were observed in cells of the internal enamel epithelium in the cap stage (not shown) and bell stage of the enamel organ and ameloblasts, notably in the apical region of cells. Double-label experiments showed only negligible co-localization between EMMPRIN and MMP-2 (Fig. 1d). In the dental papilla, the immunohistochemical staining for both metalloproteinases was more evident in odontoblasts than in undifferentiated cells.
Caveolin-1
Expression of caveolin-1 occurred predominantly in epithelial cells of the internal enamel epithelium and in ameloblasts (Fig.1f, g). In addition, IR for caveolin-1 was found in endothelial cells of surrounding blood vessels. Mesenchymal cells of the dental papilla and odontoblasts were mostly immunonegative for caveolin-1 (Fig. 1f). Both the IR with the monoclonal caveolin-1 antiserum and the anti-caveolin-(1–3) antiserum exhibited the same distribution pattern of caveolin (data not shown).
Confocal microscopic images from double-label experiments appeared to show some co-localization between caveolin-1 and EMMPRIN in the cell membranes of ameloblasts as well as in the cell membranes of the internal enamel epithelium (Fig. 1g).
Detection of EMMPRIN and caveolin-1 in HAT-7 cells
Characterization of HAT-7 cells by the immunocytochemical detection of CK14 and double-lable immunofluorescence of EMMPRIN and caveolin-1
To characterize HAT-7 cells we used CK14 as a marker of ameloblast-lineage cells (Tabata et al. 1996). The intense IR of HAT-7 cells coincides with the expression pattern of CK14 in cells of the enamel organ (e.g. in cells of the internal and external enamel epithelium and in the stratum intermedium) (Fig. 2a, b). In contrast, mesenchymal cells of the dental papilla were immunonegative for CK14.
a, b Photomicrographs demonstrate immunoreactivity (IR) for CK14 in paraffin sections of rat tooth germ tissue. Cells of the enamel organ (a) as well as HAT-7 cells (b) show intense IR. c Double-label experiments of caveolin-1 (FITC-coupled) and EMMPRIN (Alexa-coupled) in paraffin-embedded HAT-7 cells reveal co-localization of both antigens in yellow-colored areas of the cell membrane. Scale bar: a, b 50 μm; c 20 μm. Abbreviations: Am ameloblast; EEE external enamel epithelium; IEE internal enamel epithelium; Od odontoblast; SR stellate reticulum.
To examine the possible co-localization of EMMPRIN with caveolin-1 in HAT-7 cells, we analyzed the immunocytochemical co-localization of both antigens in paraffin-embedded cells by double labeling technique. Merged immunofluorescence pictures indicate a partial co-localization of EMMPRIN and caveolin-1 at cell membranes (Fig. 2c).
Isolation of lipid rafts and localization of EMMPRIN and caveolin-1
Insolubility in the detergent Triton X-100 is a well-recognized biochemical characteristic of proteins localized in lipid rafts (Fig. 3). We therefore, analyzed the Triton X-100 solubility of EMMPRIN protein prepared from HAT-7 cell membrane fractions, along with the raft marker caveolin-1 and the non-raft marker T1α (an integral membrane protein expressed strongly in the plasma membrane of both type I alveolar epithelial cells and lymphatic endothelial cells in the lung (Rishi et al. 1995; Williams et al. 1996) (Fig. 3a). As expected, caveolin-1 was predominantly found in the detergent-insoluble raft fraction, and T1α was present only within the non-raft fraction of the membrane. Interestingly, although the majority of EMMPRIN protein was Triton X-100-soluble, a significant proportion was found within the Triton X-100 insoluble fraction, suggesting a partial localization in lipid rafts.
a Solubility of caveolin-1, EMMPRIN and T1α in Triton X-100. HAT-7 cells were lysed in a buffer containing 1% Triton X-100 to obtain soluble and insoluble fractions. Equal volumes of each fraction (100 μl) were analyzed by western blot analysis using caveolin-1, EMMPRIN or T1α specific antibodies. Caveolin-1 was used as marker of the lipid rafts, and T1α as a marker of the non-raft fraction. b Characterization of membrane fractions prepared by Triton X-100 from HAT-7 cells. HAT-7 cells were homogenized in a buffer containing 1% Triton X-100 and subjected to sucrose density gradient centrifugation as described under “Materials and Methods”. Thirteen fractions were collected (fraction 1, top of the gradient; fraction 13, bottom of the gradient) and aliquots of each fraction were subjected to western blot analysis with antibodies against caveolin-1, EMMPRIN, TfR, β-Cop and PDI. Caveolin-1 was enriched in fractions 4–6, representing the caveolae-enriched membrane fractions
Another main characteristic of lipid rafts is low buoyant density in sucrose gradient centrifugation. We isolated raft-like membranes using Triton X-100 treatment at 4°C (Fig. 3b). When rafts were prepared using Triton X-100 treatment followed by sucrose density gradient centrifugation, significant amounts of EMMPRIN were detected in the caveolin-1-containing low-density raft fractions (Fig. 3b, lanes 4, 5). EMMPRIN was also detected in the high-density fractions along with markers of the Golgi apparatus and endoplasmic reticulum (β-COP and PDI). In addition, isolated lipid rafts were devoid of intracellular membrane markers for the Golgi apparatus and the endoplasmic reticulum. Transferrin receptor (TfR), a marker of the non-raft fraction of the plasma membrane (Macdonald and Pike 2005), was mainly found in high-density fractions, although very small amounts (compared to caveolin-1 and EMMPRIN) were also present in the low-density fractions.
Discussion
In this study, we demonstrate the expression of EMMPRIN not only in cells of the inner enamel epithelium and stratum intermedium cells of the enamel organ as previously described by Kumamoto and Ooya (2006), but also in ameloblasts and in cells of the odontoblastic layer in the dental papilla. In addition, we tested the dental epithelial cell line HAT-7 for the expression and subcellular localization of EMMPRIN. The cell lysates revealed two major forms of EMMPRIN (55 and 57 kDa) by western blot assay, indicating that the EMMPRIN proteins in HAT-7 cells are modified by different modes of glycosylation.
Furthermore, the expression pattern of EMMPRIN coincides with the occurrence of MMPs in the enamel organ and in odontoblasts. This is, to our knowledge, the first description of EMMPRIN in normal amelo- and odontoblasts.
EMMPRIN expression has been detected in some ameloblastomas indicating that EMMPRIN might participate in the progression of some odontogenic tumors by inducing MMPs in stromal cells (Kumamoto and Ooya 2006). In contrast, our study points out the involvement of EMMPRIN in the induction of MMPs in normal dental development.
The precise molecular function of EMMPRIN is largely unclear. Beside heterophilic interactions between EMMPRIN and a putative EMMPRIN receptor on opposing cells, the MMP-inducing function of EMMPRIN involves the molecule acting as counter-receptor for itself, by homophilic interactions in cis or trans (for review see Toole 2003). An important point concernes the EMMPRIN immunoreactivity detected at contact sites between the ameloblastic cell layer and the stratum intermedium cells. The evidence that preameloblasts cannot differentiate into secretory ameloblasts without stratum intermedium cells suggests that cell–cell-interactions play an essential role in the differentiation process of ameloblasts (Sakakura et al. 1989). Several transmembrane proteins such as the gap junction protein connexin 43 and the cell adhesion receptor CD44 take part in the cross-talk between stratum intermedium cells and ameloblasts (Nakamura and Ozawa 1997; Joao and Arana-Chavez 2003). Therefore, we suggest that heterotypic interactions between cells of the stratum intermedium and those of the ameloblastic layer take part in EMMPRIN-induced expression of MMPs in ameloblasts.
The signal transduction pathways downstream of EMMPRIN interactions that result in MMP production are not yet established. In fibroblasts, the induction of MMPs by EMMPRIN occurs at the transcription level and is mediated by a mitogen activated protein kinase(MAPK) p38 pathway (Lim et al. 1998). In another study, 5-lipoxygenase and phospholipase A2 have been implicated in MMP-2 production (Yan et al. 2005). It remains unclear, which signalling pathway is utilized by EMMPRIN in ameloblasts or odontoblasts.
In this study, we demonstrated the co-expression of EMMPRIN and MMPs in cells of the enamel organ and in differentiating odontoblasts. However, it is difficult to address the expression of MMPs to the action of EMMPRIN. We assume that EMMPRIN alone does not appear to regulate the production of MMP in ameloblasts or odontoblasts. In human odontoblasts and pulp tissue, MT1-MMP is constitutively expressed and downregulated by TGFβ and BMP-2 (Palosaari et al. 2002). The stronger EMMPRIN expression in the odontoblastic layer compared to that in undifferentiated cells of the dental papilla is probably related to the cellular differentiation programme in the developing tooth germ.
In the current study, immunofluorescence double labeling experiments indicate a focal co-localization between caveolin-1 and EMMPRIN immunoreactivities in cell membranes of ameloblasts and in inner enamel epithelial cells. Furthermore, we tested the dental epithelial cell line HAT-7 for the expression of EMMPRIN and caveolin-1. EMMPRIN was detectable in the Triton-soluble as well as in the Triton-insoluble fraction of HAT-7 cells. Furthermore, experimental datas from sucrose density gradient centrifugation of Triton X-100-insoluble membranes provide evidence that support the possibility of an association of a discrete portion of EMMPRIN with lipid rafts.
Caveolin-1 constitutes not only the structural protein of caveolar raft membranes, but also concentrates many signalling molecules in caveolae and regulates their activity (Krajewska and Maslowska 2004). Therefore, we assume a regulatory role of caveolin-1 in the EMMPRIN-induced expression of MMPs. Further in vitro studies are necessary concerning the role of caveolin-1 in cells of the enamel organ.
The detection of MT1-MMP and MMP-2 in the enamel organ and in odontoblasts is in line with a growing body of findings that highlight the critical role of MMPs on the formation and mineralization of dentin and enamel matrices (Caron et al. 1998; Satoyoshi et al. 2001; Yoshiba et al. 2003; Bourd-Boittin et al. 2005). MT1-MMP is a cellular receptor for and activator of pro-MMP-2, which forms a trimolecular complex on the cell surface with TIMP-2 (for review see (Seiki 1999). In odontoblasts, MT1-MMP activates not only pro-MMP-2, but also MMP-20 (Enamelysin) (Palosaari et al. 2002). Besides its gelatinolytic activity, MMP-2 cleaves specific matrix proteins, such as amelogenin and dentin sialoprotein (Bourd-Boittin et al. 2005). In our study, the co-expression of MT1-MMP and MMP-2 at the distal pole of ameloblasts and odontoblasts indicates that both cell types take part in the pericellular remodelling of the organic matrix.
Taken together, the immunodetection of EMMPRIN in the tooth germ and the co-expression of MT1-MMP and MMP-2 provide evidence for an involvement in signaling cascades regulating the expression of MMPs in differentiating odontoblasts and ameloblasts.
References
Bourd-Boittin K, Fridman R, Fanchon S, Septier D, Goldberg M, Menashi S (2005) Matrix metalloproteinase inhibition impairs the processing, formation and mineralization of dental tissues during mouse molar development. Exp Cell Res 304:493–505
Caron C, Xue J, Bartlett JD (1998) Expression and localization of membrane type 1 matrix metalloproteinase in tooth tissues. Matrix Biol 17:501–511
Chin JR, Werb Z (1997) Matrix metalloproteinases regulate morphogenesis, migration and remodeling of epithelium, tongue skeletal muscle and cartilage in the mandibular arch. Development 124:1519–1530
Ellis SM, Nabeshima K, Biswas C (1989) Monoclonal antibody preparation and purification of a tumor cell collagenase-stimulatory factor. Cancer Res 49:3385–3391
Fanchon S, Bourd K, Septier D, Everts V, Beertsen W, Menashi S, Goldberg M (2004) Involvement of matrix metalloproteinases in the onset of dentin mineralization. Eur J Oral Sci 112:171–176
Gabison EE, Hoang-Xuan T, Mauviel A, Menashi S (2005) EMMPRIN/CD147, an MMP modulator in cancer, development and tissue repair. Biochimie 87:361–368
Hamacher S, Matern S, Roeb E (2004) [Extracellular matrix—from basic research to clinical significance. An overview with special consideration of matrix metalloproteinases]. Dtsch Med Wochenschr 129:1976–1980
Iwabuchi K, Yamamura S, Prinetti A, Handa K, Hakomori S (1998) GM3-enriched microdomain involved in cell adhesion and signal transduction through carbohydrate-carbohydrate interaction in mouse melanoma B16 cells. J Biol Chem 273:9130–9138
Joao SM, Arana-Chavez VE (2003) Expression of connexin 43 and ZO-1 in differentiating ameloblasts and odontoblasts from rat molar tooth germs. Histochem Cell Biol 119:21–26
Kataoka H, DeCastro R, Zucker S, Biswas C (1993) Tumor cell-derived collagenase-stimulatory factor increases expression of interstitial collagenase, stromelysin, and 72-kDa gelatinase. Cancer Res 53:3154–3158
Kawano S, Saito M, Handa K, Morotomi T, Toyono T, Seta Y, Nakamura N, Uchida T, Toyoshima K, Ohishi M, Harada H (2004) Characterization of dental epithelial progenitor cells derived from cervical-loop epithelium in a rat lower incisor. J Dent Res 83:129–133
Koslowski R, Barth K, Augstein A, Tschernig T, Bargsten G, Aufderheide M, Kasper M (2004) A new rat type I-like alveolar epithelial cell line R3/1: bleomycin effects on caveolin expression. Histochem Cell Biol 121:509–519
Krajewska WM, Maslowska I (2004) Caveolins: structure and function in signal transduction. Cell Mol Biol Lett 9:195–220
Kumamoto H, Ooya K (2006) Immunohistochemical detection of MT1-MMP, RECK, and EMMPRIN in ameloblastic tumors. J Oral Pathol Med 35:345–351
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680–685
Lim M, Martinez T, Jablons D, Cameron R, Guo H, Toole B, Li JD, Basbaum C (1998) Tumor-derived EMMPRIN (extracellular matrix metalloproteinase inducer) stimulates collagenase transcription through MAPK p38. FEBS Lett 441:88–92
Macdonald JL, Pike LJ (2005) A simplified method for the preparation of detergent-free lipid rafts. J Lipid Res 46:1061–1067
Nakamura H, Ozawa H (1997) Immunolocalization of CD44 and the ezrin-radixin-moesin (ERM) family in the stratum intermedium and papillary layer of the mouse enamel organ. J Histochem Cytochem 45:1481–1492
Palosaari H, Ding Y, Larmas M, Sorsa T, Bartlett JD, Salo T, Tjaderhane L (2002) Regulation and interactions of MT1-MMP and MMP-20 in human odontoblasts and pulp tissue in vitro. J Dent Res 81:354–359
Rishi AK, Joyce-Brady M, Fisher J, Dobbs LG, Floros J, VanderSpek J, Brody JS, Williams MC (1995) Cloning, characterization, and development expression of a rat lung alveolar type I cell gene in embryonic endodermal and neural derivatives. Dev Biol 167:294–306
Sakakura Y, Fujiwara N, Nawa T (1989) Epithelial cytodifferentiation and extracellular matrix formation in enamel-free areas of the occlusal cusp during development of mouse molars: light and electron microscopic studies. Am J Anat 184:287–297
Satoyoshi M, Kawata A, Koizumi T, Inoue K, Itohara S, Teranaka T, Mikuni-Takagaki Y (2001) Matrix metalloproteinase-2 in dentin matrix mineralization. J Endod 27:462–466
Seiki M (1999) Membrane-type matrix metalloproteinases. Apmis 107:137–143
Simmer JP, Hu JC (2002) Expression, structure, and function of enamel proteinases. Connect Tissue Res 43:441–449
Tabata MJ, Matsumura T, Liu JG, Wakisaka S, Kurisu K (1996) Expression of cytokeratin 14 in ameloblast-lineage cells of the developing tooth of rat, both in vivo and in vitro. Arch Oral Biol 41:1019–1027
Tang W, Hemler ME (2004) Caveolin-1 regulates matrix metalloproteinases-1 induction and CD147/EMMPRIN cell surface clustering. J Biol Chem 279:11112–11118
Thesleff I, Aberg T (1999) Molecular regulation of tooth development. Bone 25:123–125
Toole BP (2003) Emmprin (CD147), a cell surface regulator of matrix metalloproteinase production and function. Curr Top Dev Biol 54:371–389
Tsuruda T, Costello-Boerrigter LC, Burnett JC Jr (2004) Matrix metalloproteinases: pathways of induction by bioactive molecules. Heart Fail Rev 9:53–61
Williams MC, Cao Y, Hinds A, Rishi AK, Wetterwald A (1996) T1 alpha protein is developmentally regulated and expressed by alveolar type I cells, choroid plexus, and ciliary epithelia of adult rats. Am J Respir Cell Mol Biol 14:577–585
Yan L, Zucker S, Toole BP (2005) Roles of the multifunctional glycoprotein, emmprin (basigin; CD147), in tumour progression. Thromb Haemost 93:199–204
Yoshiba N, Yoshiba K, Stoetzel C, Perrin-Schmitt F, Cam Y, Ruch JV, Lesot H (2003) Temporospatial gene expression and protein localization of matrix metalloproteinases and their inhibitors during mouse molar tooth development. Dev Dyn 228:105–112
Acknowledgments
The authors are indepted to R. Dahm (Medical University of Vienna, Center for Brain Research) for supplying fetal rat tissues. The authors thank Mrs. Bramke, Mrs. Neisser and Mrs. Streichert for the execution of immunocytochemical staining of cells and tissues and for the assistance in cell culture studies.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Schwab, W., Harada, H., Goetz, W. et al. Immunocytochemical and biochemical detection of EMMPRIN in the rat tooth germ: differentiation-dependent co-expression with MMPs and co-localization with caveolin-1 in membrane rafts of dental epithelial cells. Histochem Cell Biol 128, 195–203 (2007). https://doi.org/10.1007/s00418-007-0313-7
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00418-007-0313-7