Abstract
Objective
To describe the clinical, biochemical, and neuropathological findings of an autosomal dominant globular glial tauopathy caused by the P301T mutation at the MAPT gene.
Methods
Five patients from two unrelated pedigrees underwent clinical evaluation. Genetic analysis, brain pathological examination, and biochemical analysis of tau were performed.
Results
The patients studied were 3 men and 2 women with a mean age at onset of 52.2 years and mean disease duration of 5.2 years. Three patients presented a corticobasal syndrome, one patient an asymmetric pyramidal syndrome compatible with primary lateral sclerosis, and one patient a frontotemporal dementia. In both pedigrees (4 patients) Sanger sequencing showed the p.P301T mutation in exon 10 of the MAPT gene. Neuropathological findings consisted of atrophy of frontal and temporal lobes with marked spongiosis and astrogliosis, and abundant phosphorylated tau protein deposits in the frontal and temporal cortex, limbic area, basal ganglia, and brain stem. The most striking finding was the presence of oligodendroglial 4R phospho-tau globular positive inclusions in the white matter and cortex. Globose-type neurofibrillary neuronal tangles, and in particular astrocytic globular inclusions and coarse tufts, were present in the grey matter. Biochemical analysis of sarkosyl-insoluble fractions revealed two tau bands of 64 and 68 kDa and case-dependent bands of lower molecular weight.
Conclusion
This is the first pathological and biochemical study of the MAPT p.P301T mutation showing variable clinical manifestation and neuropathological phenotype of globular glial tauopathy not only among different families but also within families.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Globular glial tauopathy (GGT) is a recently described primary tauopathy manifested clinically by frontotemporal dementia, pyramidal signs, progressive supranuclear palsy (PSP) syndrome, corticobasal syndrome, or motor neuron disease [1,2,3,4,5,6,7,8,9,10,11]. The neuropathological hallmark of GGT is the abnormal accumulation of hyper-phosphorylated microtubule-binding protein tau in neurons, widespread tau-containing globular glial inclusions (GGIs) and white matter degeneration. GGIs in astrocytes (GAIs) differ from tufted astrocytes of PSP and astrocytic plaques of corticobasal degeneration [1,2,3,4,5,6,7,8,9,10,11]. However, tufted astrocytes and, rarely, astrocytic plaques can be found in GGT. Globular inclusions in oligodendrocytes (GOIs) differ from coiled bodies, although the two types of oligodendroglial inclusions may co-exist in GGT [12].
An international consensus committee proposed three subtypes of GGT based on the regional distribution of GGIs and resultant neuronal loss and atrophy [11]. Type I cases have predominant frontal symptoms and pathology, abundant GOIs, and scarce GAIs. Type III display involvement of frontotemporal and motor cortex, corticospinal tract, and anterior horn of the spinal cord, with abundant GOIs and GAIs; and type II has intermediate features [11]. Parkinsonism linked to involvement of the substantia nigra is frequently found in types II and III.
The majority of GGTs are sporadic [11], but a few familial cases have been reported bearing different mutations in MAPT. [13,14,15,16].
Here, we describe the clinical, biochemical, and neuropathological findings of familial GGT corresponding to two separate pedigrees, both bearing the P301T mutation in MAPT.
Materials and methods
Clinical records and results of neuroimaging of the five patients were assessed by experienced neurologists, following the ethics guidelines of our institution. Written informed consent was obtained from all participants or relatives to perform genetic and pathological brain studies.
The analysis of human brain samples was carried out according to the current national legislation (Royal Decree ref. RD1716/2011).
Neuropathological study
Immediately after removal of the brain from the skull, fresh samples from the frontal cortex and frontal white matter were frozen and stored at − 80 °C for biochemical studies. The rest of the brain was fixed in 4% buffered formalin, and selected samples of the brain and spinal cord were embedded in paraffin. Tissue sections, 4-μm-thick, were obtained with a sliding microtome. The sections were stained with haematoxylin and eosin, Klüver–Barrera and Gallyas, and processed for immunohistochemistry.
Inmunohistochemistry
Formalin-fixed 4-μm-thick sections were mounted on slides and de-waxed. After antigen retrieval, sections were incubated overnight with one of the primary antibodies: glial fibrillary acidic protein (/500, Dako, Glostrup, UK), Aβ-amyloid (1/50, Dako, Glostrup), AT8 (1/50, Immunogenetics, Ghent, BE), 3Rtau (1/50, Merck-Millipore, Billerica, MA, USA), 4Rtau (1/50, Merck-Millipore), p62 (1/500 MBL, Japan), α-synuclein (1/500, Chemicon, Billerica, MA, USA) and TDP-43 (1/200Abcam, Cambridge, UK). The reaction product was visualized using an automated slide immunostainer (Leica Bond Max) with Bond Polymer Refine Detection (Leica Biosystems Newcastle Ltd) and slight counterstaining with hematoxylin.
Western blotting of sarkosyl-insoluble fractions
Frozen samples from frontal cortex of about 1 g were lysed in 10 volumes (w/v) with cold suspension buffer (10 mM Tris–HCl, pH 7.4, 0.8 M NaCl, 1 mM EGTA) supplemented with 10% sucrose, protease, and phosphatase inhibitors (Roche, GE). The homogenates were first centrifuged at 20,000×g for 20 min (Ultracentrifuge Beckman with 70Ti rotor) and the supernatant (S1) was saved. The pellet was re-homogenized in 5 volumes of homogenization buffer and re-centrifuged at 20,000×g for 20 min (Ultracentrifuge Beckman with 70Ti rotor). The two supernatants (S1 + S2) were then mixed and incubated with 0.1% N-lauroylsarkosynate (sarkosyl) for 1 h at room temperature while being shaken. Samples were then centrifuged at 100,000×g for 1 h (Ultracentrifuge Beckman with 70Ti rotor). Sarkosyl-insoluble pellets (P3) were re-suspended (0.2 ml/g) in 50 mM Tris–HCl (pH 7.4). Protein concentrations were quantified with the bicinchoninic acid assay (BCA) assay (Pierce, Waltham, MA, USA). Samples were mixed with loading sample buffer and heated at 95 °C for 5 min. 60 µg of protein was separated by electrophoresis in SDS-PAGE gels and transferred to nitrocellulose membranes (200 mA per membrane, 90 min). The membranes were blocked for 1 h at room temperature with 5% non-fat milk in TBS containing 0.2% Tween and were then incubated with one of the primary antibodies: anti-tau Ser422 (diluted 1:1000; Thermo Fisher, Waltham, MA, USA) and tau 46 (diluted 1:1000; Thermo Fisher). After washing with TBS-T, blots were incubated with the appropriate secondary antibody (anti-mouse/anti-rabbit IgG conjugated with horseradish peroxidase, diluted at 1:2000, DAKO, DE) for 45 min at room temperature. Immune complexes were revealed by incubating the membranes with chemiluminescence reagent (Amersham, GE Healthcare, Buckinghamshire, UK).
Genetic analysis
In pedigree 1, genomic DNA was isolated from peripheral blood of patient 1.III.3 following a standard phenol/chloroform protocol. In pedigree 2, genomic DNA was obtained from peripheral blood samples of patients 2.III.7 and 2.III.8 and from fresh-frozen post-mortem brain tissue of patient 2.III.6 according to a standard phenol/chloroform protocol. Two primers, Forward 5′GGCGTGTCACTCATCCTTTT3′and Reverse 5′TGCCCTATTCTGTCCACACA3, were used for amplifying and sequencing rs63751438 variant with the Sanger method. rs63751438 variant results from a C to A substitution at position Chr17:44087754 in exon 10 of the MAPT gene and is predicted to change a proline (CCG) to threonine (ACG) leading to a p.P301T mutation [17].
Results
Family history and clinical features
Both pedigrees (Fig. 1) were from Navarra, a region of 638,213 inhabitants in Northern Spain. A summary of the main clinical data is shown in Table 1. Mean age at onset was 52.2 years (range 43–68 years). Mean duration of the disease was 5.2 years (range 4–7 years).
Pedigree 1
Two members were clinically evaluated.
Patient 1.II.2 was a woman diagnosed with corticobasal syndrome at age 68 who died 4 years after disease onset. Her father died at the age of 53 after a rapidly progressive cognitive decline. She suffered a slowly progressive clumsiness in the right arm that began 1 year before initial consultation, with depression, mood changes, and language difficulties. Levodopa treatment was initiated without any clinical benefit. She progressively developed gait impairment. Neurological examination revealed non-fluent aphasia, vertical ophthalmoparesis, hyperreflexia, postural tremor in the right arm, rigidity, ideomotor limb apraxia and reflex myoclonus in her right hand. Cranial magnetic resonance imaging (MRI) showed left parietotemporal atrophy. She did not undergo pathological or genetic exams.
Patient 1.III.3, he is the son of patient 1.II.2, who at the age of 45, began with a 1-year history of progressive gait disturbance. Neurological examination disclosed generalized spasticity and hyperreflexia with bilateral ankle clonus in absence of muscle atrophy and fasciculations. Brain MRI disclosed frontotemporal atrophy, asymmetric cortical atrophy more prominent in the left hippocampus, mesencephalic atrophy, and white matter lesions (Fig. 2). Spinal MRI was normal. Cranial magnetic stimulation (CMS) revealed pyramidal tract impairment affecting both legs and the left arm. Standard blood and cerebrospinal fluid (CSF) analysis were within normal limits. A diagnosis of primary lateral sclerosis syndrome was established. During the following years, he developed speech difficulties, progressive slowness, cognitive decline, and abnormal behavior. Neurological examination revealed mask-like facial expression, non-fluent speech with echolalia, paresis of the vertical gaze, and increased latency to initiate lateral gaze, in addition to axial rigidity with a spontaneous cervical extension. He showed an asymmetric tetraparesis with severe spasticity. SPECT-HMPAO showed left hemisphere hypoperfusion with slight frontal predominance. The patient died at the age of 49. The brain underwent a pathological exam.
Brain resonance imaging and 18[F]-fluorodeoxyglucose (FDG)-PET. a Axial T2-weighted image of patient 1.III.3 showing diffuse cortical atrophy, especially in the frontotemporal cortex with white matter hyperintense lesions. b Coronal T1-weighted image of patient 1.III.3 showing asymmetric atrophy of the left hippocampus. c18[F]-FDG-PET of patient 2.III.6 showing glucose hypometabolism in the left frontotemporal cortex
Pedigree 2
Three members of this family were examined. The mother died at the age of 54 following a rapidly progressive cognitive decline of unknown origin. Two uncles and one of four aunts, as well as the grandmother, had suffered from neurological deficits according to the clinical reports, but the symptoms were not specified.
Patient 2.III.6 was a 49-year-old man who sought help for gait instability. He also reported a lack of motor coordination in the right hand. He progressively developed severe right hand apraxia and alien hand. Reflex myoclonus and dystonic postures were observed in the right extremities. Cranial MRI showed mild frontotemporal atrophy. CMS revealed increased threshold stimulation time. 18Fluorodeoxyglucose (FDG)-PET showed hypometabolism in the left frontotemporal cortex (Fig. 2). Frequent falls and cognitive and behavioral decline were shown during the disease course. A diagnosis of corticobasal syndrome was established. The patient died at the age of 55.
Patient 2.III.7 was a 43-year-old man who presented a short history of cognitive decline and speech difficulties. He also reported motor difficulties, especially with the right hand. Behavioral changes characterized by impulsivity and aggressive behavior progressively appeared. Neurological exam showed non-fluent speech, increased latency in initiating lateral and vertical gaze movements, and rigidity and bradykinesia in the right arm and leg, together with alien phenomenon and reflex myoclonus. A corticobasal syndrome diagnosis was established. Cranial MRI showed left posterior parietal atrophy and white matter involvement. The patient died at the age of 47.
Patient 2.III.8 was a 55-year-old woman who sought help for dysexecutive syndrome accompanied by anomia, memory deficits, apathy, anhedonia, and mood disorder. The language deficit progressed, evolving to dumbness 2 years later, and stereotypic motor behavior appeared as well. At this moment she was fully dependent for basic daily activities. Froment sign was positive but no motor asymmetries or ophthalmoparesis was detected. She was diagnosed of a frontotemporal dementia. Cranial MRI showed nonspecific white matter lesions. The patient died at age 61, 3 years after the last neurological exam.
SPECT-HMPAO showed left hemisphere hypoperfusion in all three patients mainly in parietotemporal cortex. Post-mortem neuropathological examination was available in the three cases.
Genetic analysis
Sanger sequencing of exon 10 of the MAPT gene of patients 1.III.3, patients 2.III.6, 2.III.7, and 2.III.8 revealed a heterozygous missense mutation causing a C to A substitution at position Chr17:44087754 (GRCh37/hg19 assembly coordinates) of MAPT which in turn led to a change from proline (CCG) to threonine (ACG) in codon 301 of the protein (P301T) (Fig. 3a). Genetic analysis was not able to be performed in patient 1.II.2, who was defined only by clinical reports.
MAPT mutation and western-blot findings. aMAPT p.P301T mutation, which predicts and amino acid change from proline (CCG) to threonine (ACG). b Western blotting of sarkosyl-insoluble fractions of case 1.III.3 revealing two bands of 68 kDa and 64 kDa, the latter forming a doublet. Several lower bands between 50 and 37 kDa, and even lower bands of about 20–25 kDa, are also observed. A case of Alzheimer’s disease was run in parallel showing the pattern of three bands of 68 kDa, 64 kDa, and 60 kDa
Tau biochemistry
Western blotting of sarkosyl-insoluble fractions revealed two bands of 68 kDa and 64 kDa, the latter forming a doublet in case 1.III.3. This was in contrast with the pattern of three bands of 68 kDa, 64 kDa, and 60 kDa observed in a case of Alzheimer’s disease run in parallel. There were several lower bands between 50 kDa and 37 kDa; lower bands of about 20–25 kDa were observed in cases 1.III.3 and 2.III.8, but not in the other two cases (Fig. 3b).
Neuropathological findings
Macroscopic examination of the brains showed moderate-to-severe atrophy of the frontal lobes with enlargement of the ventricular system in all the cases. Atrophy of the parietal lobe was also found in cases from pedigree 2 (Fig. 4a). The left hippocampus was severely atrophic in case 1.III.3. The substantia nigra and the locus coeruleus were moderately de-pigmented in every case. The cerebellum, pons, medulla oblongata, and spinal cord were largely unremarkable. A summary of the main neuropathological features is shown in Table 2.
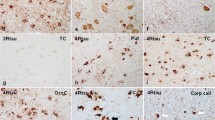
Neuropathological findings. a Macroscopic view of fresh specimen showing severe atrophy of the parietal lobes. b Neurons and astrocytes (arrowheads) with AT8-immunoreactive deposits in the frontal cortex. c Globular oligodendroglial inclusions in white matter (arrows). d Globular astrocytic inclusions immunoreactive with AT8 antibodies in the frontal cortex (arrowheads). e AT8 globular oligodendroglial inclusions in the white matter (arrows). f Tau deposition in neurons and glial cells in the anterior horn (asterisk) of the spinal cord as revealed with the AT8 antibody. g Positive staining of 4Rtau in globular oligodendroglial inclusions (arrows), globular astrocytic inclusions (arrowheads) and neuropil threads. h Negative staining of 3Rtau in (tau-containing) neurons in Meynert nucleus
Histology and immunohistochemistry
Neuron loss, spongiosis of layers II and III, and astrocytic gliosis were observed in the frontal cortex in every case. The primary motor cortex showed mild superficial spongiosis of layers I and II, loss of Betz cells, and astrocytic gliosis. Ballooned neurons were observed in the prefrontal cortex, as well as neurons with tangles in many other regions which were better identified with immunohistochemistry. The parietal cortex in affected members of pedigree 2 showed marked loss of neurons and severe gliosis, particularly in case 2.III.7. The left hippocampus showed severe neuronal loss and gliosis in case 1.III.3. The striatum and thalamus were well preserved with some patchy neuronal loss and gliosis. Neuron loss and gliosis in the substantia nigra was found in every case but it was particularly marked in 2.III.6. Neuronal loss in the anterior horn of the spinal cord was found in every case with the exception of 2.III.6 in which the spinal cord was not available for study. The white matter of the cerebral hemispheres showed variable demyelination, mild astrocytic gliosis, and the presence of glial cells with globular cytoplasm. Demyelination of the corticospinal tracts was noted in all cases.
The immunohistochemical study revealed extensive neuronal and glial deposits of hyper-phosphorylated tau. Neuronal deposits were consistent with neurofibrillary tangles and pre-tangles (Fig. 4b); astrocytic inclusions conformed globular inclusions (globular astrocytic inclusions: GAIs) and tufted inclusions (tufted astrocytes); oligodendroglial inclusions were globular (globular oligodendroglial inclusions: GOIs) and coiled bodies (Fig. 4c–e). Tau deposits were found in neurons and glial cells in the anterior horn of the spinal cord (Fig. 4f). In addition, threads were found in the grey matter and white matter. Tau inclusions were 4Rtau (Fig. 4g); 3Rtau was not detected in any case (Fig. 4h). P62 immunohistochemistry highlighted abnormal oligodendroglial inclusions in the dentate nucleus of the cerebellum. Gallyas silver staining also demonstrated pathological tau deposits in neurons, astrocytes, and oligodendrocytes. The distribution and quantity of lesions were variable from one case to another as detailed in Table 2. Case 1.III.3 had more severe lesions than cases from pedigree 2. No TDP-43, α-synuclein, or Aβ-amyloid deposits were found in any case.
Discussion
We present the clinical, pathological, biochemical and genetic characteristics of a rapidly progressive autosomal dominant tauopathy due to the P301T mutation in exon 10 of the MAPT gene, characterized by pathological phenotype of GGT. This mutation was previously described in another family [17], but neuropathological and biochemical studies were lacking. Therefore, this is the first description of the neuropathological substrate of the P301T mutation in two apparently unrelated pedigrees.
The cases manifested with heterogeneous clinical and pathological phenotype, in line with the clinical and pathological variability associated with MAPT mutations in different families and even within the same family.
The age of onset in men was the fifth decade while in women the debut occurred in the sixth or seventh decade. This sex different age of onset has not been previously mentioned in other MAPT mutations and it may be simply due to chance. The duration of the disease was similar and ranged between 4 and 7 years. Motor symptoms first appeared as gait disorder or as motor clumsiness of one hand in three of the five patients. One patient began with language disorder, and his sister with dysexecutive cognitive decline. Asymmetrical motor symptoms were common: pyramidal signs with dystonic postures in one patient and corticobasal syndrome with apraxia and myoclonus in three of them. All the patients presented variable degrees of ophthalmoparesis, parkinsonism, and cognitive impairment with abnormal behavior. Lower motor neuron signs were absent. However, clinical symptoms varied from one case to another, not only among members of the distinct pedigrees but also among members of the same family.
The patient described by Lladó et al. [17]. bearing the same mutation began with cognitive and abnormal behavior followed by gait disturbance and supranuclear paralysis. No mention was made of the presence of motor neuron involvement or asymmetry.
From the pathological point of view, all the cases of this series showed globular deposits of tau in astroglia and mainly in oligodendroglia, meeting the pathological criteria established for the diagnosis of GGT [11]. Like clinical symptoms, neuropathological changes varied from one case to another. Cases 1.III.3, 2.III.6, and 2.III.7 could correspond to subtype II of GGT where the limits of tau pathology between grey and white matter were difficult to establish. Patient 1.III.3 developed a pyramidal syndrome with features of primary lateral sclerosis associated with severe ophthalmoparesis, parkinsonism, and dementia. The other two cases of pedigree 2 presented a corticobasal syndrome (similar to patient 1.II.2, whose brain could not be studied). Oligodendroglial deposits were found mainly in the motor cortex and corticospinal tract. GAI were scarcely present. Therefore, they are similar to one of the cases described by Ahmed in 2011 [10] and Joseph’s 12 cases [7]. Unlike them, we found tau deposits in motor neurons of the anterior horn. The clinical syndrome of case 2.III.8 corresponded to a frontotemporal dementia and the brain showed some features of GGT subtype I (scarce astroglial deposits) [2] while oligodendroglial deposits were not abundant and some tau deposits were found in motor neurons of the anterior horn, which are features of subtype III. Therefore, classification of the present GGT cases into subtypes is difficult and sheds little light on understanding of the individual variations in patients suffering from the same mutation in MAPT, even among members of the same family, which is in consonance with previous criticisms regarding GGT classification [18].
GGT was initially considered to be a sporadic tauopathy, but since 2015 different mutations of the MAPT gene have been described that are associated with a pathological phenotype of GGT (Table 3): p.K317 N in exon 11 [13], p.P301L in exon 10 [14,15,16], and IVS10 + 16 C > T in intron 10 [16]. Other mutations of the MAPT gene described before the 2013 consensus that retrospectively meet the criteria of GGT include exon 10 mutation at position p.N296H [19], p.R5H in exon 1 [20], and p.K317 M in exon 11 [21].
This study describes for the first time the neuropathological findings associated with the P301T mutation of MAPT, and shows variable clinical symptoms and neuropathological characteristics not only among members of different pedigrees but also among members of the same family. Further studies are needed to identify genetic and epigenetic factors that determine phenotypic diversity in this disease.
References
Molina JA, Probst A, Villanueva C et al (1998) Primary progressive aphasia with glial cytoplasmic inclusions. Eur Neurol 40:71–77. https://doi.org/10.1159/000007961
Bigio EH, Lipton AM, Yen S-H et al (2001) Frontal lobe dementia with novel tauopathy: sporadic multiple system tauopathy with dementia. J Neuropathol Exp Neurol 60:328–341. https://doi.org/10.1093/jnen/60.4.328
Ferrer I, Hernández I, Boada M et al (2003) Primary progressive aphasia as the initial manifestation of corticobasal degeneration and unusual tauopathies. Acta Neuropathol 106:419–435. https://doi.org/10.1007/s00401-003-0756-4
Powers JM, Byrne NP, Ito M et al (2003) A novel leukoencephalopathy associated with tau deposits primarily in white matter glia. Acta Neuropathol 106:181–187. https://doi.org/10.1007/s00401-003-0719-9
Piao Y-S, Tan C-F, Iwanaga K et al (2005) Sporadic four-repeat tauopathy with frontotemporal degeneration, parkinsonism and motor neuron disease. Acta Neuropathol 110:600–609. https://doi.org/10.1007/s00401-005-1086-5
Tan C-F, Piao Y-S, Kakita A et al (2005) Frontotemporal dementia with co-occurrence of astrocytic plaques and tufted astrocytes, and severe degeneration of the cerebral white matter: a variant of corticobasal degeneration? Acta Neuropathol 109:329–338. https://doi.org/10.1007/s00401-004-0933-0
Josephs KA, Katsuse O, Beccano-Kelly DA et al (2006) Atypical progressive supranuclear palsy with corticospinal tract degeneration. J Neuropathol Exp Neurol 65:396–405. https://doi.org/10.1097/01.jnen.0000218446.38158.61
Kovacs GG, Majtenyi K, Spina S et al (2008) White matter tauopathy with globular glial inclusions: a distinct sporadic frontotemporal lobar degeneration. J Neuropathol Exp Neurol 67:963–975. https://doi.org/10.1097/NEN.0b013e318187a80f
Fu Y-J, Nishihira Y, Kuroda S et al (2010) Sporadic four-repeat tauopathy with frontotemporal lobar degeneration, Parkinsonism, and motor neuron disease: a distinct clinicopathological and biochemical disease entity. Acta Neuropathol 120:21–32. https://doi.org/10.1007/s00401-010-0649-2
Ahmed Z, Doherty KM, Silveira-Moriyama L et al (2011) Globular glial tauopathies (GGT) presenting with motor neuron disease or frontotemporal dementia: an emerging group of 4-repeat tauopathies. Acta Neuropathol 122:415–428. https://doi.org/10.1007/s00401-011-0857-4
Ahmed Z, Bigio EH, Budka H et al (2013) Globular glial tauopathies (GGT): consensus recommendations. Acta Neuropathol 126:537–544. https://doi.org/10.1007/s00401-013-1171-0
Ferrer I (2018) Oligodendrogliopathy in neurodegenerative diseases with abnormal protein aggregates: the forgotten partner. Prog Neurobiol 169:24–54. https://doi.org/10.1016/j.pneurobio.2018.07.004
Tacik P, DeTure M, Lin W-L et al (2015) A novel tau mutation, p. K317N, causes globular glial tauopathy. Acta Neuropathol 130:199–214. https://doi.org/10.1007/s00401-015-1425-0
Tacik P, Sanchez-Contreras M, DeTure M et al (2017) Clinicopathologic heterogeneity in frontotemporal dementia and parkinsonism linked to chromosome 17 (FTDP-17) due to microtubule-associated protein tau (MAPT) p. P301L mutation, including a patient with globular glial tauopathy. Neuropathol Appl Neurobiol 43:200–214. https://doi.org/10.1111/nan.12367
Borrego-Écija S, Morgado J, Palencia-Madrid L et al (2017) Frontotemporal dementia caused by the P301L Mutation in the MAPT gene: clinicopathological features of 13 cases from the same geographical origin in Barcelona, Spain. Dement Geriatr Cogn Disord 44:213–221. https://doi.org/10.1159/000480077
Forrest SL, Kril JJ, Stevens CH et al (2018) Retiring the term FTDP-17 as MAPT mutations are genetic forms of sporadic frontotemporal tauopathies. Brain 141:521–534. https://doi.org/10.1093/brain/awx328
Lladó A, Ezquerra M, Sánchez-Valle R et al (2007) A novel MAPT mutation (P301T) associated with familial frontotemporal dementia. Eur J Neurol 14:e9–e10. https://doi.org/10.1111/j.1468-1331.2007.01763.x
Burrell JR, Forrest S, Bak TH et al (2016) Expanding the phenotypic associations of globular glial tau subtypes. Alzheimer’s Dementia: Diagn, Assess Dis Monit 4:6–13. https://doi.org/10.1016/j.dadm.2016.03.006
Iseki E, Matsumura T, Marui W et al (2001) Familial frontotemporal dementia and parkinsonism with a novel N296H mutation in exon 10 of the tau gene and a widespread tau accumulation in the glial cells. Acta neuropathologica 102:285–292
Hayashi S, Toyoshima Y, Hasegawa M et al (2002) Late-onset frontotemporal dementia with a novel exon 1 (Arg5His) tau gene mutation. Ann Neurol 51:525–530. https://doi.org/10.1002/ana.10163
Zarranz JJ, Ferrer I, Lezcano E et al (2005) A novel mutation (K317 M) in the MAPT gene causes FTDP and motor neuron disease. Neurology 64:1578–1585. https://doi.org/10.1212/01.WNL.0000160116.65034.12
Acknowledgements
The authors thank Valle Coca and Miren Roldan for their technical support. They are also grateful for the collaboration the Neurological Tissue Bank of Navarrabiomed (Pamplona, Spain). We wish to thank T. Yohannan for editorial assistance.
Funding
The study did not receive funding.
Author information
Authors and Affiliations
Contributions
Erro ME and Ferrer I: designed and conceptualized study, analyzed the data and drafted the manuscript for intellectual content. Zelaya MV and Mendioroz M: performed and analyzed experimental work, provided administrative support and technical contributions. Larumbe R: acquired data. Ortega-Cubero S: performed experimental work and acquired data. Lanciego JL: performed experimental work. Lladó A: performed experimental work. Cabada T: acquired data. Tuñón T: revised manuscript for intellectual content. García-Bragado F: revised manuscript for intellectual content. Luquin MR: acquired data and revised manuscript for intellectual content. Pastor P: performed experimental work and reviewed the manuscript for intellectual content. All the authors reviewed the final version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Informed consent
Consent was obtained from all participants or relatives prior to their inclusion in the study.
Rights and permissions
About this article
Cite this article
Erro, M.E., Zelaya, M.V., Mendioroz, M. et al. Globular glial tauopathy caused by MAPT P301T mutation: clinical and neuropathological findings. J Neurol 266, 2396–2405 (2019). https://doi.org/10.1007/s00415-019-09414-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-019-09414-w