Abstract
Objectives
To describe the neurological phenotype of children with prenatal diagnosis of agenesis of corpus callosum (ACC) and interhemispheric cysts associated with malformations of cortical development (MCD).
Methods
We reviewed the neuroimaging, neurologic, EEG, and genetic data of 36 patients (21 males, mean age 7 years) with ACC and interhemispheric cysts. Associations were tested with Chi-squared and Fisher exact tests.
Results
According to the 2001 Barkovich classification, we found 4 type 1c (11.1%), 6 type 2a (16.6%), 18 type 2b (50%, 6/18 girls with Aicardi syndrome), and 9 type 2c cysts (22.2%). EEG showed specific epileptic activity in 27/36 patients (75%). Epilepsy was diagnosed in 16 subjects (16/36, 44.4%), including all Aicardi patients, and was associated with cognitive impairment (p = 0.032). Severe intellectual disability and epilepsy were associated with type 2b cysts, always due to Aicardi patients (p < 0.05). After excluding Aicardi patients, all subjects with type 2b cysts had mild neurological phenotype. Patients with 2a and 2c cysts more frequently had normal cognition (83.3% and 62.5% of cases, respectively). Patients with type 1c cyst mostly had mild/moderate cognitive impairment. Severe neurologic deficits were associated with 1c cysts and 2b cysts with Aicardi syndrome (p < 0.05). Multilobar and/or bilateral MCD were associated with severe neurological and epileptic phenotypes (p < 0.05).
Conclusion
Once excluded Aicardi syndrome, most patients with ACC and interhemispheric cysts have a mild clinical phenotype characterized by borderline/normal cognition and minor neurological signs. Despite the high prevalence of EEG epileptic abnormalities, epilepsy in these cases is infrequent and usually responsive to antiepileptic drugs.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Agenesis of corpus callosum (ACC) is one of the most frequent brain malformations with an incidence of 0.05–0.7% [1,2,3], and interhemispheric cysts represent an important feature of such malformation, being associated in 14–33.3% of cases [4,5,6]. ACC is no longer viewed as a narrowly defined specific disorder, but as a spectrum of genetic or acquired malformative conditions variably associated with other brain or extracranial malformations [2, 7]. Accordingly, a wide array of clinical phenotypes has been described, ranging from normal development or mild neurological impairment (usually in isolated ACC) to severe cognitive deficits, motor disabilities, and epilepsy (mostly in syndromic ACC) [8,9,10,11,12,13,14]. Therefore, the accurate characterization of concomitant brain anomalies and stratification of cases based on clinical and imaging features are pivotal for defining the prognosis [4, 7]. Rarely, ACC may be associated with interhemispheric cysts resulting from midline meningeal dysplasia. The current classification scheme, proposed by Barkovich et al. [15], categorizes interhemispheric cysts into two types based on the presence (Type 1) or absence (Type 2) of communication with the ventricular system [15]. Further subdivision into subgroups depends on the presence of other associated brain anomalies or clinical features including macro- or microcephaly, hydrocephalus, malformations of cortical developments (MCD) [16], and multiloculated or arachnoid cysts [15].
ACC and interhemispheric cysts are easily identified in utero. Thanks to the improvement of prenatal ultrasound screening and fetal MRI, the identification of associated MCD has increased during pregnancy, raising relevant prognostic questions. Indeed, the presence of MCD is potentially associated with an increased risk of mental disability, epilepsy and cerebral palsy in subjects with ACC [8, 9]. Of note, ACC associated with multiple cysts (type 2b cysts), MCD, ocular anomalies, and infantile spasms in girls corresponds to the Aicardi syndrome, one of the most severe epileptic encephalopathies [9, 15, 17, 18]. Conversely, variable clinical phenotypes have been described in patients with non-syndromic ACC and interhemispheric cysts [15, 19]. Unfortunately, limited data on neurological characterization and long-term follow-up of these patients are available in the literature [20,21,22,23]. Therefore, the clinical significance of ACC with interhemispheric cysts and MCD remains elusive, with important consequences for prenatal parental counselling and clinical prognosis.
In this study, we aimed to describe the clinical and neuroradiological phenotypes, EEG patterns, and neurological outcomes in a group of 36 children diagnosed prenatally or soon after birth with ACC and interhemispheric cysts variably associated with MCD, and to provide information on 185 additional published cases. Moreover, we explored the associations between clinical and neuroimaging data based both on the 2001 Barkovich classification and on single malformative features.
Methods
This was a double-center retrospective observational study performed at the Istituto Giannina Gaslini Children’s Hospital (Genoa, Italy) and McGill University (Montreal, Canada). Ethical Committees of both institutions waived written parental consent due to the retrospective nature of the study.
Subjects
We selected subjects with ACC and interhemispheric cysts who were consecutively diagnosed from January 1996 to January 2018 in the two Institutions. Subjects who met all the following inclusion criteria were included in the study: (1) prenatal or immediate postnatal diagnosis of ACC and interhemispheric cysts, confirmed on postnatal brain MRI; (2) available data on extra-CNS malformations detected at postnatal imaging or clinical evaluation; and (3) neurological evaluation with EEG examinations. Exclusion criteria were: (1) history of perinatal asphyxia or other acquired injuries and (2) poor MR imaging quality.
Neuroimaging assessment
Brain MRI studies were performed on 1.5T and 3T scanners with different protocols; however, all included 3D T1-weighted, 3 mm-thick axial and coronal T2-weighted, axial FLAIR, and diffusion weighted imaging sequences. Two neuroradiologists blinded to the clinical status reviewed all images in consensus and classified patients according to the 2001 Barkovich classification [15]. In particular, type 1a presents with macrocephaly and hydrocephalus, type 1b with macrocephaly and hydrocephalus associated with third ventricular obstruction due to diencephalic anomalies (thalamic fusion, hamartoma), and type 1c presents with microcephaly. Type 2a is characterized by hydrocephalus and a grossly normal brain, while type 2b is made of multiple cysts different from CSF with malformations of cortical development (MCD), such as polymicrogyria and periventricular nodular heterotopias [16], type 2c usually affects males and presents with multiloculated cysts and large subcortical heterotopia, and type 2d corresponds to a histologically proven arachnoid cyst [15]. Of note, we decided to classify into type 2a group also patients with minor neuroimaging features, such as mild pontine hypoplasia or minor basal ganglia dysmorphisms, since the main discriminant distinguishing type 2a from type 2b and 2c cysts is the absence of MCD, while type 2d cysts may be proven only by histology [15]. Moreover, in some patients cortical thinning around the cysts was visible only after ventricular shunting on high-resolution 3D T1-weighted images.
The cyst signal, distribution, and extension were recorded. The type of ACC and other commissural anomalies were noted. Agenesis or hypoplasia of Probst bundles, fornices, cingulate gyri and mammillary bodies were qualitatively evaluated on coronal reformatted 3D T1-weighted images. Hemispheric and/or cortico-spinal tract hypoplasia were also assessed. The type and location of MCD or cortical thinning around the cysts were considered. Finally, other associated brain anomalies were described.
Clinical and epileptologic evaluation
Clinical, neurological, and genetic data were extracted from patient medical records. Neurological examinations were performed by experienced pediatric neurologists in both Institutions. Clinical follow-up was performed every 6–12 months on average (depending on clinical state), always including a neurological examination and EEG recording. For children younger than 1 year, neurological follow-up was performed at 1 month, 3 months, 6 months, and 12 months with EEG every 6 months. Developmental milestones were evaluated by the Griffith’s Mental Developmental Scales-Extended and Revised (GMDS-ER) below the age of 5 years, while cognitive impairment was assessed by the Wechsler scales in older children or with the Leiter scales in non-verbal or severely intellectually disabled patients. On average, GMDS-ER scales were performed every 12 months, while children older than 5 years were re-evaluated at least once with Weschler scales or Leiter scale. Global developmental delay (GDD) was defined according to the Diagnostic and Statistical Manual of Mental Disorders-5th edition (DSM-5) as a significant persistent (at least for 6 months) delay in two or more developmental domains, including motor, speech, cognition, social functioning, and activities of daily living, in children below the age of 5 years [24]. Conversely, the term intellectual disability (ID) was applied to older children in whom IQ testing could be performed. Of note, ID was defined as a disability originating before age 18 years, characterized by significant limitations both in intellectual functioning and in adaptive behavior as expressed in conceptual, social, and practical adaptive skills [24]. Patients were thus divided into global categories of normal intelligence, borderline intelligence, or mild, moderate, or severe intellectual disability (ID), according to the DSM-5 definitions. Finally, we provided for all patients a global measure of developmental or cognitive disability, called disability outcome score, considering the latest available neuropsychological assessment. In particular, for patients too young to perform a IQ testing, we considered only the GMDS-ER subscales associated with cognitive outcome, such as the C (language), D (eye–hand coordination), and E (performance) scales [25]. Patients were thus divided as having a global normal functioning (corresponding to a score of 0); borderline functioning (corresponding to a score of 1), mild impairment (corresponding to a score of 2), moderate impairment (corresponding to a score of 3), and severe impairment (corresponding to a score of 4).
Sleep disorders and psychiatric comorbidities were also considered. In particular, psychiatric symptoms were investigated by specific tests, including the Multidimensional Anxiety Scale for Children (MASC) and the Children’s Depression Inventory (CDI2) for depressive symptoms.
Attention-deficit hyperactivity disorder (ADHD) was diagnosed when frequency and duration of the patient’s symptoms fulfilled the criteria set out in the DSM-5. The diagnosis of Aicardi syndrome was based on the criteria proposed by Aicardi [17, 18]. The presence of extra-CNS malformations and facial dysmorphisms was recorded. Results of genetic tests, such as basal metabolic screening, karyotype, array-CGH, and target genetic testing were mentioned when available. Data regarding neurosurgical interventions (when performed) were recorded, including indications, age at intervention, type of surgery, and outcome.
Data on the age of onset of epilepsy and types of epileptic seizures were recorded, taking into account the type and number of antiepileptic drugs (AE), drug sensitivity (DS) or drug resistance (DR), and episodes of status epilepticus (SE). Drug resistance was defined as failure of adequate trials of two tolerated, appropriately chosen and used antiepileptic drug schedules whether as monotherapies or in combination [26]. All patients underwent EEG recordings during wakefulness and sleep. Parameters taken into consideration were: (1) the presence and localization (focal, multifocal, or generalized) of epileptiform abnormalities; (2) activation of the abnormalities during sleep; (3) presence of continuous spike and wave occupying more than 85% of slow-wave sleep associated with cognitive impairment (CSWS); and (4) presence of hypsarrhythmia. Aspecific EEG abnormalities, such as focal or diffuse slow activity or voltage asymmetry, were also reported.
Statistical analysis
Continuous variables were summarized as mean, and categorical variables were summarized as frequencies and percentages. The associations between clinical, EEG, and neuroradiological findings based both on the 2001 Barkovich classification and on single malformative features were evaluated by the Chi-squared and Fisher exact test. Statistical significance was set at p = 0.05. Statistical analyses were performed using SPSS Statistics software, v21 (IBM, Armonk, NY, USA).
Results
36 patients with ACC and interhemispheric cysts satisfied the inclusion criteria (21 males, mean age at last follow-up 7.08 years, range 7 month–22 years) (Online Fig. 1). The mean follow-up period was 7.08 years (range 7 months–22 years). One patient (Pt#18), who was previously developing normally, died of hemolytic-uremic syndrome at age 2 years. The vast majority of patients were diagnosed in utero by fetal US screening (32/36, 88.8%) and confirmed by fetal MRI in almost half of the cases (15/32). In the remaining four cases, the diagnosis was made in the neonatal period in two subjects who presented with macrocephaly and seizures, respectively, and at 6 months of age in two other patients presenting with motor deficits. Online Table 1 shows clinical, neuroimaging, and genetic data for every patient.
Neuroradiological data
According to the 2001 Barkovich classification, four patients were classified with type 1c cysts (4/36, 11.1%), 6 with type 2a cysts (6/36, 16.6%), 18 with type 2b cysts (18/36, 50%), and eight patients with 2c cysts (8/36, 22.2%). Six girls with type 2b cysts were diagnosed with Aicardi syndrome (6/18, 30%). Table 1 shows the imaging features based on the type of cysts.
Neurological and epileptic phenotype
GDD was observed in 24/36 patients (66.6%) and was rated as borderline in 1/24 (4.2%) cases, mild in 13/24 (54.2%), moderate in 4/24 (16.6%), and severe in 6/24 (25%) subjects. In 22 patients, the cognitive outcome was re-assessed after the age of 5 years, revealing normal cognition in 14/22 (63.6%) subjects, borderline cognition in 4/22 (18.2%) patients, mild ID in 2/22 (9.1%), and severe ID in 2/22 (9.1%) cases. Two patients with normal IQ had specific learning disorders. Mild ID was present in 2/21 (9.5%) cases, while severe ID was found in 2 other cases (9.5%). Individualized teaching was needed in 15/25 (60%) subjects attending school or preschool.
Overall, the disability outcome score was stratified as follows: normal cognitive functioning in 17/36 (47.2%) subjects, borderline functioning in 5/36 (13.8%) cases, mild cognitive impairment in 7/36 (19.4%) patients, moderate cognitive impairment in 1/36 (2.7%) subjects, and severe cognitive impairment 6/36 (16.6%) patients (all affected by Aicardi syndrome).
Neurological deficits were present in 26/36 (72.2%) cases: minor neurological signs in 18/26 (69.2%), tetraparesis in 6/26 (23%), and hemiparesis in 2/26 of patients (7.8%).
Epilepsy was diagnosed in 16 subjects (16/36, 44.4%), including all Aicardi patients. Age of onset was variable, ranging from birth to 16 years, and the frequency of seizures varied from multiple per each day to a few seizures per year. Concerning seizure outcome, 3/16 (18.7%) patients became seizure free at the last follow-up and were off treatment. The remaining patients (13/16, 81.3%) were still under AE therapy. All patients with Aicardi syndrome and one without Aicardi syndrome were drug resistant. Concerning drug-responsive patients (9/10 non-Aicardi patients with epilepsy), AE monotherapy was initially introduced, with good seizure control in all except three patients: in one (#35), substitution of the AE drug was sufficient in controlling epilepsy; in the other two (#2 and #22), polytherapy with two AE drugs was effective. No specific AE strategy was associated with a higher likelihood of seizure freedom. Epilepsy was associated both with developmental delay and cognitive impairment (p = 0.032).
Table 2 describes the seizure characteristics in patients with and without Aicardi syndrome. The median age at seizure onset for Aicardi syndrome was earlier compared with those without Aicardi syndrome (p = 0.001). Most patients from both groups had multiple coexisting seizure types. Focal seizures, mostly clonic or tonic–clonic, sometimes with secondary generalization, were present in 9/36 (25%). Infantile spasms were present in 7/36 (19%) female patients, 6 of whom were diagnosed with Aicardi syndrome, as by definition, and the remaining one received a molecular diagnosis of frontonasal dysplasia. Seizure frequency was higher in Aicardi syndrome (all patients having more than one seizure/day) compared with non-Aicardi patients (only 1 patient having more than one seizure/day) (p = 0.007).
EEG recording displayed voltage asymmetry of the background activity in 24/36 (66%) cases, with decrease of the amplitude on derivations located near the cysts. All Aicardi subjects (6/36, 16%) showed additional typical asynchrony of the EEG hemispheric activity. The remaining 6/36 (16%) had a normal EEG background activity. We found a hypsarrhythmic pattern in 6/6 Aicardi patients alone.
Epileptiform abnormalities were present in the majority of cases (27/36 patients, 75%); in 17/36 (47%) the abnormalities were concordant to the area of PMG and in 13/36 (36%) concordant to the subcortical gray matter heterotopia (GMH) localization. Activation of the epileptic abnormalities during sleep was depicted in 11/36 (30.5%). Of interest, no CSWS was found in our cohort (Fig. 1). Psychiatric comorbidities were present in 12/36 patients (33.3%), including anxiety disorder (6/36; 16.6%), ADHD (3/36; 8.3%), and autism spectrum disorders (3/36; 8.3%).
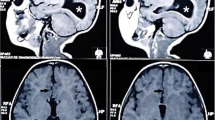
Brain MRI and EEG recordings of every cyst type: bipolar montage, high-frequency filter sets at 70 Hz and time constant sets at 0.1 s. Vertical marker 100 μV, horizontal marker, 1 s. a Type 1c (EEG at 4 years of age in therapy with VPA e TPR). Wake. Slowing posterior background activity and nonspecific abnormalities (theta waves) prevalent in the posterior regions of the right hemisphere. b Type 2b Aicardi syndrome (EEG at 22 years of age, DR epilepsy, in the past infantile spasms). Wake. Eyes opened; subcontinuous synchronous and asynchronous spikes and spike waves of medium and high amplitude predominant on the right frontal regions. c Type 2a (EEG at 6 years of age). Wake. Eyes opened, despite presence of muscle artefacts, no clear-cut epileptic abnormalities are evident. d Type 2b. Craniofrontonasal syndrome (EEG at 12 months of age). Wake. Asymmetric infantile spasms recorded. e Type 2b (EEG at 12 years of age). Wake. Eyes closed, normal background activity. f Type 2c. Drowsiness. Slowing and epileptic anomalies in the left posterior regions
The karyotype was performed in all patients, revealing chromosomal abnormalities in 3/36 (8.3%) cases. In particular, we found an inherited pericentric chromosome Y inversion (Y:46, XY, inv(Y)(p11.2q11.23) in patient #13, an inherited pericentric inversion of the chromosome 5 (46XY inv(5)(p13.1-q13-1) in patient #14, and partial 14q tetrasomy in patient #30. Array-CGH tests were performed in 31 out of 36 patients, and were normal in 29/31 cases (93.5%). In patient #30, they confirmed the partial 14q tetrasomy, while in the remaining subject (patient #11) they revealed 4q34.3 and 15q11.2 deletions inherited from the healthy mother.
22 patients were surgically treated (22/36, 61.1%): 7 cases with endoscopic fenestration of the cysts, 10 with cystoperitoneal shunting, and 5 with both fenestration and cystoperitoneal shunting.
Clinical–radiological associations
Table 3 summarizes the clinical features according to the type of cysts. In particular, a quarter of patients with type 1c cysts were affected by epilepsy, never with infantile spasms (p = 0.034) or requiring polytherapy (p = 0.034). All of these patients had neurological deficits, in particular half presented with hemiplegia (p = 0.042). In one of the subjects with hemiparesis, there was a contralateral cyst associated with PMG in the motor area. Conversely, in the other patient, a gliotic cavity in the contralateral basal ganglia and a hypoplastic internal capsule indicative of a prenatal ischemic infarct were noted. Most patients had borderline, mild or moderate cognitive impairment, while none had normal cognition (p = 0.047) or severe cognitive impairment (p = 0.042). One-third of patients with type 2a cysts were affected by epilepsy, but never with infantile spasms or requiring polytherapy (p = 0.034). Half of patients presented minor neurological deficits, while none had hemi- or tetraparesis (p = 0.04). The majority (83.3%) had normal cognition (p = 0.047), and none had severe cognitive impairment (p = 0.042). More than half of patients with type 2b cysts presented with epilepsy (55.5%). Infantile spasms and AE polytherapy were associated with this group (p = 0.034) due to the presence of Aicardi patients (p < 0.001). Similarly, tetraparesis was noted only in this group (p = 0.049) due to Aicardi syndrome (p < 0.001). Severe cognitive impariment was more frequent than in other groups (p = 0.042), again due to Aicardi patients (p < 0.001). In addition, subjects with Aicardi syndrome more frequently presented status epilepticus (p = 0.002), AE drug resistance (p < 0.001), sleep problems (p = 0.02), and retinal/optic nerve malformations (< 0.001). Finally, more than one-third of patients with type 2c cysts had epilepsy (37.5%), but never experienced infantile spasms or required polytherapy (p = 0.034). The majority of these patients showed minor neurological signs (87.5%) and none had hemi- or tetraparesis (p = 0.04). More than half of subjects had normal cognition (p = 0.047), while none presented severe cognitive impairment (p = 0.042).
Tables 4 and 5 report clinical–radiological correlations in our cohort based on single brain malformative features. In particular, regarding the epileptic phenotype, bilateral PMG was associated with EEG abnormalities, epilepsy, status epilepticus, infantile spasms, AE polytherapy, and drug resistance (p < 0.05). PMG involving multiple lobes was associated with EEG abnormalities, status epilepticus, infantile spasms, and AE polytherapy (p < 0.05). Bilateral GMH and GMH involving multiple lobes were associated with epilepsy, status epilepticus, infantile spasms, AE polytherapy, and drug resistance (p < 0.05). Hemispheric asymmetry and cortico-spinal tract (CST) hypoplasia were associated with status epilepticus and infantile spasms, and need of AE polytherapy (p < 0.05).
Tetraparesis was associated with bilateral PMG (p < 0.001), PMG involving multiple lobes (p = 0.024), bilateral GMH (p < 0.001), and GMH involving multiple lobes (p = 0.003). Developmental delay was associated with bilateral PMG (p = 0.016). Cognitive problems were associated with bilateral GMH (p = 0.020) and GMH involving multiple lobes (p = 0.049). In particular, severe ID was associated with bilateral PMG (p < 0.001), PMG involving multiple lobes (p = 0.024), bilateral GMH (p < 0.001), GMH involving multiple lobes (p = 0.003), and CST hypoplasia (p = 0.016). Psychiatric problems were associated with bilateral PMG (p = 0.005).
Discussion
In this study, we describe the clinical course of ACC with interhemispheric cysts in a cohort of 36 patients diagnosed prenatally or soon after birth, and in 185 patients reported in the literature (Table 6), thus providing relevant information regarding outcome and prognosis of this malformative condition.
Neurological phenotype
Several studies have confirmed that the prognosis of ACC depends mainly on whether associated brain anomalies and/or syndromic conditions are present [8]. Similarly, in our cohort, the most important factor correlating with an adverse neurological outcome was the diagnosis of Aicardi syndrome that was always associated with severe cognitive impairment, tetraparesis, and epilepsy. Conversely, the majority of patients without Aicardi syndrome presented normal or borderline cognition (73.4%), and only a quarter of them (26.6%) had mild or moderate cognitive impairment. Interestingly, several patients with mild or borderline GDD did not receive the diagnosis of intellectual disability at a later date. In particular, of the eight patients with mild GDD who were re-assessed after the age of 5 years, six had a normal cognitive level (2/6 with specific learning disability), while two had a borderline cognitive level. Of note, although moderate or severe GDD is generally related with a poor cognitive outcome, an initial diagnosis of GDD is not necessarily associated with objective cognitive impairment in all cases [25]. These data suggest a potential role of early identification and appropriate intervention and treatment of GDD in infants and young children with ACC and interhemispheric cysts to improve the cognitive prognosis.
Minor neurological signs such as clumsiness were relatively frequent (56%), but only two patients were hemiplegic (6%). These data confirm prior reports on ACC and interhemispheric cysts [5, 13,14,15, 19,20,21,22,23, 27,28,29,30,31,32,33,34,35,36,37,38,39,40,41]. Indeed, from the present literature review, the neurological and cognitive profile after a median follow-up of 7 years was satisfactory with normal or borderline cognition in 69.1% of cases, while relevant neurological problems such as hemiplegia or other focal motor abnormalities were reported in 24% of subjects. Moreover, despite the high prevalence of macrocrania (70.7%), overt signs of raised intracranial pressure with headaches, vomiting, and papilledema were rarely reported, due to the compliance of the infant skull. Of note, the neurological phenotype of non-syndromic patients with ACC and interhemispheric cysts might be even milder, since almost half the reported cases (47%) were diagnosed after birth. Indeed, postnatal studies are likely to be biased by the fact that the large majority of patients were referred because of symptoms, including macrocrania, developmental delay, or seizures.
Seizures were found in 44% of our patients. However, when Aicardi patients were excluded, this percentage decreased to 27.7%. Remarkably, we found that cognitive impairment was significantly related to epilepsy. Similarly, 31.2% of subjects with ACC and interhemispheric cysts reported in the literature were affected by epilepsy. As expected and previously reported by other authors [9], Aicardi subjects had different epileptic and EEG phenotypes characterized by earlier seizure onset, higher seizure frequencies, infantile spasms, drug resistance despite AE polytherapy, and hypsarrhythmia and background asynchrony at EEG. Conversely, patients without Aicardi syndrome exhibited a milder epileptic phenotype, characterized by occasional focal seizures with sometime secondary generalization, activated during sleep only in half of cases, with good response to single AE therapy, and resolving in 9/10 cases. Status epilepticus was observed in 9/16 (56.2%); of interest, 4/10 non Aicardi epileptic patients presented at least one episode of SE, by the way presenting a final good outcome (only 1/10 patient was DR).
EEG studies revealed mainly multifocal and/or generalized epileptiform abnormalities. Aspecific EEG abnormalities such as amplitude asymmetry or slow activities were reported in half of non-epileptic patients, without difference between the type of interhemispheric cysts. Of interest, 12/36 (33,3%) of non-epileptic patients showed focal or multifocal abnormalities on the EEG.
Neuroimaging correlations and classification issues
One important issue when dealing with prognosis of ACC and interhemispheric cysts regards the lack of systematic use of classification schemes. Indeed, apart from our study, only four other studies [19, 23, 42, 43] have used the classification of Barkovich et al. [15], whereas the majority did not provide information on the type of cysts and often lacked detailed descriptions of associated brain malformations. Interestingly, we found that patients with 2a and 2c cysts more frequently had normal cognition (respectively, in 83.3% and 62.5% of cases), while no patients with type 1c cyst had normal IQ. Severe ID was associated only with type 2b cysts, due to the presence of Aicardi patients in that group. Remarkably, after excluding Aicardi patients, all subjects with type 2b cysts had normal IQ, borderline cognition, or mild ID. Hemiplegia was associated with type 1c cysts, while tetraparesis was associated with type 2b cysts, again due to the presence of Aicardi patients. Conversely, there were no significant differences regarding the prevalence of minor neurological signs among different cyst types. Likewise, we found similar prevalence of developmental delay (50–75%), sleep problems (33–50%), psychiatric disorders (16–50%), mild facial dysmorphisms, and extracranial malformations in all cyst types. Finally, there was no significant relationship between epilepsy and any specific type of cyst.
Interestingly, we noticed a discrepancy between the severity of neuroimaging features and the neurological phenotype in patients with type 2b and 2c cysts. Indeed, despite the presence of large areas of PMG and subcortical gray matter heterotopia (GMH) in type 2c cysts, only 37.5% of subjects developed epilepsy, mostly characterized by focal seizures with secondary generalization and well controlled by AE monotherapy, and associated with status epilepticus in only one case. Moreover, none of these patients were affected by hemiplegia, but only showed clumsiness and/or poor eye–hand coordination. In addition, we did not find a perfect correspondence between type 2b cysts and the diagnosis of Aicardi syndrome. Indeed, ten male patients with neuroradiological findings typical of type 2b cysts presented a significantly milder clinical phenotype, while two girls did not fulfill the diagnostic criteria of Aicardi syndrome. These findings raise important considerations regarding prenatal counseling, since not all patients with type 2b cysts will have a poor neurological outcome, especially if they are males. Furthermore, these data suggest the need of implementing previous classification schemes with further stratification of patients according to clinical and genetic data. In particular, ophthalmological data are fundamental to identify the typical abnormalities described in Aicardi syndrome, such as chorioretinal lacunae, eye and optic nerve anomalies, persistent pupillary membrane, and anterior synechiae [44].
Independently of the type of cyst, we found that specific neuroimaging features correlated well with the neurological and epilepsy profile. Intriguingly, neurological problems or epilepsy was not simply associated with PMG or GMH, but rather related to bilateral extension of MCD, involvement of multiple lobes, and other associated neuroradiological features such as hemispheric asymmetry and/or CST hypoplasia. Indeed, bilateral PMG and/or PMG involving more lobes was frequently associated with developmental delay, severe ID, tetraparesis, epilepsy (especially infantile spasms and status epilepticus), AE polytherapy, drug resistance, EEG epileptic abnormalities, psychiatric disorders, and ocular anomalies. Similarly, bilateral GMH and/or GMH involving multiple lobes were related to severe ID, tetraparesis, epilepsy (especially infantile spasms and status epilepticus), AE polytherapy, drug resistance, and ocular anomalies. As a descriptive observation, we noted that patients with PMG and/or GMH involving the frontal precentral regions, the insular regions, and temporal areas more frequently presented a severe neurologic and epileptic phenotype associated with ocular anomalies.
Similarly, hemispheric and CST hypoplasia were noted more frequently in patients with status epilepticus, infantile spasms, and AE polytherapy. Of note, the combination of all these neuroimaging features was found almost exclusively in patients with Aicardi syndrome, and might be useful for raising the diagnostic suspicion on fetal MRI. Indeed, ocular anomalies are very rarely identified in utero both with US and MRI, thus making this malformative condition almost impossible to diagnose before birth.
When compared with literature data, a higher prevalence of MCD was found in our cohort (75%). Indeed, PMG and/or GMH was described in only 25% of reported cases [13,14,15, 19, 20, 22, 30, 33, 38, 39]. This discrepancy is likely explained by the low resolution of MR studies and CT scans used in the past. Moreover, MCD may not be evident at presentation due to the mass effect of large interhemispheric cysts on the surrounding brain parenchyma, and are more easily detected after cystic fenestration or shunting, as we observed in seven cases. These data underlie the need of accurate neuroimaging evaluation using high spatial resolution (possibly 3D) MR sequences, both pre- and postnatally, and after surgery.
Genetic data and embryological considerations
Besides Aicardi syndrome, only a few syndromes have been associated with commissural anomalies, intracranial cysts, and/or MCD. Overall, these syndromes are very rare and only a minority of patients with ACC and interhemispheric cysts are affected by a genetic condition (Online Table 2). In ciliopathies, such as orofaciodigital syndrome type I and acrocallosal syndrome, recent data established that callosal anomalies are due to specific patterning defects of the cortical septum boundary leading to altered distribution of guidepost cells required to guide the callosal axons through the midline [45, 46]. Conversely, in other disorders characterized by ACC and interhemispheric cysts, such as Aicardi and oculocerebrocutaneous syndromes, the underlying molecular mechanism remains to be elucidated.
In the present study, most patients presented minor facial dysmorphisms and/or extracranial anomalies. Apart from the six patients with Aicardi syndrome, one patient was diagnosed with craniofrontonasal syndrome due to a frameshift EFNB1 mutation, and two other cases presented clinical features consistent with a complex phenotype. Overall, karyotype and array-CGH studies were mostly negative (91.3%) or showed copy number variants (CNVs) of unknown significance. Craniofrontonasal syndrome (MIM 304,110) is an X-linked disorder due to mutation in ephrin-B1 gene (EFNB1), characterized paradoxically by a more severe phenotype in heterozygous females than in hemizygous males. EFNB1 encodes a transmembrane ligand for Eph receptor tyrosine kinases that is crucial for cell adhesion and axon guidance during CNS development [47]. Heterozygous females have craniofrontonasal dysplasia and possible extracranial manifestations including midline defects and skeletal abnormalities, whereas hemizygous males usually show mild features such as hypertelorism and cleft lip or palate. Interestingly, while ACC has been described in craniofrontonasal syndrome, interhemispheric cysts have never been reported before.
While abnormal neuronal migration, axonal guidance, cell division, and patterning during early CNS development are common pathomechanism among syndromic cases of interhemispheric cysts/ACC, the etiology of the non-syndromic cases, that are the majority, remains unknown.
Nowadays, it is still controversial whether the constellation of associated features seen in the ACC-interhemispheric cyst spectrum are a consequence of a primary meningeal defect that prevents commissuration and proper development of other midline structures or, rather, an epiphenomenon of a complex (acquired or genetic) defect during early development. Indeed, while noncommunicating interhemispheric cysts (often multiloculated) have been considered as extra-axial cysts due to defects of the arachnoid/neuroepithelial membrane, communicating cysts are thought to be an expansion of the diencephalic roof plate, with possible associated anterior commissure defects when falx celebri development is impaired. Accordingly, the roof of the third ventricle is displaced inferiorly and superiorly in the noncommunicating and communicating cysts, respectively [48]. However, these embryological speculations are based on a dated classification that needs to be revisited, integrating novel neuroradiogical, histopathological, and genetic data.
Limitations
This study has several limits, including the relatively small number of cases and the retrospective design. Moreover, the correlation with the 2001 Barkovich classification scheme was partial since patients with 1a and 1b cysts were not included. Finally, genetic testing with array-CGH and WES was not performed in all cases, thus limiting the possibility of genotype–phenotype correlations. Future prospective studies on larger cohort of prenatally diagnosed patients with ACC, interhemispheric cysts, and MCD are awaited to clarify the natural history and neurological outcome of this rare malformative condition.
Conclusions
Despite the underlined limitations, the strength of our study is principally related to the amount of detailed clinical, neuroradiological, and electrophysiological information. Moreover, the long-term follow-up in this population has allowed us to pinpoint some clinical relevant aspects. In particular, our findings and data from the literature indicate that, once Aicardi syndrome and other rare genetic conditions have been ruled out, ACC with intehemispheric cysts may be associated with a favorable neurological outcome with borderline or normal cognition and no major neurological signs in the majority of patients. Despite the presence of EEG abnormalities, the occurrence of epilepsy in these cases is low and usually responsive to antiepileptic drugs. A careful neuroradiologic assessment is pivotal pre- and postnatally as well as after surgery to detect associated MCD that, when multilobar and/or bilateral, may influence the neurological and epileptic phenotype. Finally, current neuroimaging classification schemes must be implemented, stratifying patients also according to clinical and genetic data, thus prompting a multidisciplinary approach to these rare malformations.
References
Paul LK, Brown WS, Adolphs R et al (2007) Agenesis of the corpus callosum: genetic, developmental and functional aspects of connectivity. Nat Rev Neurosci 8:287–299. https://doi.org/10.1038/nrn2107
Edwards TJ, Sherr EH, Barkovich AJ, Richards LJ (2014) Clinical, genetic and imaging findings identify new causes for corpus callosum development syndromes. Brain 137:1579–1613. https://doi.org/10.1093/brain/awt358
Guillem P, Fabre B, Cans C et al (2003) Trends in elective terminations of pregnancy between 1989 and 2000 in a French county (the Isère). Prenat Diagn 23:877–883. https://doi.org/10.1002/pd.711
Hetts SW, Sherr EH, Chao S et al (2006) Anomalies of the corpus callosum: an MR analysis of the phenotypic spectrum of associated malformations. AJR Am J Roentgenol 187:1343–1348. https://doi.org/10.2214/AJR.05.0146
Raybaud C, Girard N (1998) Anatomic MRI study of commissural agenesis and dysplasia of the telencephalon (agenesis of the corpus callosum and related anomalies). Clinical correlations and morphogenetic interpretation. Neurochirurgie 44:38–60
Byrd SE, Radkowski MA, Flannery A, McLone DG (1990) The clinical and radiological evaluation of absence of the corpus callosum. Eur J Radiol 10:65–73
Raybaud C (2010) The corpus callosum, the other great forebrain commissures, and the septum pellucidum: anatomy, development, and malformation. Neuroradiology 52:447–477. https://doi.org/10.1007/s00234-010-0696-3
Romaniello R, Marelli S, Giorda R et al (2017) Clinical characterization, genetics, and long-term follow-up of a large cohort of patients with agenesis of the corpus callosum. J Child Neurol 32:60–71. https://doi.org/10.1177/0883073816664668
Govil-Dalela T, Kumar A, Agarwal R, Chugani HT (2017) Agenesis of the corpus callosum and aicardi syndrome: a neuroimaging and clinical comparison. Pediatr Neurol 68:44–48.e2. https://doi.org/10.1016/j.pediatrneurol.2016.12.002
DAntonio F, Pagani G, Familiari A et al (2016) Outcomes associated with isolated agenesis of the corpus callosum: a meta-analysis. Pediatrics 138:e20160445–e20160445. https://doi.org/10.1542/peds.2016-0445
Folliot-Le Doussal L, Chadie A, Brasseur-Daudruy M et al (2018) Neurodevelopmental outcome in prenatally diagnosed isolated agenesis of the corpus callosum. Early Hum Dev 116:9–16. https://doi.org/10.1016/j.earlhumdev.2017.10.004
Al-Hashim AH, Blaser S, Raybaud C, MacGregor D (2016) Corpus callosum abnormalities: neuroradiological and clinical correlations. Dev Med Child Neurol 58:475–484. https://doi.org/10.1111/dmcn.12978
Yeh H-R, Park H-K, Kim H-J et al (2018) Neurodevelopmental outcomes in children with prenatally diagnosed corpus callosal abnormalities. Brain Dev 40:634–641. https://doi.org/10.1016/j.braindev.2018.04.012
Chadie A, Radi S, Trestard L et al (2008) Neurodevelopmental outcome in prenatally diagnosed isolated agenesis of the corpus callosum. Acta Paediatr 97:420–424. https://doi.org/10.1111/j.1651-2227.2008.00688.x
Barkovich AJ, Simon EM, Walsh CA (2001) Callosal agenesis with cyst: a better understanding and new classification. Neurology 56:220–227
Desikan RS, Barkovich AJ (2016) Malformations of cortical development. Ann Neurol 80:797–810. https://doi.org/10.1002/ana.24793
Aicardi J, Chevrie JJ, Rousselie F (1969) Spasma-in-flexion syndrome, callosal agenesis, chorioretinal abnormalities. Arch Fr Pediatr 26:1103–1120
Aicardi J (2005) Aicardi syndrome. Brain Dev 27:164–171. https://doi.org/10.1016/j.braindev.2003.11.011
Pavone P, Barone R, Baieli S et al (2005) Callosal anomalies with interhemispheric cyst: expanding the phenotype. Acta Paediatr 94:1066–1072. https://doi.org/10.1080/08035250510027372
Griebel ML, Williams JP, Russell SS et al (1995) Clinical and developmental findings in children with giant interhemispheric cysts and dysgenesis of the corpus callosum. Pediatr Neurol 13:119–124
Lena G, van Calenberg F, Genitori L, Choux M (1995) Supratentorial interhemispheric cysts associated with callosal agenesis: surgical treatment and outcome in 16 children. Childs Nerv Syst 11:568–573
Haverkamp F, Heep A, Woelfle J (2002) Psychomotor development in children with early diagnosed giant interhemispheric cysts. Dev Med Child Neurol 44:556–560
Oh KY, Kennedy AM, Selden NR et al (2012) Asymmetric ventriculomegaly, interhemispheric cyst, and dysgenesis of the corpus callosum (AVID): an imaging triad. J Ultrasound Med 31:1811–1820
Moeschler JB, Shevell M, Committee on Genetics (2014) Comprehensive evaluation of the child with intellectual disability or global developmental delays. Pediatrics 134:e903–e918. https://doi.org/10.1542/peds.2014-1839
Riou EM, Ghosh S, Francoeur E, Shevell MI (2009) Global developmental delay and its relationship to cognitive skills. Dev Med Child Neurol 51:600–606. https://doi.org/10.1111/j.1469-8749.2008.03197.x
Kwan P, Arzimanoglou A, Berg AT et al (2010) Definition of drug resistant epilepsy: consensus proposal by the ad hoc Task Force of the ILAE commission on therapeutic strategies. Epilepsia 51:1069–1077. https://doi.org/10.1111/j.1528-1167.2009.02397.x
Caldarelli M, Di Rocco C (1996) Surgical options in the treatment of interhemispheric arachnoid cysts. Surg Neurol 46:212–221
Pilu G, Falco P, Perolo A et al (1997) Differential diagnosis and outcome of fetal intracranial hypoechoic lesions: report of 21 cases. Ultrasound Obstet Gynecol 9:229–236. https://doi.org/10.1046/j.1469-0705.1997.09040229.x
Bannister C, Russell S, Rimmer S, Mowle D (1999) Fetal arachnoid cysts: their site, progress, prognosis and differential diagnosis. Eur J Pediatr Surg 9:27–28. https://doi.org/10.1055/s-2008-1072308
Tange Y, Aoki A, Mori K et al (2000) Interhemispheric glioependymal cyst associated with agenesis of the corpus callosum–case report. Neurol Med Chir (Tokyo) 40:536–542
Blaicher W, Prayer D, Kuhle S et al (2001) Combined prenatal ultrasound and magnetic resonance imaging in two fetuses with suspected arachnoid cysts. Ultrasound Obstet Gynecol 18:166–168. https://doi.org/10.1046/j.1469-0705.2001.00503.x
Cinalli G, Peretta P, Spennato P et al (2006) Neuroendoscopic management of interhemispheric cysts in children. J Neurosurg Pediatr 105:194–202. https://doi.org/10.3171/ped.2006.105.3.194
Murphy A-M, Brenner C, Ann Lynch S (2006) Agenesis of the corpus callosum with interhemispheric cyst, hepatic haemangioma and trisomy 21. Clin Dysmorphol 15:149–151. https://doi.org/10.1097/01.mcd.0000198933.16953.db
Rizk E, Awad AJ, Tubbs RS et al (2013) Dorsal third ventricular cysts revisited. Child’s Nerv Syst 29:2271–2274. https://doi.org/10.1007/s00381-012-2009-0
Dall’Asta A, van Oostrum N, Basheer S et al (2018) Etiology and prognosis of severe ventriculomegaly diagnosed at late gestation. Ultraschall der Medizin Eur J Ultrasound. https://doi.org/10.1055/a-0627-7173
Solt LC, Deck JH, Baim RS, TerBrugge K (1980) Interhemispheric cyst of neuroepithelial origin in association with partial agenesis of the corpus callosum. Case report and review of the literature. J Neurosurg 52:399–403. https://doi.org/10.3171/jns.1980.52.3.0399
Morimoto T, Kaneko M, Nishikawa R et al (1986) Ependymal cyst–case report. No Shinkei Geka 14:351–356
Ulu MO, Kafadar AM, Dashti R et al (2010) Treatment of symptomatic interhemispheric arachnoid cysts by cystoperitoneal shunting. J Clin Neurosci 17:700–705. https://doi.org/10.1016/j.jocn.2009.09.029
Fuchs F, Moutard ML, Blin G et al (2008) Prenatal and postnatal follow-up of a fetal interhemispheric arachnoid cyst with partial corpus callosum agenesis, asymmetric ventriculomegaly and localized polymicrogyria. Fetal Diagn Ther 24:385–388. https://doi.org/10.1159/000165511
Uematsu Y, Kubo K, Nishibayashi T et al (2000) Interhemispheric neuroepithelial cyst associated with agenesis of the corpus callosum. Pediatr Neurosurg 33:31–36. https://doi.org/10.1159/000028972
Inagaki H, Kurosaki M, Hori T et al (1992) [Interhemispheric choroidal epithelial cyst associated with partial agenesis of the corpus callosum: case report and review of the literature]. No Shinkei Geka 20:1301–1306
Korsic M, Jugović D, Porcnik A (2013) Endoscopic treatment of in utero diagnosed multiloculated interhemispheric cyst in a newborn: case report. Acta Clin Croat 52:119–124
Mankotia DS, Sardana H, Sinha S et al (2016) Pediatric interhemispheric arachnoid cyst: an institutional experience. J Pediatr Neurosci 11:29–34. https://doi.org/10.4103/1817-1745.181258
Fruhman G, Eble TN, Gambhir N et al (2012) Ophthalmologic findings in Aicardi syndrome. J AAPOS 16:238–241. https://doi.org/10.1016/j.jaapos.2012.01.008
Laclef C, Anselme I, Besse L et al (2015) The role of primary cilia in corpus callosum formation is mediated by production of the Gli3 repressor. Hum Mol Genet 24:4997–5014. https://doi.org/10.1093/hmg/ddv221
Putoux A, Baas D, Paschaki M et al (2018) Altered GLI3 and FGF8 signaling underlies Acrocallosal syndrome phenotypes in Kif7 depleted mice. Hum Mol Genet. https://doi.org/10.1093/hmg/ddy392
Wieland I, Jakubiczka S, Muschke P et al (2004) Mutations of the Ephrin-B1 Gene Cause Craniofrontonasal Syndrome. Am J Hum Genet 74:1209–1215. https://doi.org/10.1086/421532
Utsunomiya H, Yamashita S, Takano K et al (2006) Midline cystic malformations of the brain: imaging diagnosis and classification based on embryologic analysis. Radiat Med 24:471–481. https://doi.org/10.1007/s11604-006-0049-7
Acknowledgements
The authors thank Carola Martinetti, Radiology Unit, University of Genoa, Genoa, Italy. They are also thank the patients and family members, and the ASSACCI (Associazione Anomalie Corpo Calloso Italia) Association.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare that they have no conflict of interest.
Ethical standards
This was a double-center retrospective observational study performed at the Istituto Giannina Gaslini Children’s Hospital (Genoa, Italy) and McGill University (Montreal, Canada). The Ethical Committees of both institutions waived written parental consent due to the retrospective nature of the study. This study has been performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Uccella, S., Accogli, A., Tortora, D. et al. Dissecting the neurological phenotype in children with callosal agenesis, interhemispheric cysts and malformations of cortical development. J Neurol 266, 1167–1181 (2019). https://doi.org/10.1007/s00415-019-09247-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-019-09247-7