Abstract
Our previous research has shown that the extracellular matrix metalloproteinase inducer (EMMPRIN) is expressed during and may function in the early development of tooth germs. In the present study, we observed the specific expression of EMMPRIN in ameloblasts and odontoblasts during the middle and late stages of tooth germ development using immunohistochemistry. Furthermore, to extend our understanding of the function of EMMPRIN in odontogenesis, we used an anti-EMMPRIN function-blocking antibody to remove EMMPRIN activity in tooth germ culture in vitro. Both the formation and mineralisation of dental hard tissues were suppressed in the tooth germ culture after the abrogation of EMMPRIN. Meanwhile, significant reductions in VEGF, MMP-9, ALPL, ameloblastin, amelogenin and enamelin expression were observed in antibody-treated tooth germ explants compared to control and normal serum-treated explants. The current results illustrate that EMMPRIN may play a critical role in the processing and maturation of the dental matrix.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Extracellular matrix metalloproteinase inducer (EMMPRIN) is a highly glycosylated transmembrane molecule belonging to the immunoglobulin superfamily; EMMPRIN is also known as CD147 or basigin (Berditchevski et al. 1997) and was originally identified as a tumour surface protein that is capable of inducing matrix metalloproteinase (MMP) expression in fibroblasts (Biswas and Nugent 1987; Ellis et al. 1989). Further studies revealed that EMMPRIN could play a crucial role in the progression of malignancies by regulating the expression of MMPs in stromal cells (Tang et al. 2004). Purified EMMPRIN from tumour cells and recombinant EMMPRIN have been shown to induce the expression of interstitial collagenase (MMP-1), gelatinase A (MMP-2), stromelysin 1 (MMP-3), gelatinase (MMP-9), and membrane type 1- and type 2-MMPs (MT1- and MT2-MMP) by fibroblasts (Sameshima et al. 2000; Li et al. 2001; Sun and Hemler 2001; Nabeshima et al. 2004; Cao et al. 2009). Although numerous studies have shown that EMMPRIN is able to activate MMP gene expression and mediate the secretion or activation of pre-existing MMP proteins, the precise mechanism by which EMMPRIN induces MMP gene expression remains unclear. However, it has been reported that activation of both the p38 and ERK1/2 pathways is necessary for EMMPRIN expression to regulate the production of MMPs in phorbol myristate acetate (PMA)-induced THP-1 cells (Huang et al. 2008). EMMPRIN is mainly found embedded in the cell membrane, but a small proportion of EMMPRIN expressed in breast cancer cells is secreted into the cell culture medium (Taylor et al. 2002). This soluble EMMPRIN is also detected in non-cancerous tissues, for example, in gingival crevicular fluid (Emingil et al. 2006) and ocular fluid (Määttä et al. 2006). EMMPRIN is known to be expressed at varying levels in many cell types, including haematopoietic, epithelial and endothelial cells. EMMPRIN is particularly critical for mammalian reproduction, angiogenesis, neuronal and lymphatic development and maintenance as well as for tissue repair and remodelling (Igakura et al. 1998; Agrawal and Yong 2011; Kanyenda et al. 2011; Mishra et al. 2012).
Odontogenesis originates from adjacent layers of epithelial (ectodermal) and mesenchymal (mesodermal or neural-crest-derived) tissues. As in many organs, the cytodifferentiation and morphogenesis of teeth are governed by sequential and reciprocal epithelial-mesenchymal interactions and are mediated by a variety of pathways (Line 2003; Mitsiadis and Smith 2006; Wang et al. 2014). Many factors govern the proliferation and differentiation of tooth germ cells in the different stages of tooth development (Pispa and Thesleff 2003; Tang et al. 2013; Lei et al. 2014). Our previous results showed that the genetic ablation of EMMPRIN activity form the tooth germ leads to alterations in growth and morphogenesis and results in abnormalities of enamel organ development (Xie et al. 2010). The differentiation-dependent co-expression of MMPs with EMMPRIN was also observed in the enamel organ and in odontoblasts, which indicated that EMMPRIN takes part in the induction of proteolytic enzymes in the rat tooth germ (Schwab et al. 2007). Furthermore, a number of recent reports have pointed to a link between EMMPRIN and the remodelling of periodontal tissue under pathological and physiological conditions (Liu et al. 2010). Thus, it seems that EMMPRIN plays an important role in several different stages of tooth development; however, it remains unclear how EMMPRIN, as a multifunctional molecule, exerts a specific role in the formation and mineralisation of the dental extracellular matrix in the secretory stage of mouse lower first molars (from the newborn state onwards).
The present study used an organotypic culture of embryonic mouse tooth germs to investigate the implications of EMMPRIN expression in the cell differentiation and morphogenesis of late-stage tooth germ development. This model allows the visualisation of the onset of matrix apposition and early mineralisation. Our results provide evidence for previously unknown functions of EMMPRIN in odontogenesis. In particular, using an anti-EMMPRIN function-blocking antibody, we found that loss of EMMPRIN affects enamel and dentin matrix formation and inhibits enamel mineralisation, and these changes were associated with the inhibition of both VEGF and enamel matrix proteins. These findings suggest that EMMPRIN may be involved in the terminal morphogenesis of murine molars.
Materials and methods
Animals
BALB/c mice at embryonic days 15.0 (E15.0) and 18.0 (E18.0) after gestation and postnatal days 1 (PN1) and 3 (PN3) were used in this study. Adult BALB/c mice were obtained from the Shanghai Laboratory Animal Center, Chinese Academy of Sciences (Shanghai, China). All mice were maintained on a 12-h light/12-h dark cycle with food and water provided ad libitum. Female BALB/c mice (10–30 weeks) were caged together with male mice. After 3 h, successful insemination was determined based on the presence of a post-copulatory plug in the vagina, and embryonic day was defined as E0 after such a plug was observed. The embryos were removed from pregnant mice under ether anaesthesia. The lower first molar tooth germs at E15.0 and E18.0 were dissected form the embryos under a stereomicroscope in cold Hank’s buffer and then explanted into culture.
Immunohistochemistry
The removed heads of newborn mice (PN1, n = 5; PN3, n = 5) were fixed in 4 % paraformaldehyde in phosphate-buffered saline (PBS, pH 7.4) for 12 h at 4 °C and embedded in Tissue-Tek OCT (Sakura Finetek, USA). Serial cryostat sections were cut at a thickness of 6 μm and were mounted on silane-coated glass slides. At least four specimens of each developmental stage were used for immunohistochemistry. After the cryostat sections were dried at room temperature for 1 h, the sections were rinsed with PBS containing 0.1 % Triton X-100 for 10 min. To block non-specific immunoreactivity, the sections were incubated with 5 % donkey serum (GTX27475; GeneTex, San Antonio, TX, USA) in PBS for 30 min. The sections were then incubated with a primary antibody against EMMPRIN (goat polyclonal G-19, Santa Cruz Biotechnology, Santa Cruz, CA, USA) diluted 1:200 in PBS. The sections were washed four times with PBS for 10 min each and incubated with Alexa 568-labelled donkey anti-goat IgG (Invitrogen, Carlsbad, CA, USA) diluted 1:2,000 in PBS for 2 h at room temperature. The sections were rinsed five times with PBS and then incubated with 4′, 6-diamino-2-phenylindole (DAPI, 0.5 μg/ml; Wako, Osaka, Japan) for 10 min at room temperature. As a specificity control, the primary antibody was omitted from the staining procedure for some samples. These sections were examined under an OlympusIX71 fluorescence microscope (OLYMPUS, Tokyo, Japan), and immunofluorescent images were acquired using an Olympus DP-71 camera (OLYMPUS).
Organ culture
Tooth germs were removed micro-surgically and cultured for periods of up to 8 days. Untreated explants and explants treated with normal goat serum (GS) were used as controls. Preliminary experiments of organ culture for tooth germs showed mineralisation by the 8th day of culture. However, no further development of the tooth germ was observed after the 8th day. At least 90 tooth germs each were dissected from E15.0 and E18.0 embryos and cultured for a period of 8 days using a modification of Trowell’s system. These explants were supported by a filter (0.8 μm pore size, Millipore, MA, USA) mounted on metal mesh and incubated in Fitton-Jackson’s modified BGJb medium (Invitrogen) supplemented with 5 % foetal bovine serum (Filtron, Brooklyn, Australia), 100 μg/ml ascorbic acid (Invitrogen) and 100 U/ml penicillin–streptomycin (Invitrogen) under 100 % humidity in an atmosphere containing 5 % CO2 and 95 % air at 37 °C. For the antibody inhibition experiments, the concentration of neutralising antibody (NA) was estimated as described by Mitsiadis et al. (1995). Where indicated, the culture medium was supplemented with a NA against the EMMPRIN protein (40 µg/ml). The EMMPRIN antibody (G-19, Santa Cruz Biotechnology, Santa Cruz, CA, USA) is an affinity-purified goat polyclonal antibody raised against a peptide mapping to the C-terminus of mouse EMMPRIN. Sodium azide was removed from the antibody by dialysis prior to use. In the positive control cultures, normal goat serum (GeneTex) was added to the medium in an amount identical to the anti-EMMPRIN antibody. The culture media were changed every 24 h. For time course experiments, cultures were stopped at 4, 6 and 8 days. All experiments were performed at least 3 times with approximately 10 tooth germs for each treatment.
Primary cell culture of tooth germs
Tooth germs were isolated from E18.0 embryos using a stereoscopic microscope and collected in phosphate-buffered saline (PBS) at 4 °C. Tooth germs were washed three times in PBS, chopped and dissociated with trypsin (0.025 % trypsin and 0.001 % EDTA) for 5 min at 37 °C. The organoid units remaining after treatment were filtered through 70-μm filters (Falcon, BD Labware, Franklin Lakes, NJ). The living cells were counted using a Malassez haemocytometer, and the cell number was calculated as the average of three counts. The cells collected form digestions three, four and five were pooled and plated at a concentration of 2.5104 cells/cm2 into 96-well plates (Falcon, CA, USA) in Fitton-Jackson’s modified BGJb medium supplemented with 5 % foetal bovine serum, 100 μg/ml ascorbic acid and 100 U/ml penicillin–streptomycin. All cell cultures were maintained in a humidified atmosphere of 5 % CO2 at 37 °C. After 24 h of incubation, the growth medium was changed to remove cell debris and nonviable cells. Cells at passage two were treated with an anti-EMMPRIN antibody for 6 days. The culture media was changed every 24 h. Cell lysates for biochemical analysis were then collected. All protein concentrations were determined using BCA assays (Pierce, Rockford, IL).
Quantitative real-time PCR
Total RNA was isolated from 15 8-day cultured E18.0 tooth germ explants for each group using the SV Total RNA Isolation System (Promega, Madison, WI, USA). cDNA was prepared by a reverse transcription (RT) reaction using the SuperScript III First Strand Synthesis System (Invitrogen) according to the manufacturer’s instructions. Real-time PCR was performed in a 20-μl mixture consisting of 10 μl SYBR Premix Ex Taq II (TAKARA, Shiga, Japan) containing Taq DNA polymerase, oligonucleotide primers (0.2 μM each) and 1 μl of template cDNA. The amplification consisted of a two-step procedure: denaturation at 95 °C for 10 s and 40 cycles with denaturation at 95 °C for 5 s followed by annealing/elongation at 60 °C for 31 s using an ABI PRISM® 7300 Sequence Detection System (Applied Biosystems, Foster City, CA, USA). β-actin was used as an endogenous control. The primer sequences were designed using the Primer Express Software Version 1.0 (Applied Biosystems). The sequences of the forward and reverse primers used for real-time PCR amplification are shown in Table 1.
Western blot analysis
Primary tooth germ cells were washed twice with PBS (pH 7.4). After centrifugation, the cell pellets were lysed in RIPA buffer (50 mM Tris pH 8.0, 150 mM NaCl, 1 % Triton X-100, 1 mM EDTA pH 8.0, and 0.1 % SDS) supplemented with protease inhibitor cocktail (50 μM), lactacystin (20 μM), β-glycerophosphate (25 mM) and sodium orthovanadate (1 mM). The resulting protein samples (20 μg) were separated on 10 % SDS–polyacrylamide gels and transferred to Immun-Blot PVDF membranes (Bio-Rad, Hercules, CA, USA). The membranes were blocked with 5 % non-fat milk in TBST buffer (Bio-Rad, CA), probed with primary antibodies against MMP-9 (H-129 Santa Cruz) or VEGF (A-20 Santa Cruz) at a dilution of 1:1,000, and incubated at 4 °C overnight. After washing, the blots were incubated for 1 h with secondary goat anti-mouse IgG conjugated to horseradish peroxidase (Amersham Pharmacia Biotech, Piscataway, NJ, USA). Finally, the signal was visualised with an ECL kit (Amersham Biosciences).
Histological analysis
Fifteen explants in each experimental group were used for histological analysis. All cultured tissues were fixed in 4 % paraformaldehyde for 24 h. The tissues were then dehydrated in an ethanol series and embedded in paraffin. After embedding, 5-μm-thick sections were prepared for histology, stained with haematoxylin and eosin, and examined by light microscopy.
Statistical analysis
Analysis of variance (ANOVA) was used to determine the significance of differences in multiple comparisons of gene expression. The values are expressed as the mean ± standard deviation (SD). Fisher’s exact test was used to evaluate the differences in tooth germ formation in the organ culture experiments. Differences with probability values less than 0.05 were considered significant.
Results
Developmental distribution of EMMPRIN in the mouse tooth germ
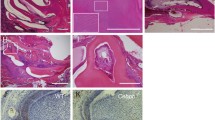
We have previously described the expression of EMMPRIN in the mouse first lower molar from E14 to E18 (21). In this paper, we detected the specific expression of EMMPRIN in the tooth germ at PN1 and PN3. In the secretory stage of PN1, an intense EMMPRIN immunofluorescence signal was detected, primarily concentrated in the ameloblasts and the odontoblasts underlying the dentino-enamel junction. Some staining was also noted in the scattered stellate reticulum cells and the stratum intermedium cells (Fig. 1a). In PN3 tooth germs, the staining pattern of the EMMPRIN signal was restricted to the polarised, elongated ameloblasts and the odontoblasts, especially the cells facing the matrix formation sites (Fig. 1b). No specific fluorescent products were observed in the negative controls incubated only with the secondary antibody (Fig. 1c, d). Higher magnification views of tooth germs showed that EMMPRIN protein was present on the surface and in the cytoplasm of the ameloblasts and the odontoblasts (Fig. 1c, d).
The expression of EMMPRIN in the postnatal tooth germ. Immunofluorescent signals corresponding to EMMPRIN (red fluorescence) were detected in the secretory stage of tooth germs; the nuclei were counterstained by DAPI (blue fluorescence). a At secretory stage PN1, intense EMMPRIN expression was observed in the ameloblasts, the odontoblasts, the stellate reticulum cells and the stratum intermedium cells. b The expression of EMMPRIN was observed in the ameloblasts as well as odontoblasts at PN3. c, d Negative control tests were carried out using PBS instead of the primary antibody. e, f Higher magnification views of PN1 and PN3 tooth germs showing EMMPRIN immunofluorescence. The scale bars represent 100 μm (a, b, c, and d) or 20 μm (e, f). DP dental papilla, SI stratum intermedium, SR stellate reticulum, A ameloblast, O odontoblast
NA against EMMPRIN inhibits tooth germ development and morphogenesis
Based on the immunohistochemistry results for EMMPRIN, we examined the role of EMMPRIN in tooth germ development using a NA against the EMMPRIN protein. After 4 days of culture, the morphogenesis of the tooth cusps was observed in the untreated and GS-treated tooth germs (Fig. 2a, b, g, and h). However, no obvious formation of tooth cusps was identified in the tooth germs treated with NA (Fig. 2m, n). During the first 6 days of culture, most of the untreated and GS-treated tooth germs showed a normal bell-like structure, and the tooth cusps became sharp (Fig. 2c, d, i, and j). On the other hand, although the tooth germs treated with NA had increased in size after 6 days, no further development was observed, and the NA-treated tooth germs were smaller than those of controls (Fig. 2o, p). After culturing for 8 days, the ameloblasts and odontoblasts were more matured in the GS-treated and untreated tooth germs. In addition, a thin layer of extracellular matrix was observed at the interface between the ameloblastic and odontoblastic layers (Fig. 2e, f, k, and i). However, no characteristics of the secretory stage of tooth germ, such as high columnar ameloblasts and odontoblasts and secretion of extracellular matrix, were observed in any of the samples treated with NA for 8 days. The cultured explants were small and severely misshapen, and the differentiation of the inner epithelial cells and the dental papilla cells in these explants was suspended (Fig. 2q, r). Meanwhile, no obvious differences were found between tooth germs treated with GS and untreated tooth germs.
EMMPRIN-NA blocks morphogenesis in E15.0 tooth germ explants. a, b, g, and h After 4 days of culture, the formation of tooth cusps could be observed in tooth germs cultured with normal GS and in untreated tooth germs. m, n The formation of tooth cusps was not observed in tooth germs treated with NA after 4 days of culture. c, d, i, and j Bell-like structures were observed in untreated and GS-treated tooth germs after 6 days of culture. o, p No further morphological development to the bell stage was detected in tooth germs treated with NA for 6 days. e, f, k, and i After 8 days of culture, high columnar ameloblasts and odontoblasts and secretion of extracellular matrix could be observed in untreated and GS-treated tooth germs. q, r After culture for 8 days, the NA-treated tooth germs were smaller and showed an abnormal histological structure. The scale bars represent 200 (a, c, e, g, i, k, m, o, and p) or 30 μm (b, d, f, h, j, l, n p, and r)
NA against EMMPRIN affects dental matrix formation and mineralisation
To examine the effects of EMMPRIN-NA on the deposition of enamel and dentin matrices in the secretory stage of tooth germs, E18.0 tooth germs were dissected form mouse embryos and cultured for 4, 6 and 8 days in medium containing EMMPRIN-NA. At the onset of culture, the tooth germs of E18.0 were at the early bell stage. The addition of EMMPRIN-NA caused a change in the morphogenesis of the E18.0 tooth germs after 2 days of culture; few neovascularised blood vessels were observed on the surface of the tooth germ explants treated with NA compared to the GS-treated tooth germs (Fig. 3a, b). To examine whether exogenous EMMPRIN-NA was taken up by the cells of tooth germ explants in vitro, the penetration of the antibody into the cultured tooth germs was examined by immunohistochemical detection with a secondary antibody. An immunofluorescence signal was observed in the inner epithelial cells and the mesenchymal cells of the dental papilla, indicating that the EMMPRIN antibody had reached the central portions of the explants (Fig. 3c), whereas tooth germs cultured in control medium did not show immunostaining (Fig. 3d).
EMMPRIN-NA inhibits EMMPRIN protein in E18.0 tooth germ explants. a NA inhibited the morphogenesis of the tooth germ explants after 2 days of culture. b In the medium supplemented with normal GS, the morphogenesis of the tooth germ explant was advanced, and a cuspal pattern was evident after 2 days in culture. c EMMPRIN-NA was identified in cryostat sections of E18.0 tooth germ explants cultured for 2 days with the secondary antibody, indicating that EMMPRIN-NA was distributed throughout the cells of the explants. d Cryostat section of an explant from E18.0 tooth germ cultured for 2 days in control medium. The scale bars represent 250 μm. E enamel epithelium, M dental papilla mesenchyme
After 4 days of culture, the untreated and GS-treated tooth germs showed development into the secretory stage. The secretion of predentin was observed between the ameloblastic and odontoblastic layers (Fig. 4a, b, g, and h). The tooth germs cultured with NA also showed the formation of extracellular matrix, although a slight delay in development was observed in comparison to the tooth germs without NA treatment (Fig. 4m, n). After 6 days of culture, the tooth germs treated with or without NA showed a thin layer of dentin at the interface between the ameloblastic and odontoblastic layers. In untreated and GS-treated tooth germs, the inner enamel epithelial cells had differentiated into high columnar ameloblasts with polarised nuclei, and the mesenchymal cells adjacent to the cells of inner enamel epithelium had differentiated into odontoblasts with a columnar appearance (Fig. 4c, d, i, and j). On the other hand, no further maturation of ameloblasts was observed in the tooth germs treated with NA. The ameloblasts maintained their columnar appearance, and the arrangement of the cells was slightly disorganised, with no elongation or polarisation. However, no obvious morphological changes were detected in the columnar odontoblasts with or without NA treatment (Fig. 4o, p). After culturing for 8 days, well-differentiated ameloblasts and secretory odontoblasts were more prominent in the untreated and GS-treated tooth germs, and visible enamel and dentin layers were formed by differentiated polarised, high columnar-appearing ameloblasts and columnar-appearing odontoblasts, respectively (Fig. 4e, f, k, and l). However, the formation and mineralisation of the enamel matrix were not observed in the tooth germs treated with NA, and the mesiodistal diameters of the tooth germs treated with NA were shorter than those of the control (p < 0.01; Table 2). Furthermore, the NA-treated ameloblasts were smaller and more irregular, with disordered polarisation (Fig. 4q, r).
The effect of an EMMPRIN-NA on the maturation of ameloblasts and the formation of enamel in E18.0 tooth germ explants. a, b, g, and h Tooth germ explants treated with normal GS supplement or untreated tooth germs revealed a secretory-stage shape after 4 days of culture. m, n A slight delay in development was observed in the tooth germ explants treated with NA for 4 days. c, d, i, and j Well-differentiated ameloblasts and odontoblasts were observed in tooth germ explants treated with GS and in untreated tooth germ explants after 6 days of culture. The dentin matrix layers were observed in the interstitial space between the ameloblastic layers and the odontoblastic layers. o, p After 6 days of culture with NA, dentin matrix and functional odontoblasts were observed in the tooth germ, but no elongation or polarisation was observed in the ameloblasts. e, f, k, l Well-differentiated ameloblasts and odontoblasts, as well as the visible dentin and enamel matrix layers, were distinct in the tooth germs cultured with GS or normal medium for 8 days. q, r No formation or mineralisation of the enamel matrix was observed in the tooth germ explants cultured with NA for 8 days. Meanwhile, the ameloblasts were smaller and showed irregular shapes with disordered polarisation. The scale bars represent 200 (a, c, e, g, i, k, m, o, and q) or 30 μm (b, d, f, h, j, l, n, p, and r)
The inhibition of EMMPRIN activation down-regulates gene expression in ameloblasts
Real-time PCR was performed to detect the differential expression of odontogenesis-related genes between the EMMPRIN-NA treated group and the other groups. Mouse β-actin was used as an internal control to standardise the mRNA expression of the selected genes. After 6 days of culture, the mRNA expression of MMP-9, ALPL, ameloblastin, amelogenin, enamelin and VEGF in NA-treated E18.0 tooth germs was decreased to 23, 19, 43, 35, 56, and 10 % of the control, respectively (Fig. 5). Meanwhile, a 34 % reduction of EMMPRIN mRNA expression was also detected in the NA-treated E18.0 tooth germs (data not shown).
The transcription of VEGF, MMP-9 and enamel matrix proteins was down-regulated by treatment with the EMMPRIN-NA in E18.0 tooth germ explants. Real-time PCR revealed that the mRNA expression levels of MMP-9, ALPL, ameloblastin, amelogenin, enamelin and VEGF were decreased to 23, 19, 43, 35, 56, and 10 % of the controls, respectively, in NA-treated E18.0 tooth germ explants after 6 days of culture. β-actin was used as a control; *p < 0.05, **p < 0.01
Down-regulation of MMP-9 and VEGF protein expression following the abrogation of EMMPRIN activity
Western blot analysis was used to evaluate the differential expression of MMP-9 and VEGF in E18.0 tooth germs after 6 days of primary culture with or without NA. β-actin was used as an internal control. An 86 % reduction in VEGF levels and a 72 % reduction in MMP-9 levels were detected in the NA-treated E18.0 tooth germ cells after 6 days of culture (Fig. 6a, b).
The effects of EMMPRIN-NA on the expression of VEGF and MMP-9 protein in primary cultured cells of tooth germs in vitro. a The inhibition of VEGF and MMP-9 protein expression in 6-day-cultured E18.0 tooth germ cells was demonstrated by western blot. b Western blot analysis showed that EMMPRIN-NA treatment reduced VEGF and MMP-9 protein expression by 86 and 72 %, respectively, compared to control conditions [untreated (UT) and GS]. β-actin was used as a control; *p < 0.05, **p < 0.01
Discussion
Although EMMPRIN expression is enriched in a wide variety of cancers (Caudroy et al. 1999), the presence of EMMPRIN in non-tumoural tissues, including the developing retina, blood–brain barrier, central nervous system, thymus, epithelial tissues and a variety of immune cells, suggests that EMMPRIN plays an important role in a variety of physiological processes (Fadool and Linser 1993; Fan et al. 1998). In this study, we found that the expression of EMMPRIN protein is restricted to terminally differentiated ameloblasts and odontoblasts. Higher magnification views of tooth germs showed a strong immunofluorescence signal on the surface and in the cytoplasm of the produced ameloblasts and the odontoblastic processes, which are involved in compositional changes of the enamel and dentin matrices. Based on these results, we suggest that EMMPRIN may take part in cytodifferentiation events and/or the subsequent deposition of the dental matrix in the secretory stage of the tooth germ. To gain insight into EMMPRIN function, we used an antibody inhibition assay to investigate the functions of EMMPRIN in the processing and maturation of the dental matrix. The EMMPRIN-NA is stable for at least 96 h in culture medium and did not show any obvious toxic effects on the cultured tooth germ cells, even at a final concentration of 100 μg/ml. Secondly, our previous results demonstrated that anti-EMMPRIN antibody treatment was more effective and faster than siRNA treatment for blocking the activity of EMMPRIN protein located on the surface of tooth germ cells. Therefore, EMMPRIN-NA, with its specific function-blocking activity, is advantageous as a study tool in tooth germ culture.
EMMPRIN is composed of an extracellular domain of 187 residues, a 24-residue transmembrane domain and a 40-amino acid cytoplasmic region (Biswas et al. 1995). As an early inducer of MMPs, EMMPRIN binds to itself in a homotypic cis pattern as well as to other ligands such as cyclophilin A and B (Yurchenko et al. 2005), MCT1, MCT4 (Kirk et al. 2000), α3β1 integrin and α6β1 integrin (Berditchevski et al. 1997). Both the transmembrane and cytoplasmic domains of EMMPRIN are thought to be critical for protein–protein interactions within the plasma membrane (Kirk et al. 2000). Two extracellular immunoglobulin-like domains of EMMPRIN are variably glycosylated, resulting in significant alterations in EMMPRIN function and protein interactions. The N-linked glycosylation of EMMPRIN protein is known to be crucial for its role as an MMP inducer (Sun and Hemler 2001), whilst MCT1 and MCT4 were found to require the C-terminus of EMMPRIN as a chaperone for their expression at the plasma membrane (Kirk et al. 2000). Four isoforms of MCT (MCT1-MCT4) have been functionally characterised, and they have been shown to catalyse the proton-linked transport of short-chain substituted monocarboxylates such as lactate, pyruvate and acetoacetate (Halestrap 2012). MCT1-MCT4 are reported to be associated with a glycosylated ancillary protein, either EMMPRIN or embigin, for their correct translocation to the plasma membrane (Wilson et al. 2005). A recent report demonstrated that the scFv-M6-1B9 intrabody, which can reduce EMMPRIN cell surface expression, efficiently decreased the expression levels of α3β1-integrin on the cell surface and increased the intracellular accumulation of both MCT1 and lactate in a colorectal cancer cell line (Sangboonruang et al. 2014). On the other hand, the silencing of CD147/basigin in A375 cells using an siRNA clearly abrogated the expression of MCT1 and MCT4 and significantly decreased the glycolysis rate, cell proliferation and VEGF production (Su et al. 2009). We know that tooth organogenesis is regulated by epithelial-mesenchymal interactions, and this process is also characterised by the sequential activation of specific regulatory genes (Chen et al. 2014; Huang et al. 2014). Many factors govern the proliferation and differentiation of tooth germ cells in the different stages of tooth development (Pispa and Thesleff 2003; Kero et al. 2014). In our present study, the EMMPRIN-NA, which was raised against a peptide mapping to the C-terminus of mouse EMMPRIN, significantly hindered the development of tooth germ explants. We therefore hypothesise that the inhibition of tooth germ cell differentiation and the reduction in the size of tooth germs cultured with EMMPRIN-NA could be explained by the microenvironmental metabolic alterations caused by the inhibition of the interaction between the C-terminus of EMMPRIN and other related factors in cultured tooth germ explants where EMMPRIN is negligible. However, it is also possible that the inhibition of EMMPRIN activity results in changes in the expression of important growth factors in the tooth germ, which would lead to a reduction in tooth germ development.
After EMMPRIN expression was blocked using the NA from E18.0, the inner epithelial cells failed to polarise and differentiate into secretory ameloblasts. As a result, the formation of the enamel matrix did not occur. Meanwhile, the expression levels of MMP-9, ALPL, ameloblastin, amelogenin, enamelin, VEGF as well as EMMPRIN were also found to be markedly decreased by EMMPRIN-NA treatment. Experiments on transgenic null mice have demonstrated that mutations in the secreted proteins amelogenin, enamelin, and enamelysin result in visibly, structurally, or mechanically defective enamel. The morphology of cultured tooth germs in secretory stages showed that EMMPRIN-NA dramatically inhibited the differentiation of ameloblasts and the mineralisation of enamel. However, there have been no reports describing the function of EMMPRIN in the secretory stage of tooth germ, and this is the first report of an association between EMMPRIN and amelogenesis.
Our results also showed a link between the function of EMMPRIN in tooth germ development and VEGF regulation. The expression of VEGF mRNA and protein was found to be markedly decreased by EMMPRIN inhibition in E18.0 tooth germ culture and primary cell culture, respectively. VEGF is a key regulator of blood vessel growth; it is expressed in angiogenesis, organ development and differentiation during embryogenesis (Carmeliet et al. 1996; Ferrara et al. 1996). EMMPRIN has been shown to participate in angiogenesis through the hypoxia-inducible factor 2α-mediated regulation of soluble VEGF (Bougatef et al. 2009). Recently, it has also been reported that both endogenously expressed EMMPRIN and exogenously added recombinant EMMPRIN are capable of stimulating VEGF production in tumour and fibroblast cells, respectively, via the PI3 K-Akt pathway (Tang et al. 2006). In addition, MMPs have also been implicated in the regulation of VEGF bioavailability from extracellular stores (Lee et al. 2005). The increased expression of VEGF, MMP-2 and MMP-9 together stimulates continuous remodelling of the extracellular matrix and angiogenesis and thereby promotes new bone formation (Rocha et al. 2014). The expression of VEGF has also been observed in ameloblasts and in odontoblasts underlying the dentino-enamel junction (DEJ) of the human first molar tooth germ; this pattern is similar to that of EMMPRIN (Miwa et al. 2008). Combined with previously published data, it is conceivable that the expression of EMMPRIN may be related to the direct or indirect regulation of the expression of VEGF and thus plays an important role in tooth germ biology beyond the regulation of MMPs. However, the inhibition of EMMPRIN expression by NA did not show any noticeable effects on the differentiation of or hard tissue formation by odontoblasts. Meanwhile, the results of real-time PCR analysis of the expression of DSPP, DMP-1, TIMP-1 and TIMP-2 did not show significant differences after treatment with EMMPRIN-NA in cultured tooth germs at E18.0 (data not shown). These results implied that odontoblasts were less sensitive to EMMPRIN disruption than ameloblasts. Thus, EMMPRIN seems to play no effective role in the differentiation of odontoblasts. Several possible reasons for this result are as follows: (1) odontoblasts may have some ability to resist EMMPRIN disruption; (2) dentin formation could be resumed though the bypass pathway; or (3) the concentration of EMMPRIN-NA may not have been high enough to infiltrate into the dentin matrix and to neutralise its expression in odontoblasts.
In summary, these findings demonstrate that EMMPRIN functions during the secretory stage of tooth germ development to facilitate the differentiation and maturation of ameloblasts. This function of EMMPRIN allows the enamel matrix to grow in width and thickness until it becomes mineralised, likely via the regulated expression of VEGF and MMPs in ameloblasts in a direct or indirect manner. However, the function of EMMPRIN in ameloblasts, currently, remains unknown, and further studies are required to determine the detailed mechanism of how EMMPRIN regulates the functions of enamel proteins to exert its control on mineralisation.
References
Agrawal SM, Yong VW (2011) The many faces of EMMPRIN—roles in neuroinflammation. Biochim Biophys Acta 1812(2):213–219. doi:10.1016/j.bbadis.2010.07.018
Berditchevski F, Chang S, Bodorova J, Hemler ME (1997) Generation of monoclonal antibodies to integrin associated proteins. Evidence that α3β1 complexes with EMMPRIN/basigin/OX47/M6. J Biol Chem 272(46):29174–29180
Biswas C, Nugent MA (1987) Membrane association of collagenase stimulatory factor(s) from B-16 melanoma cells. J Cell Biochem 35(3):247–258. doi:10.1002/jcb.240350307
Biswas C, Zhang Y, DeCastro R, Guo H, Nakamura T, Kataoka H, Nabeshima K (1995) The human tumor cell-derived collagenasestimulatory factor (renamed EMMPRIN) is a member of theimmunoglobulin superfamily. Cancer Res 55(2):434–439
Bougatef F, Quemener C, Kellouche S, Naïmi B, Podgorniak MP, Millot G, Gabison EE, Calvo F, Dosquet C, Lebbé C, Menashi S, Mourah S (2009) EMMPRIN promotes angiogenesis through hypoxia-inducible factor-2alpha-mediated regulation of soluble VEGF isoforms and their receptor VEGFR-2. Blood 114(27):5547–5556. doi:10.1182/blood-2009-04-217380
Cao Z, Xiang J, Li C (2009) Expression of extracellular matrix metalloproteinase inducer and enhancement of the production of matrix metalloproteinase-1 in tongue squamous cell carcinoma. Int J Oral Maxillofac Surg 38(8):880–885. doi:10.1016/j.ijom.2009.03.004
Carmeliet P, Ferreira V, Breier G, Pollefeyt S, Kieckens L, Gertsenstein M, Fahrig M, Vandenhoeck A, Harpal K, Eberhardt C, Declercq C, Pawling J, Moons L, Collen D, Risau W, Nagy A (1996) Abnormal blood vessel development and lethality in embryos lacking a single VEGF allele. Nature 380(6573):435–439. doi:10.1038/380435a0
Caudroy S, Polette M, Tournier JM, Burlet H, Toole B, Zucker S, Birembaut P (1999) Expression of the extracellular matrix metalloproteinase inducer (EMMPRIN) and the matrix metalloproteinase-2 in bronchopulmonary and breast lesions. J Histochem Cytochem 47(12):1575–1580. doi:10.1177/002215549904701209
Chen X, Chen G, Feng L, Jiang Z, Guo W, Yu M, Tian W (2014) Expression of Nfic during root formation in first mandibular molar of rat. J Mol Histol (Epub ahead of print). doi:10.1007/s10735-014-9588-x
Ellis SM, Nabeshima K, Biswas C (1989) Monoclonal antibody preparation and purification of a tumor cell collagenase-stimulatory factor. Cancer Res 49(12):3385–3391
Emingil G, Tervahartiala T, Mãntylã P, Määttä M, Sorsa T, Atilla G (2006) Gingival crevicular fluid matrix metalloproteinase (MMP)-7, extracellular MMP inducer, and tissue inhibitor of MMP-1 levels in periodontal disease. J Periodontol 77(12):2040–2050. doi:10.1902/jop.2006.060144
Fadool JM, Linser PJ (1993) Differential glycosylation of the 5A11/HT7 antigen by neural retina and epithelial tissues in the chicken. J Neurochem 60(4):1354–1364. doi:10.1111/j.1471-4159.1993.tb03296.x
Fan QW, Kadomatsu K, Uchimura K, Muramatsu T (1998) Embigin/basigin subgroup of the immunoglobulin superfamily: different modes of expression during mouse embryogenesis and correlated expression with carbohydrate antigenic markers. Dev Growth Differ 40(3):277–286. doi:10.1046/j.1440-169X.1998.t01-1-00003.x
Ferrara N, Carver-Moore K, Chen H, Dowd M, Lu L, O’Shea KS, Powell-Braxton L, Hillan KJ, Moore MW (1996) Heterozygous embryonic lethality induced by targeted inactivation of the VEGF gene. Nature 380(6573):439–442. doi:10.1038/380439a0
Halestrap AP (2012) The monocarboxylate transporter family—structure and functional characterization. IUBMB Life 64(1):1–9. doi:10.1002/iub.573
Huang Z, Wang C, Wei L, Wang J, Fan Y, Wang L, Wang Y, Chen T (2008) Resveratrol inhibits EMMPRIN expression via P38 and ERK1/2 pathways in PMA-induced THP-1 cells. Biochem Biophys Res Commun 374(3):517–521. doi:10.1016/j.bbrc.2008.07.058
Huang Z, Hu X, Lin C, Chen S, Huang F, Zhang Y (2014) Genome-wide analysis of gene expression in human embryonic tooth germ. J Mol Histol (Epub ahead of print). doi:10.1007/s10735-014-9580-5
Igakura T, Kadomatsu K, Kaname T, Muramatsu H, Fan QW, Miyauchi T, Toyama Y, Kuno N, Yuasa S, Takahashi M, Senda T, Taguchi O, Yamamura K, Arimura K, Muramatsu T (1998) A null mutation in basigin, an immunoglobulin superfamily member, indicates its important roles in peri-implantation development and spermatogenesis. Dev Biol 194(2):152–165. doi:10.1006/dbio.1997.8819
Kanyenda LJ, Verdile G, Boulos S, Krishnaswamy S, Taddei K, Meloni BP, Mastaglia FL, Martins RN (2011) The dynamics of CD147 in Alzheimer’s disease development and pathology. J Alzheimers Dis 26(4):593–605. doi:10.3233/JAD-2011-110584
Kero D, Kalibovic Govorko D, Vukojevic K, Cubela M, Soljic V, Saraga-Babic M (2014) Expression of cytokeratin 8, vimentin, syndecan-1 and ki-67 during human tooth development. J Mol Histol 45(6):627–640. doi:10.1007/s10735-014-9592-1
Kirk P, Wilson MC, Heddle C, Brown MH, Barclay AN, Halestrap AP (2000) CD147 is tightly associated with lactate transporters MCT1 and MCT4 and facilitates their cell surface expression. EMBO J 19(15):3896–3904. doi:10.1093/emboj/19.15.3896
Lee S, Jilani SM, Nikolova GV, Carpizo D, Iruela-Arispe ML (2005) Processing of VEGF-A by matrix metalloproteinases regulates bioavailability and vascular patterning in tumors. J Cell Biol 169(4):681–691. doi:10.1083/jcb.200409115
Lei H, Liu H, Ding Y, Ge L (2014) Immunohistochemical localization of Pax6 in the developing tooth germ of mice. J Mol Histol 45(4):373–379. doi:10.1007/s10735-014-9564-5
Li R, Huang L, Guo H, Toole BP (2001) Basigin (murine EMMPRIN) stimulates matrix metalloproteinase production by fibroblasts. J Cell Physiol 186(3):371–379. doi:10.1002/1097-4652(2000)9999:999<000:AID-JCP1042>3.0.CO;2-8
Line SR (2003) Variation of tooth number in mammalian dentition: connecting genetics, development, and evolution. Evol Dev 5(3):295–304. doi:10.1046/j.1525-142X.2003.03036.x
Liu L, Li C, Cai X, Xiang J, Cao Z, Dong W (2010) The temporal expression and localization of extracellular matrix metalloproteinase inducer (EMMPRIN) during the development of periodontitis in an animal model. J Periodontal Res 45(4):541–549. doi:10.1111/j.1600-0765.2010.01269.x
Määttä M, Tervahartiala T, Kaarniranta K, Tang Y, Yan L, Tuukkanen J, Sorsa T (2006) Immunolocalization of EMMPRIN (CD147) in the human eye and detection of soluble form of EMMPRIN in ocular fluids. Curr Eye Res 31(11):917–924. doi:10.1080/02713680600932290
Mishra B, Kizaki K, Sato T, Ito A, Hashizume K (2012) The role of extracellular matrix metalloproteinase inducer (EMMPRIN) in the regulation of bovine endometrial cell functions. Biol Reprod 87(6):149. doi:10.1095/biolreprod.112.102152
Mitsiadis TA, Smith MM (2006) How do genes make teeth to order through development? J Exp Zool B Mol Dev Evol 306(3):177–182. doi:10.1002/jez.b.21104
Mitsiadis TA, Muramatsu T, Muramatsu H, Thesleff I (1995) Midkine (MK), a heparin-binding growth/differentiation factor, is regulated by retinoic acid and epithelial-mesenchymal interactions in the developing mouse tooth, and affects cell proliferation and morphogenesis. J Cell Biol 129(1):267–281. doi:10.1083/jcb.129.1.267
Miwa Y, Fujita T, Sunohara M, Sato I (2008) Immunocytochemical localization of vascular endothelial growth factor and vascular endothelial growth factor receptor-2 of the human deciduous molar tooth germ development in the human fetus. Ann Anat 190(3):246–251. doi:10.1016/j.aanat.2007.11.006
Nabeshima K, Suzumiya J, Nagano M, Ohshima K, Toole BP, Tamura K, Iwasaki H, Kikuchi M (2004) Emmprin, a cell surface inducer of matrix metalloproteinases (MMPs), is expressed in T-cell lymphomas. J Pathol 202(3):341–351. doi:10.1002/path.1518
Pispa J, Thesleff I (2003) Mechanisms of ectodermal organogenesis. Dev Biol 262(2):195–205. doi:10.1016/S0012-1606(03)00325-7
Rocha CA, Cestari TM, Vidotti HA, de Assis GF, Garlet GP, Taga R (2014) Sintered anorganic bone graft increases autocrine expression of VEGF, MMP-2 and MMP-9 during repair of critical-size bone defects. J Mol Histol 45(4):447–461. doi:10.1007/s10735-014-9565-4
Sameshima T, Nabeshima K, Toole BP, Yokogami K, Okada Y, Goya T, Koono M, Wakisaka S (2000) Glioma cell extracellular matrix metalloproteinase inducer (EMMPRIN) (CD147) stimulates production of membrane-type matrix metalloproteinases and activated gelatinase A in co-cultures with brain-derived fibroblasts. Cancer Lett 157(2):177–184. doi:10.1016/S0304-3835(00)00485-7
Sangboonruang S, Thammasit P, Intasai N, Kasinrerk W, Tayapiwatana C, Tragoolpua K (2014) EMMPRIN reduction via scFv-M6-1B9 intrabody affects α3β1-integrin and MCT1 functions and results in suppression of progressive phenotype in the colorectal cancer cell line Caco-2. Cancer Gene Ther 21(6):246–255. doi:10.1038/cgt.2014.24
Schwab W, Harada H, Goetz W, Nowicki M, Witt M, Kasper M, Barth K (2007) Immunocytochemical and biochemical detection of EMMPRIN in the rat tooth germ: differentiation-dependent co-expression with MMPs and co-localization with caveolin-1 in membrane rafts of dental epithelial cells. Histochem Cell Biol 128(3):195–203. doi:10.1007/s00418-007-0313-7
Su J, Chen X, Kanekura T (2009) A CD147-targeting siRNA inhibits the proliferation, invasiveness, and VEGF production of human malignant melanoma cells by down-regulating glycolysis. Cancer Lett 273(1):140–147. doi:10.1016/j.canlet.2008.07.034
Sun J, Hemler ME (2001) Regulation of MMP-1 and MMP-2 production through CD147/extracellular matrix metalloproteinase inducer interactions. Cancer Res 61(5):2276–2281
Tang W, Chang SB, Hemler ME (2004) Links between CD147 function, glycosylation, and caveolin-1. Mol Biol Cell 15(9):4043–4050. doi:10.1091/mbc.E04-05-0402
Tang Y, Nakada MT, Rafferty P, Laraio J, McCabe FL, Millar H, Cunningham M, Snyder LA, Bugelski P, Yan L (2006) Regulation of vascular endothelial growth factor expression by EMMPRIN via the PI3 K-Akt signaling pathway. Mol Cancer Res 4(6):371–377. doi:10.1158/1541-7786.MCR-06-0042
Tang R, Wang Q, Du J, Yang P, Wang X (2013) Expression and localization of Nell-1 during murine molar development. J Mol Histol 44(2):175–181. doi:10.1007/s10735-012-9472-5
Taylor PM, Woodfield RJ, Hodgkin MN, Pettitt TR, Martin A, Kerr DJ, Wakelam MJ (2002) Breast cancer cell-derived EMMPRIN stimulates fibroblast MMP2 release through a phospholipase A(2) and 5-lipoxygenase catalyzed pathway. Oncogene 21(37):5765–5772. doi:10.1038/sj.onc.1205702
Wang B, Li H, Liu Y, Lin X, Lin Y, Wang Y, Hu X, Zhang Y (2014) Expression patterns of WNT/β-CATENIN signaling molecules during human tooth development. J Mol Histol 45(5):487–496. doi:10.1007/s10735-014-9572-5
Wilson MC, Meredith D, Fox JE, Manoharan C, Davies AJ, Halestrap AP (2005) Basigin (CD147) is the target for organomercurial inhibition of monocarboxylate transporter isoforms 1 and 4: the ancillary protein for the insensitive MCT2 is EMBIGIN (gp70). J Biol Chem 280(29):27213–27221. doi:10.1074/jbc.M411950200
Xie M, Jiao T, Chen Y, Xu C, Li J, Jiang X, Zhang F (2010) EMMPRIN (basigin/CD147) is involved in the morphogenesis of tooth germ in mouse molars. Histochem Cell Biol 133(5):585–594. doi:10.1007/s00418-010-0697-7
Yurchenko V, Pushkarsky T, Li JH, Dai WW, Sherry B, Bukrinsky M (2005) Regulation of CD147 cell surface expression: involvement of the proline residue in the CD147 transmembrane domain. J Biol Chem 280(17):17013–17019. doi:10.1074/jbc.M412851200
Acknowledgments
This work was supported by the National Natural Science Foundation of China (Grant No. 30900848), the Science and Technology Commission of Shanghai (Grant No. 09DZ2271100), and the Shanghai Leading Academic Discipline Project (Grant No. T0202).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Xie, M., Xing, G., Hou, L. et al. Functional role of EMMPRIN in the formation and mineralisation of dental matrix in mouse molars. J Mol Hist 46, 21–32 (2015). https://doi.org/10.1007/s10735-014-9603-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10735-014-9603-2