Abstract
Introduction
Extrapyramidal deficits are poorly characterised in amyotrophic lateral sclerosis (ALS) despite their contribution to functional disability, increased fall risk and their quality-of-life implications. Given the concomitant pyramidal and cerebellar degeneration in ALS, the clinical assessment of extrapyramidal features is particularly challenging.
Objective
The comprehensive characterisation of postural instability in ALS using standardised clinical assessments, gait analyses and computational neuroimaging tools in a prospective study design.
Methods
Parameters of gait initiation in the anticipatory postural adjustment phase (APA) and execution phase (EP) were evaluated in ALS patients with and without postural instability and healthy controls. Clinical and gait analysis parameters were interpreted in the context of brain imaging findings.
Results
ALS patients with postural instability exhibit impaired gait initiation with an altered APA phase, poor dynamic postural control and significantly decreased braking index. Consistent with their clinical profile, “unsteady” ALS patients have reduced caudate and brain stem volumes compared to “steady” ALS patients.
Interpretation
Our findings highlight that the ALS functional rating scale (ALSFRS-r) does not account for extrapyramidal deficits, which are major contributors to gait impairment in a subset of ALS patients. Basal ganglia degeneration in ALS does not only contribute to cognitive and behavioural deficits, but also adds to the heterogeneity of motor disability.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Amyotrophic lateral sclerosis (ALS) is a relentlessly progressive neurodegenerative disorder with no effective disease-modifying therapies at present [1]. Functional rating scales [2], electrophysiology studies [3] and neuroimaging studies of ALS [4] overwhelmingly focus on upper and lower motor neuron degeneration. ALS, however, is now widely recognised as a multisystem condition affecting extra-motor frontotemporal regions [5], cerebellar [6] and subcortical grey matter regions [7]. Backward falls, impaired postural reflexes, retropulsion, bradykinesia, and decreased arm swing in ALS have all been reported in early-stage ALS and are often linked to basal ganglia alterations [8]. While extrapyramidal deficits are relatively often observed in ALS [9], they are difficult to appraise clinically due to concomitant pyramidal weakness, lower motor neuron involvement, vestibular [10] and cerebellar deficits [11]. Gait impairment in ALS [12], especially if coupled with cognitive deficits presents a considerable risk for falls and fractures [13,14,15]. Postural instability contributes significantly to the heterogeneity of clinical disability in ALS, requiring specific rehabilitation and physiotherapy strategies [16] and careful appraisal of fall risk [17]. Despite its considerable clinical ramifications, the pathophysiology of gait impairment in ALS remains poorly characterised. The coexistence of cerebellar, vestibular, pyramidal and extra-pyramidal processes precludes the accurate clinical evaluation of gait impairment in ALS and requires quantitative gait assessment approaches. Computational gait analyses have contributed to the characterisation of gait pathology in Parkinson’s disease (PD) [18], and gait rhythm alterations and stride length fluctuations have also been described in ALS [19]. The methods for evaluating anticipatory postural adjustments [20, 21] are well established and have been applied to a variety of neurological conditions [22, 23].
Given the striking paucity of gait analysis studies in ALS, our objective is to evaluate gait impairment in ALS using a multimodal approach, including standardised clinical assessments, kinematic recordings of gait initiation and brain imaging. Based on the available literature, our hypothesis is that ALS patients with postural instability (PI-ALS) exhibit altered gait initiation and have distinctive imaging signatures compared to ALS patients without gait impairment (NPI-ALS).
Materials and methods
Participants
Thirty-one ALS patients and 14 age-matched healthy controls were recruited in a prospective gait analysis and neuroimaging study. All participants provided informed written consent in accordance with the Declaration of Helsinki and the study was approved by the local ethics committee (CPP Ile-de-France Paris VI; INSERM promotion RBM C12-13).
Patients underwent standardised clinical assessments, gait analyses, neuropsychological testing and neuroimaging. The demographic and clinical details of study participants, including site-of-onset, cognitive performance, functional rating scale scores, handedness, age, and gender are presented in Table 1. Inclusion criteria included definite or probable ALS according to the revised El Escorial criteria [24], age between 18 and 70 years, right-handedness, ability to walk at least 10 m without assistance, and ability to tolerate MR scanning in a supine position for the duration of the T1-weighted structural MR sequence. Exclusion criteria included frontotemporal dementia based on current diagnostic criteria [25], coexisting musculoskeletal conditions that interfere with functional evaluation, patients on medications that would potentially affect gait analyses such as dopamine antagonist, benzodiazepines, opiates and tricyclic antidepressants.
Amyotrophic lateral sclerosis patients were stratified in PI-ALS and NPI-ALS groups, based on the pull test [26] during a standardised neurological assessment by an experienced neurologist. The patient’s shoulders were pulled abruptly while standing in an upright position with eyes open and feet slightly apart. The observed response was scored from 0 to 4 points (0: normal response; 1: retropulsion, but unaided recovery; 2: absence of postural response and would fall if not caught by the examiner; 3: very unstable, tends to lose balance spontaneously; 4: unable to stand without assistance). A score of 1 or more was considered abnormal and ALS patients were then classified as having postural instability (PI-ALS) clinically.
Clinical evaluation
Each subject was administered the revised ALS Functional Rating Scale (ALSFRS-R) as a measure of their overall motor disability [2]. Progression rates were calculated using the following formula: disease progression rate = (48 − ALSFRS-R score)/(disease duration in months).
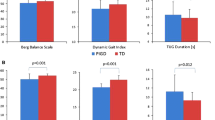
Lower limb muscle strength was tested manually using the MRC muscle scale in the hip flexors, knee extensors, hip abductors, knee flexors, ankle plantar flexors, ankle dorsiflexors, and extensor hallucis longus. Stiffness during passive leg, arm and neck flexion–extension was evaluated using the Ashworth scale [27]. Balance impairment was assessed using the Berg Balance Scale [28].
All participants underwent a comprehensive neuropsychological assessment using the California verbal learning test (CVLT II), the Stroop test, verbal fluency tests (both categorical and phonemic), the Wisconsin card sorting test, and the forward and backward digit span.
Gait initiation test
Biomechanical parameters of gait initiation were recorded using a force platform (0.9 × 1.8 m, AMTI, Advanced Mechanical Technology Inc. Watertown, MA, USA) with analogue signals digitized at 1000 Hz. Subjects, who were barefoot and standing upright, were instructed to start walking for 5 metres following a visual cue projected onto a screen in front of them at a distance of 6 metres at eye level. Twenty consecutive trials were recorded for each subject. Assessment took place under standardised conditions, using the same equipment, supervised by the same clinical staff. The high respiratory ALSFRS-r subscores in both ALS group indicate that fatigue secondary to hypoxia was not a major confounder.
The accelerations and velocities of the centre of mass (CoM) and centre of foot pressure (CoP) displacement of the first two steps were calculated offline (Fig. 1). Data were processed using in-house software running under MATLAB (The MathWorks, Inc., Natick, MA, USA). Two phases of gait initiation were analysed: the anticipatory postural adjustment phase (APA) and the execution phase (EP). The anticipatory postural adjustment phase (APA) is the period between the first biomechanical event [t0] and the foot-off of the swing leg [FO]. The execution phase (EP) is defined as the period between the foot-off of the swing leg and the foot-off of the stance leg [FO2].
Gait initiation process in a representative control subject and an ALS patient with postural instability. The traces represent, from top to bottom: VZg (m s−1): vertical velocity of the centre of mass (CoM); VXg (m s−1): anterior–posterior velocity of the CoM; Xg (m): anterior–posterior displacement of the CoM; Yp (mm): medial–lateral displacement of the centre of foot pressure (CP); Xp (mm): anterior–posterior displacement of the CP. T0 (s): time of the onset of the first mechanical phenomena; TTO (s): time of toe-off of the swing leg; TTO2 (s): time of toe-off of the standing leg; APA duration (s): time between TTO and T0; Min Xp (mm): peak magnitude of the backward displacement of the CP; L (m): step length; Vm (m s− 1): peak progression velocity of the CoM; V1 (m s−1): minimum negative vertical velocity of the CoM; V2 (m s−1): vertical velocity of the CoM at time of foot contact TFC (s); PI-ALS: ALS patients with postural instability
During APA, anteroposterior and lateral displacements of the CoP were measured, as well as the duration of this phase. During EP, the length (L) and velocity (Vm) at the end of the first step were recorded.
The measurement of vertical CoM velocity allows the calculation of the braking index, which reflects the dynamic balance control during step execution and is, therefore, a proxy of postural instability. During the swing phase, the vertical CoM velocity, measured in m/s, follows a V shape with a fall (V1) and a reduction of this fall before the foot touches the ground (V2). The braking index is calculated as follows: (V1 − V2)/V1 × 100 (Fig. 1). A braking index less than 25% is considered abnormal, reflecting impaired active postural control during gait initiation.
Magnetic resonance imaging data acquisition
Magnetic resonance imaging data were acquired on a 3.0 T Siemens platform, using a 32-channel head coil. Each examination included the acquisition of sagittal 3D T1-weighted anatomical images using a magnetization-prepared rapid acquisition with gradient echo (MP-RAGE) sequence (repetition time/echo time = 2300/4.2 ms, inversion time = 900 ms, flip angle = 9°, averages = 1, field of view = 256 × 248 × 176, isotropic 1 × 1 × 1 mm voxel size).
Statistical analysis
Kinematics parameters of gait initiation
The average values of the gait initiation parameters were calculated for each subject based on 20 trials, and the median values and interquartile for ALS patients with and without postural instability, and controls were then calculated. Gait initiation parameters were compared between controls, ALS patients with postural instability (PI-ALS) and ALS patients without postural instability (No PI-ALS) using the nonparametric Kruskall–Wallis test. Pairs of means were compared using the Mann–Whitney test where significant effects were identified. Post hoc Bonferroni corrections were used for each pairwise comparison to adjust for the family-wise error (FWE). Statistical threshold was set at p < 0.05. Analyses of gait parameters were performed with the Statistica® software (StatSoft, Inc).
Magnetic resonance imaging
Basal ganglia volumetric analyses
Volumes of subcortical structures were estimated using the subcortical segmentation and registration tool FIRST [29] of the FMRIB’s Software Library (FSL) as described previously [7]. Seven subcortical grey matter structures were evaluated: the thalamus, caudate nucleus, hippocampus, amygdala, nucleus accumbens, putamen, and pallidum. Segmentation and volumetric estimation were also performed for the brain stem. A two-stage affine registration approach was used to register raw T1 data to the Montreal Neurological Institute standard space (MNI152) and a model-based approach was then utilised for the segmentation of subcortical structures. Subcortical mesh and volumetric outputs were generated following automatic boundary corrections. Comparative statistics were carried out with IBM SPSS Statistics Version 22. Analyses of covariance (ANCOVA) were used to compare volumes of subcortical structures between study groups. Group membership was used as the categorical independent variable, volumes of subcortical grey matter structures were included as dependent variables, and age at the time of MRI scan, and gender were used as covariates. Boxplots of volumes and plots of estimated marginal means of volumes were generated to illustrate intergroup volumetric differences. For subcortical structures with significant intergroup differences, pairwise Sidak corrected post hoc comparisons were performed. A p value ≤ 0.05 was considered significant.
Morphometric analyses
Voxel-based morphometry analyses were carried out to compare grey matter density patterns between the study groups using FSL [30]. Following brain extraction and tissue-type segmentation, grey matter partial-volume images were aligned to the MNI152 standard space using affine registration. The grey matter partial-volume estimates were subsequently non-linearly co-registered to a study-specific template, modulated by a Jacobian field warp and smoothed with an isotropic Gaussian kernel with a sigma of 3 mm. The threshold-free cluster-enhancement (TFCE) method [31] and permutation-based nonparametric inference were used for group comparisons controlling for age and gender [32, 33]. The FWE-corrected statistical significance was set at p < 0.05.
Results
Clinical features
Key clinical, demographic and neuropsychological variables are presented in Table 1. Based on the standardised clinical examination, 14 ALS patients had postural instability (PI-ALS) and 17 showed no clinical signs of postural instability (NPI-ALS). No significant differences were identified between the two groups in age, functional disability, ALSFRS-R subscores, disease duration, progression rates, lower limb muscle strength and cognitive performance. The modified Ashworth scale of the lower limbs was significantly higher and the Berg Balance scale significantly lower in PI-ALS patients than in NPI-ALS patients (Table 2).
Gait initiation recordings
During the APA, the posterior displacement of the CoP was significantly lower and the duration of this phase significantly longer in PI-ALS patients than in both NPI-ALS patients and healthy controls (Table 3). There was no significant difference in these parameters between NPI-ALS patients and controls.
During the EP, the velocity and length of the first step were significantly reduced in PI-ALS patients compared to both NPI-ALS patients and controls (Table 3). Similarly to the observations in APA, the parameters of EP were not significantly different between NPI-ALS patients and healthy controls.
In 4 out of 14 PI-ALS patients, the stride length was less than 35 cm with no change in CoM, and thus no detectable active braking. In the ten remaining PI-ALS patients, the mean braking index was significantly reduced with a braking index close to zero in all patients. In NPI-ALS patients, the mean braking index was also reduced compared to healthy controls (Table 3).
The volumetric profile of subcortical regions
PI-ALS patients exhibited significant reductions in brain stem volume compared to NPI-ALS patients (p = 0.049) as illustrated in Fig. 2. Interestingly, NPI-ALS patients had significantly increased caudate nucleus volumes compared to controls (p = 0.031 left, p = 0.04 right) which may be interpreted as a compensatory or adaptive mechanism as PI-ALS patients also exhibited caudate hypertrophy compared to controls, but to a lesser extent. Figure 2. Intergroup differences in the volumes of other subcortical structures did not reach statistical significance. Raw volumetric data are presented in Table 4.
Volumetric differences in the brain stem and caudate nuclei between study groups. Boxplots of volumes (left) and plots of estimated marginal means adjusted for age (right) are shown to illustrate intergroup differences. Green colour represents healthy controls (HC), blue colour shows no PI-ALS patients and orange colour shows PI-ALS patients with. P value indicates intergroup ANCOVA outcome correcting for age and gender
Standard, voxel-based morphometry analyses did not identify cortical density reductions in either ALS group compared to healthy controls. The direct comparison of the two ALS groups did not identify cortical grey matter differences either.
Discussion
Postural instability and gait impairment is one of the least characterised aspects of ALS which is difficult to appraise by clinical examination alone due to concomitant pyramidal degeneration. Despite sporadic reports [8, 9], extra-pyramidal features of ALS are understudied and formal gait analyses are hardly ever carried out. As per best practice recommendations, ALS is diagnosed and managed by specialist multidisciplinary teams focussing on the motor, nutritional, respiratory and cognitive aspects of the condition and a movement disorder perspective is seldom adopted. Our study demonstrates that ALS patients with postural instability have an abnormal preparatory phase to gait initiation with reduced stride length and velocity, and impaired balance control during the execution phase. Based on quantitative MRI metrics brain stem and caudate volume reductions distinguish PI-ALS patients from NPI-ALS patients. We did not identify any cortical changes in the cerebellum, motor cortex or supplementary motor cortex, suggesting that the extrapyramidal deficits observed clinically are not confounded by cerebellar or motor cortex pathology.
Our findings highlight considerable functional and structural extrapyramidal involvement in ALS. The systematic clinical and instrumental evaluation of gait impairment in ALS is not only relevant for fall prevention and individualised rehabilitation, but potentially signals alternative therapeutic strategies. There are anecdotal accounts of initiating DOPA agonists and MAO inhibitors in ALS patients with Parkinsonian features, but no established evidence exists for effective pharmacological fall prevention or gait improvement. Additionally, while DAT scans are routinely used in atypical Parkinson presentations, they are seldom considered in ALS. Overlap syndromes of ALS-like and PD features are not infrequent and encompass a variety of manifestations [30]. Specific phenotypes such as the lytico–bodig syndrome have been linked to reduced striatal 18F-6-fluorodopa uptake [31]. Dopaminergic deficits, however, have been consistently shown in ALS beyond what would be expected by the incidence profile of the two neurogenerative condition [32, 33]. A few number of non-PET imaging studies have looked specifically into basal ganglia degeneration in ALS to explore the underpinnings of extrapyramidal features. Among these, a pioneering magnetic resonance spectroscopy (MRS) study captured reduced N-acetylaspartate (Naa)/choline (Ch) ratio in the caudate, lentiform nucleus and thalamus and associations with clinical measures [34]. Subsequent large whole-brain MRS studies confirmed reduced Naa/Cr ratios in the caudate nucleus [35]. Alternative imaging approaches such as transcranial B-mode sonography have also been used to explore extra-pyramidal degeneration in ALS and substantia nigra hyperechogenicity was reported by multiple studies [36, 37]. Substantia nigra degeneration is a relatively established pathological feature of ALS and has been linked to fast progression rates [38]. The pars compacta of the substantia nigra and the caudate nuclei are recognised sites to pTDP-43 pathology in ALS [39].
The notion of shared genetic susceptibility between PD and ALS has been proposed by family aggregation studies [40, 41] and more recently has been also linked to Ataxin-2 mutations [42]. From an evolutionary perspective, shared phylogenetic factors have been proposed for the aetiology of both ALS and PD, implicating the vulnerability of recently developed brain structures [43, 44].
We observed altered gait initiation in ALS patients with postural instability similar to those observed in Parkinson disease [45]. The strikingly reduced stride length and CoM velocity could stem from reduced lower limb muscle strength during the execution of the first step. However, manual muscle testing revealed no significant difference between the two ALS groups. While the two ALS groups were comparable in their overall functional disability as reflected by ALSFRS-R, patients with postural impairment exhibited increased tone, which may contribute to the observed gait impairment. Increased muscle tone in ALS patients with postural instability may impede propelling the body forward and supporting the leg during gait initiation. ALS patients with postural instability also exhibited reduced dynamic postural control, with an absence of active braking. Similarly to patients with axial rigidity, such as progressive supranuclear palsy, the fall of the CoM during the execution phase of gait initiation is mechanically stopped when the foot hits the ground without active control [46, 47]. Passive braking of the CoM before foot contact could be interpreted as a feature of balance impairment in our ALS group.
Considerable APA abnormalities were detected in PI-ALS patients despite comparable functional disability (ALSFRS-R), cognitive performance, disease duration, and muscle strength to the NPI-ALS group. This observation confirms that APA parameters are not solely driven by motor impairments. APA impairment may be linked to increased tone in the lower limbs as it is responsible for moving the CoM both to the side to transfer the body weight to the supporting leg and forward to start walking [47]. The prolonged anticipation phase and the reduction of APA magnitude may also reflect an adaptive strategy allowing superior gait control to avoid falling. The adaptation of a stooped posture during gait initiation could also contribute to our findings, as similarly to PD patients, PI-ALS patients were inclined forward and were more likely to stand on their toes than no PI-ALS patients.
Interestingly, we identified increased caudate nucleus (CN) volume in no PI-ALS ALS patients compared with both PI-ALS patients and controls and PI-ALS patients did not differ significantly from controls. The CN is heavily involved in balance control [48] and gait initiation [49] in humans, in particular for shifting the CoM forward [50]. The caudate nucleus also relays high cortical processes such as integrating sensory information with appropriate behavioural responses. A recent magnetoencephalography (MEG) study has shown that the caudate nucleus is involved in anticipatory postural adjustments, limbs posture and speed of target-oriented movements [51]. Data from both functional MRI and MEG studies confirm the integrative role of the CN in movement preparation and lesion studies also highlight the role of the caudate nucleus in posture control [52].
Our findings suggest that the higher CN volume in no PI-ALS patients compared to PI-ALS patients is likely to contribute to superior APA performance. While the increased GM volume in the no-PI group compared to controls should only be cautiously interpreted as a compensatory mechanism, it is likely that the low caudate volumes in the PI group can be linked to poorer APA performance. Our findings also showcase that PI-ALS patients not only exhibit APA deficits, but also a decreased CoM velocity, shorter first step and poor dynamic postural control.
Basal ganglia degeneration in ALS has been linked to cognitive deficits [53], C9orf72 hexanucleotide repeats [7], motor disability [54], but has not been specifically linked to extrapyramidal deficits to date. Our two ALS groups, which are matched for motor disability and cognitive performance, and have no evidence of motor cortex, supplementary motor cortex or cerebellar involvement, exhibit distinctly different basal ganglia profiles which are likely to explain the observed extrapyramidal deficits.
This study is not without limitations. Patient stratification was based on clinical evaluation, which is a common approach in PD studies, but alternative approaches such as cluster analysis of patients based on gait parameters could have been also considered. While the interrater reliability of the pull test is often regarded as suboptimal [55], in our study the same neurologist (PFP) assessed all the patients ensuring standardised assessments. Additional clinical tests such as the push and release test may have been desirable [56]. PI-ALS patients exhibited impaired braking, and while the breaking index of NPI-ALS patients was in the normal range, it was significantly lower than the breaking index of healthy controls. These findings suggest that our patients were appropriately classified, and homogeneous groups were defined on clinical grounds. The inclusion of ALS patients who could tolerate MR scanning led to the selection of patients with no significant respiratory insufficiency. (Table 1.) This is a common selection bias of all ALS imaging studies, where patients without orthopnoea tend to be overrepresented [57].
Supplementary imaging analyses would have been of interest, but the main focus and novelty of this paper was to present detailed gait analyses in clinically well-characterised patients to draw attention to extrapyramidal deficits in ALS. Another obvious limitation of this study is the sample size, which despite its relatively small size confirms considerable extrapyramidal deficits. Based on our proof of concept study, future directions can be outlined to assess the extrapyramidal profile of ALS in more detail. Future research strategies would include the targeted genetic screening of ALS patients with gait impairment, combined PET-MR studies, connectivity-based segmentation of basal ganglia structures, [58] inclusion of disease controls [57] such as PD patients without ALS and longitudinal study designs [54, 59].
Conclusions
We demonstrate considerable extrapyramidal deficits in a small group of clinically well-characterised ALS patients using standardised clinical, gait and imaging assessments. Extrapyramidal deficits in ALS are important contributors of disease heterogeneity and have considerable impact on fall risk, rehabilitation efforts, individualised pharmacological treatment and quality of life. The comprehensive assessment of extrapyramidal deficits should be incorporated in routine clinical evaluation and also included in future clinical trials of ALS.
References
Turner MR, Hardiman O, Benatar M, Brooks BR, Chio A, de Carvalho M, Ince PG, Lin C, Miller RG, Mitsumoto H, Nicholson G, Ravits J, Shaw PJ, Swash M, Talbot K, Traynor BJ, Van den Berg LH, Veldink JH, Vucic S, Kiernan MC (2013) Controversies and priorities in amyotrophic lateral sclerosis. Lancet Neurol 12:310–322
Cedarbaum JM, Stambler N, Malta E, Fuller C, Hilt D, Thurmond B, Nakanishi A, Grp BAS (1999) The ALSFRS-R: a revised ALS functional rating scale that incorporates assessments of respiratory function. J Neurol Sci 169:13–21
de Carvalho M, Chio A, Dengler R, Hecht M, Weber M, Swash M (2005) Neurophysiological measures in amyotrophic lateral sclerosis: markers of progression in clinical trials. Amyotroph Lateral Scler Other Motor Neuron Disord 6:17–28
Bede P, Bokde A, Elamin M, Byrne S, McLaughlin RL, Jordan N, Hampel H, Gallagher L, Lynch C, Fagan AJ, Pender N, Hardiman O (2013) Grey matter correlates of clinical variables in amyotrophic lateral sclerosis (ALS): a neuroimaging study of ALS motor phenotype heterogeneity and cortical focality. J Neurol Neurosurg Psychiatry 84:766–773
Phukan J, Elamin M, Bede P, Jordan N, Gallagher L, Byrne S, Lynch C, Pender N, Hardiman O (2012) The syndrome of cognitive impairment in amyotrophic lateral sclerosis: a population-based study. J Neurol Neurosurg Psychiatry 83:102–108
Bede P, Elamin M, Byrne S, McLaughlin RL, Kenna K, Vajda A, Fagan A, Bradley DG, Hardiman O (2015) Patterns of cerebral and cerebellar white matter degeneration in ALS. J Neurol Neurosurg Psychiatry 86:468–470
Bede P, Elamin M, Byrne S, McLaughlin RL, Kenna K, Vajda A, Pender N, Bradley DG, Hardiman O (2013) Basal ganglia involvement in amyotrophic lateral sclerosis. Neurology 81:2107–2115
Desai J, Swash M (1999) Extrapyramidal involvement in amyotrophic lateral sclerosis: backward falls and retropulsion. J Neurol Neurosurg Psychiatry 67:214–216
Pradat P-F, Bruneteau G, Munerati E, Salachas F, Le Forestier N, Lacomblez L, Lenglet T, Meininger V (2009) Extrapyramidal stiffness in patients with amyotrophic lateral sclerosis. Mov Disord 24:2143–2148
Sanjak M, Hirsch MA, Bravver EK, Bockenek WL, Norton HJ, Brooks BR (2014) Vestibular deficits leading to disequilibrium and falls in ambulatory amyotrophic lateral sclerosis. Arch Phys Med Rehabil 95:1933–1939
Prell T, Grosskreutz J (2013) The involvement of the cerebellum in amyotrophic lateral sclerosis. Amyotroph Lateral Scler Frontotemporal Degener 14:507–515
Radovanovic S, Milicev M, Peric S, Basta I, Kostic V, Stevic Z (2014) Gait in amyotrophic lateral sclerosis: is gait pattern differently affected in spinal and bulbar onset of the disease during dual task walking? Amyotroph Lateral Scler Frontotemporal Degener 15:488–493
Elamin M, Bede P, Byrne S, Jordan N, Gallagher L, Wynne B, O’Brien C, Phukan J, Lynch C, Pender N (2013) Cognitive changes predict functional decline in ALS. A population-based longitudinal study. Neurology 80:1590–1597
Elamin M, Phukan J, Bede P, Jordan N, Byrne S, Pender N, Hardiman O (2011) Executive dysfunction is a negative prognostic indicator in patients with ALS without dementia. Neurology 76:1263–1269
Olney RK, Murphy J, Forshew D, Garwood E, Miller BL, Langmore S, Kohn MA, Lomen-Hoerth C (2005) The effects of executive and behavioral dysfunction on the course of ALS. Neurology 65:1774–1777
Majmudar S, Wu J, Paganoni S (2014) Rehabilitation in amyotrophic lateral sclerosis: why it matters. Muscle Nerve 50:4–13
Montes J, Cheng B, Diamond B, Doorish C, Mitsumoto H, Gordon PH (2007) The Timed Up and Go test: predicting falls in ALS. Amyotroph Lateral Sclerosis 8:292–295
Peterson DS, Horak FB (2016) Neural control of walking in people with Parkinsonism. Physiology (Bethesda Md) 31:95–107
Hausdorff JM, Lertratanakul A, Cudkowicz ME, Peterson AL, Kaliton D, Goldberger AL (2000) Dynamic markers of altered gait rhythm in amyotrophic lateral sclerosis. J Appl Physiol (Bethesda, Md: 1985) 88:2045–2053
Lepers R, Breniere Y (1995) The role of anticipatory postural adjustments and gravity in gait initiation. Exp Brain Res 107:118–124
Anand M, Seipel J, Rietdyk S (2017) A modelling approach to the dynamics of gait initiation. J R Soc Interface 14:20170043
Isaias IU, Dipaola M, Michi M, Marzegan A, Volkmann J, Rodocanachi Roidi ML, Frigo CA, Cavallari P (2014) Gait initiation in children with Rett syndrome. PLoS One 9:e92736
Welter ML, Do MC, Chastan N, Torny F, Bloch F, du Montcel ST, Agid Y (2007) Control of vertical components of gait during initiation of walking in normal adults and patients with progressive supranuclear palsy. Gait posture 26:393–399
Brooks BR, Miller RG, Swash M, Munsat TL, World Federation of Neurology Research Group on Motor Neuron D (2000) El Escorial revisited: revised criteria for the diagnosis of amyotrophic lateral sclerosis. Amyotroph Lateral Scler Other Motor Neuron Disord 1:293–299
Rascovsky K, Hodges JR, Knopman D, Mendez MF, Kramer JH, Neuhaus J, van Swieten JC, Seelaar H, Dopper EGP, Onyike CU, Hillis AE, Josephs KA, Boeve BF, Kertesz A, Seeley WW, Rankin KP, Johnson JK, Gorno-Tempini ML, Rosen H, Prioleau-Latham CE, Lee A, Kipps CM, Lillo P, Piguet O, Rohrer JD, Rossor MN, Warren JD, Fox NC, Galasko D, Salmon DP, Black SE, Mesulam M, Weintraub S, Dickerson BC, Diehl-Schmid J, Pasquier F, Deramecourt V, Lebert F, Pijnenburg Y, Chow TW, Manes F, Grafman J, Cappa SF, Freedman M, Grossman M, Miller BL (2011) Sensitivity of revised diagnostic criteria for the behavioural variant of frontotemporal dementia. Brain J Neurol 134:2456–2477
Movement Disorder Society Task Force on Rating Scales for Parkinson’s Disease (2003) The Unified Parkinson’s Disease Rating Scale (UPDRS): status and recommendations. Mov Disord 18:738–750
Bohannon RW, Smith MB (1987) Interrater reliability of a modified Ashworth scale of muscle spasticity. Phys Ther 67:206–207
Berg K, Wood-Dauphinee S, Williams JI (1995) The Balance Scale: reliability assessment with elderly residents and patients with an acute stroke. Scand J Rehabil Med 27:27–36
Patenaude B, Smith SM, Kennedy DN, Jenkinson M (2011) A Bayesian model of shape and appearance for subcortical brain segmentation. NeuroImage 56:907–922
Gilbert RM, Fahn S, Mitsumoto H, Rowland LP (2010) Parkinsonism and motor neuron diseases: twenty-seven patients with diverse overlap syndromes. Mov Disord 25:1868–1875
Snow BJ, Peppard RF, Guttman M, Okada J, Martin WR, Steele J, Eisen A, Carr G, Schoenberg B, Calne D (1990) Positron emission tomographic scanning demonstrates a presynaptic dopaminergic lesion in Lytico-Bodig. The amyotrophic lateral sclerosis-parkinsonism-dementia complex of Guam. Arch Neurol 47:870–874
Takahashi H, Snow BJ, Bhatt MH, Peppard R, Eisen A, Calne DB (1993) Evidence for a dopaminergic deficit in sporadic amyotrophic lateral sclerosis on positron emission scanning. Lancet 342:1016–1018
Borasio GD, Linke R, Schwarz J, Schlamp V, Abel A, Mozley PD, Tatsch K (1998) Dopaminergic deficit in amyotrophic lateral sclerosis assessed with [I-123] IPT single photon emission computed tomography. J Neurol Neurosurg Psychiatry 65:263–265
Sharma KR, Saigal G, Maudsley AA, Govind V (2011) 1H MRS of basal ganglia and thalamus in amyotrophic lateral sclerosis. NMR Biomed 24:1270–1276
Verma G, Woo JH, Chawla S, Wang S, Sheriff S, Elman LB, McCluskey LF, Grossman M, Melhem ER, Maudsley AA, Poptani H (2013) Whole-brain analysis of amyotrophic lateral sclerosis by using echo-planar spectroscopic imaging. Radiology 267:851–857
Fathinia P, Hermann A, Reuner U, Kassubek J, Storch A, Ludolph AC (2013) Parkinson’s disease-like midbrain hyperechogenicity is frequent in amyotrophic lateral sclerosis. J Neurol 260:454–457
Prell T, Schenk A, Witte OW, Grosskreutz J, Gunther A (2014) Transcranial brainstem sonography as a diagnostic tool for amyotrophic lateral sclerosis. Amyotroph Lateral Scler Frontotemporal Degener 15:244–249
Kato S, Oda M, Tanabe H (1993) Diminution of dopaminergic neurons in the substantia nigra of sporadic amyotrophic lateral sclerosis. Neuropathol Appl Neurobiol 19:300–304
Brettschneider J, Arai K, Del Tredici K, Toledo JB, Robinson JL, Lee EB, Kuwabara S, Shibuya K, Irwin DJ, Fang L, Van Deerlin VM, Elman L, McCluskey L, Ludolph AC, Lee VM, Braak H, Trojanowski JQ (2014) TDP-43 pathology and neuronal loss in amyotrophic lateral sclerosis spinal cord. Acta Neuropathol 128:423–437
Majoor-Krakauer D, Ottman R, Johnson WG, Rowland LP (1994) Familial aggregation of amyotrophic lateral sclerosis, dementia, and Parkinson’s disease: evidence of shared genetic susceptibility. Neurology 44:1872–1877
Longinetti E, Mariosa D, Larsson H, Ye W, Ingre C, Almqvist C, Lichtenstein P, Piehl F, Fang F (2017) Neurodegenerative and psychiatric diseases among families with amyotrophic lateral sclerosis. Neurology 89:578–585
Elden AC, Kim HJ, Hart MP, Chen-Plotkin AS, Johnson BS, Fang X, Armakola M, Geser F, Greene R, Lu MM, Padmanabhan A, Clay-Falcone D, McCluskey L, Elman L, Juhr D, Gruber PJ, Rub U, Auburger G, Trojanowski JQ, Lee VM, Van Deerlin VM, Bonini NM, Gitler AD (2010) Ataxin-2 intermediate-length polyglutamine expansions are associated with increased risk for ALS. Nature 466:1069–1075
Eisen A, Calne D (1992) Amyotrophic lateral sclerosis, Parkinson’s disease and Alzheimer’s disease: phylogenetic disorders of the human neocortex sharing many characteristics. Can J Neurol Sci 19:117–123
Eisen A, Turner MR, Lemon R (2014) Tools and talk: an evolutionary perspective on the functional deficits associated with amyotrophic lateral sclerosis. Muscle Nerve 49:469–477
Maillet A, Pollak P, Debu B (2012) Imaging gait disorders in parkinsonism: a review. J Neurol Neurosurg Psychiatry 83:986–993
la Fougere C, Zwergal A, Rominger A, Forster S, Fesl G, Dieterich M, Brandt T, Strupp M, Bartenstein P, Jahn K (2010) Real versus imagined locomotion: a [18F]-FDG PET-fMRI comparison. NeuroImage 50:1589–1598
Karim HT, Sparto PJ, Aizenstein HJ, Furman JM, Huppert TJ, Erickson KI, Loughlin PJ (2014) Functional MR imaging of a simulated balance task. Brain Res 1555:20–27
Visser JE, Bloem BR (2005) Role of the basal ganglia in balance control. Neural Plast 12:161–174 (discussion 263–272)
Zhang L, Li TN, Yuan YS, Jiang SM, Tong Q, Wang M, Wang JW, Chen HJ, Ding J, Xu QR, Zhang KZ (2016) The neural basis of postural instability gait disorder subtype of Parkinson’s disease: A PET and fMRI Study. CNS Neurosci Ther 22:360–367
Wagner J, Stephan T, Kalla R, Bruckmann H, Strupp M, Brandt T, Jahn K (2008) Mind the bend: cerebral activations associated with mental imagery of walking along a curved path. Exp Brain Res 191:247–255
Ng TH, Sowman PF, Brock J, Johnson BW (2013) Neuromagnetic brain activity associated with anticipatory postural adjustments for bimanual load lifting. NeuroImage 66:343–352
Takakusaki K (2017) Functional neuroanatomy for posture and gait control. J Mov Disord 10:1–17
Machts J, Loewe K, Kaufmann J, Jakubiczka S, Abdulla S, Petri S, Dengler R, Heinze HJ, Vielhaber S, Schoenfeld MA, Bede P (2015) Basal ganglia pathology in ALS is associated with neuropsychological deficits. Neurol 85:1301–1309
Westeneng HJ, Verstraete E, Walhout R, Schmidt R, Hendrikse J, Veldink JH, van den Heuvel MP, van den Berg LH (2015) Subcortical structures in amyotrophic lateral sclerosis. Neurobiol Aging 36:1075–1082
Munhoz RP, Li JY, Kurtinecz M, Piboolnurak P, Constantino A, Fahn S, Lang AE (2004) Evaluation of the pull test technique in assessing postural instability in Parkinson’s disease. Neurology 62:125–127
Jacobs JV, Horak FB, Van Tran K, Nutt JG (2006) An alternative clinical postural stability test for patients with Parkinson’s disease. J Neurol 253:1404–1413
Bede P, Hardiman O (2014) Lessons of ALS imaging: Pitfalls and future directions—a critical review. NeuroImage Clin 4:436–443
Bede P, Omer T, Finegan E, Chipika RH, Iyer PM, Doherty MA, Vajda A, Pender N, McLaughlin RL, Hutchinson S, Hardiman O (2018) Connectivity-based characterisation of subcortical grey matter pathology in frontotemporal dementia and ALS: a multimodal neuroimaging study. Brain Imaging Behav. https://doi.org/10.1007/s11682-018-9837-9
Bede P, Hardiman O (2018) Longitudinal structural changes in ALS: a three time-point imaging study of white and gray matter degeneration. Amyotroph Lateral Scler Frontotemporal Degener 19:232–241
Acknowledgements
The authors are grateful for the generosity and kindness of all participating patients and healthy controls. We also thank Xavier Devrelle, Sophien Mehdi, and Sara Fernandez-Vidal (ICM Foundation) for their assistance in data recording. We thank the Center for Clinical Investigation (Instititut du Cerveau et de la Moellle Epiniere, Paris, France) and Vanessa Brochard for their role in the organisation and management of the study.
Funding
This study was funded by a grant from the Association for Research on ALS (ARSLA) and the Institut National pour la Santé et la Recherche Médicale (INSERM). The research leading to these results has also received support from the programme ‘‘Investissements d’avenir’’ ANR-10-IAIHU-06.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
The authors have no actual or potential conflict of interest to disclose, including any financial, personal, or other relationships with other individuals or organisations within 3 years of beginning the submitted work that could inappropriately influence, or be perceived to influence, their work. Marie-Laure Welter received research support from the ‘Institut du Cerveau et de la Moelle. Epinière’ (ICM) Foundation and the Agence Nationale de la Recherche. Nicolas Termoz received research support from the Laboratoire CeRSM—EA 2931. Peter Bede is supported by the Health Research Board (HRB—Ireland; HRB EIA-2017-019), the Irish Institute of Clinical Neuroscience IICN—Novartis Ireland Research (IICN—2016), the Iris O’Brien Foundation, the Perrigo Clinician-Scientist Research Fellowship, and the Research Motor Neuron (RMN-Ireland) Foundation Ireland. Pierre-François Pradat received research support from the French Association for Research in ALS (ARSla), the Institute for Research in Spinal Cord and Brain (IRME), the French Association for Myopathie (AFM-Telethon), Paris Institute of Translational Neuroscience (IHU-A-ICM), the Thierry Latran foundation, the Target ALS foundation and the Institut National pour la Santé et la Recherche Médicale (INSERM). Giovanni de Marco received research support from the Laboratoire CeRSM—EA 2931 and COMUE Université Paris Lumières.
Ethics approval
All procedures performed in this study were fully approved by the local, institutional ethics committee (CPP Ile-de-France Paris VI; INSERM promotion RBM C12-13) and were in accordance with the 1964 Helsinki Declaration and its later amendments. This study does not involve any methods or experiments with animals.
Informed consent
All study participants provided informed consent prior to inclusion in the study.
Glossary
- ALS
-
Amyotrophic lateral sclerosis
- APA
-
Anticipatory postural adjustment
- CoM
-
Centre of mass
- CoP
-
Centre of foot pressure
- EP
-
Execution phase
- FO
-
Foot-off of the swing leg
- FO2
-
Foot-off of the stance leg
- GM
-
Grey matter
- HC
-
Healthy control
- MRI
-
Magnetic resonance imaging
- CN
-
Caudate nucleus
- L
-
Stride length
- NPI-ALS
-
ALS patients without postural instability
- PI-ALS
-
ALS patients with postural instability
- PD
-
Parkinson’s disease
- PU
-
Putamen
- VBM
-
Voxel-based morphometry
- V 1
-
Minimum vertical velocity of the CoM
- V 2
-
CoM vertical velocity of the CoM at time of foot contact
- V m
-
Maximum anteroposterior velocity of the CoM
Rights and permissions
About this article
Cite this article
Feron, M., Couillandre, A., Mseddi, E. et al. Extrapyramidal deficits in ALS: a combined biomechanical and neuroimaging study. J Neurol 265, 2125–2136 (2018). https://doi.org/10.1007/s00415-018-8964-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-018-8964-y