Abstract
Frontotemporal dementia (FTD) phenotypes have distinctive and well-established cortical signatures, but their subcortical grey matter profiles are poorly characterised. The comprehensive characterisation of striatal and thalamic pathology along the ALS-FTD spectrum is particularly timely, as dysfunction of frontostriatal and cortico-thalamic networks contribute to phenotype-defining cognitive, behavioral, and motor deficits. Ten patients with behavioral-variant FTD, 11 patients with non-fluent-variant primary progressive aphasia, 5 patients with semantic-variant primary progressive aphasia, 14 ALS-FTD patients with C9orf72 hexanucleotide expansions, 12 ALS-FTD patients without hexanucleotide repeats, 36 ALS patients without cognitive impairment and 50 healthy controls were included in a prospective neuroimaging study. Striatal, thalamic, hippocampal and amygdala pathology was evaluated using volume measurements, density analyses and connectivity-based segmentation. Significant volume reductions were identified in the thalamus and putamen of non-fluent-variant PPA patients. Marked nucleus accumbens and hippocampal atrophy was observed in the behavioral-variant FTD cohort. Semantic-variant PPA patients only exhibited volumetric changes in the left hippocampus. C9-positive ALS-FTD patients showed preferential density reductions in thalamic sub-regions connected to motor and sensory cortical areas. C9-negative ALS-FTD patients exhibited striatal pathology in sub-regions projecting to rostral-motor and executive cortical areas. The bulk of striatal and thalamic pathology in non-fluent-variant PPA patients was identified in foci projecting to motor areas. Subcortical density alterations in svPPA patients were limited to basal ganglia regions with parietal projections. Striatal and thalamic changes in FTD exhibit selective, network-defined vulnerability patterns mirroring cortical pathology. Multi-modal cortico-basal imaging analyses confirm that the subcortical grey matter profiles of FTD phenotypes are just as distinct as their cortical signatures. Our findings support emerging concepts of network-wise degeneration, preferential circuit vulnerability and disease propagation along connectivity patterns.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Despite the clinical and genetic heterogeneity of frontotemporal dementia (FTD), phenotypes along the ALS-FTD spectrum are recognized by their distinctive clinical features. They are associated with unique grey-matter atrophy patterns on structural imaging and regional hypometabolism on PET. The cortical grey matter signatures of these phenotypes have been gradually complemented by the characterisation of their strikingly selective white matter involvement. (Omer et al. 2017; Lu et al. 2014) It is increasingly recognised however that cognitive and behavioral deficits should not be linked to focal cortical pathology alone, but should ideally be explored from a network perspective. (Verstraete et al. 2015) The concept of circuit-wise degeneration in not only supported by imaging studies, but also by neuropsychology and post mortem pathology data. Network failure has been elegantly demonstrated by selective language deficits in ALS-FTD, (Grossman et al. 2008) verbal abstraction deficits in FTD, (Lagarde et al. 2015) and other linguistic studies. (Bak et al. 2001).
There is now compelling evidence that impairments in specific cognitive and behavioral domains are driven by selective network failure in specific frontostriatal and cortico-basal circuits, which are relayed through specific basal ganglia nuclei. (Bede 2017) However, while the functional anatomy of cortico-basal circuitry is well established, (Bonelli and Cummings 2007; O’Callaghan et al. 2013) subcortical grey matter pathology in FTD phenotypes are surprisingly understudied. (Halabi et al. 2013) Existing striatal studies of FTD often focus on a single FTD phenotype (Macfarlane et al. 2015) and frequently evaluate overall volumes for entire structures as opposed to characterising thalamic and striatal sub-regions.(Moller et al. 2015) Furthermore, many FTD imaging studies focus on hippocampal pathology alone, without evaluating other subcortical grey matter structures. (de Souza et al. 2013).
Distinctive, genotype-specific imaging signatures have been proposed in FTD based on both cortical, (Whitwell et al. 2009; Luis et al. 2016) and subcortical grey matter involvement. (Premi et al. 2014) The practical relevance of characterising basal ganglia signatures is further highlighted by emerging machine-learning approaches. These algorithms rely on multimodal MR data and pattern-recognition methods to accurately categorise patients into diagnostic, prognostic, genotypic, and phenotypic sub-groups. (Meyer et al. 2017; Bede et al. 2017; Whitwell et al. 2012; Schuster et al. 2017) In addition to separating FTD phenotypes, the evaluation of basal ganglia mediated functional networks has potential clinical implications for distinguishing FTD from Alzheimer disease. (Zhou et al. 2010; Filippi et al. 2013, 2017), thus obviating the need for PET imaging. While subcortical grey matter degeneration has been previously linked to specific cognitive domains in ALS, (Machts et al. 2015) network-based segmentation of these structures may offer further pathological insights.
The connectivity-based characterisation of basal ganglia pathology is particularly timely in the FTD spectrum, as the concepts of network-wise degeneration (Ahmed et al. 2016; Guo et al. 2016), pre-symptomatic circuit vulnerability, (Lee et al. 2017) spread along connectivity patterns (Verstraete et al. 2013), and prion-like hypotheses (Jucker and Walker 2013) are increasingly recognised as key mechanisms of propagation. Another rationale to comprehensively characterise FTD-associated imaging signatures stems from the recognition that a long presymptomatic phase precedes symptom manifestation in FTD, which may have implications for earlier recruitment into clinical trials. (Jiskoot et al. 2016; Rohrer et al. 2015; Lee et al. 2017).
From a connectivity perspective, it is therefore particularly pertinent to determine whether concomitant degeneration of interconnected grey matter regions occurs in a cortico-basal pattern. The primary hypothesis of this study is that subcortical grey matter degeneration mirrors cortical atrophy. A secondary hypothesis is that selective and focal basal ganglia degeneration occurs in the main FTD phenotypes, similarly to the focal changes observed at a cortical level. (Omer et al. 2017) The objective of this study is the comprehensive characterisation of subcortical grey matter pathology in the main FTD phenotypes along the ALS-FTD spectrum.
Methods
Participants
Patients were recruited from the specialist behavioral clinic at St James’s Hospital, and the ALS/FTD clinic at Beaumont Hospital Dublin. A total of 138 participants were included in a prospective imaging study. Of these, 10 patients had behavioral-variant FTD (bvFTD), 11 patients had non-fluent-variant primary progressive aphasia (nfvPPA), 5 patients had semantic-variant primary progressive aphasia (svPPA), 14 ALS-FTD patients carried C9orf72 hexanucleotide repeat expansion, 12 ALS-FTD patients had normal C9orf72 repeats, 36 ALS patients without behavioral or cognitive deficits (ALSnci) and fifty healthy controls (HC). The demographic and clinical profile of the study participants is presented in Table 1. All participants provided informed consent in accordance with the Medical Ethics Approval of the research project (Ethics (Medical Research) Committee - Beaumont Hospital, Dublin, Ireland). Exclusion criteria included unexpected intracranial pathology, such as hydrocephalus or meningioma, prior cerebrovascular event, and traumatic brain injury. FTD and ALS-FTD was diagnosed based on the Rascovsky criteria (Rascovsky et al. 2011) and the behavioral profile of all ALS-FTD patients was consistent with the ALS-bvFTD. Participating ALS patients had ‘possible’, ‘probable’ or ‘definite’ ALS according to the revised El Escorial research criteria (Brooks et al. 2000; Ludolph et al. 2015). ALSnci patients had no cognitive impairment based on a comprehensive neuropsychological battery, including tests for executive function, letter fluency, category fluency, attention, memory, language, visuo-spatial skills, and behavioral domains. The neuropsychological battery, reference psychometric values and patient categorisation have been previously described. (Phukan et al. 2012; Elamin et al. 2013, 2011) While participating patients were screened for depression, no formal IQ measurements were undertaken.
Magnetic resonance imaging
Magnetic Resonance (MR) data were acquired on a 3 T Philips Achieva system using an 8-channel receive-only head coil. T1-weighted images were acquired using a 3D Inversion Recovery prepared Spoiled Gradient Recalled echo (IR-SPGR) sequence, with a field-of-view (FOV) of 256 × 256 × 160 mm, spatial resolution of 1 mm3, TR/TE = 8.5/3.9 ms, TI = 1060 ms, flip angle = 8°, SENSE factor = 1.5, and an acquisition time of 7 min 32 s.
Genetic testing
DNA samples from patients were tested for the presence of the pathogenic GGGGCC hexanucleotide repeat expansion in C9orf72 by repeat-primed PCR as described previously. (Byrne et al. 2012; Bede et al. 2013a) Amplified fragments were analysed using an Applied Biosystems (Foster City, CA, USA) 3130xl Genetic Analyser and visualised using GeneMapper version 4.0. Patients carrying more than 30 hexanucleotide repeats were considered positive for the expansion. Using targeted next-generation sequencing, patients were also screened and tested negative for established, definitively pathogenic mutations in genes previously implicated in ALS and FTD: SOD1, ALS2, SETX, SPG11, FUS, VAPB, ANG, TARDBP, FIG4, OPTN, ATXN2, VCP, UBQLN2, SIGMAR1, CHMP2B, PFN1, ERBB4, HNRNPA1, MATR3, CHCHD10, UNC13A, DAO, DCTN1, NEFH, PRPH, SQSTM1, TAF15, SPAST, ELP3, LMNB1, SARM1, C21orf2, NEK1, FUS, CHMP2B, GRN, MAPT, PSEN1, PSEN2, TBK1.
Volumetric characterisation of subcortical grey matter structures
Following standard pre-processing steps and brain extraction, the subcortical segmentation and registration tool FIRST (Patenaude et al. 2011) of the FMRIB’s Software Library (FSL) was used to estimate volumes of seven subcortical grey matter structures in the left and right hemispheres; the caudate nucleus, thalamus, nucleus accumbens, hippocampus, amygdala, putamen, and pallidum. Pipelines for subcortical segmentation and volume estimations were previously described (Bede et al. 2013b). Briefly, FSL-FIRST uses a two-stage affine registration algorithm to register input T1 data sets to the Montreal Neurological Institute 152 (MNI152) standard space and a model-based approach is then implemented for the segmentation of subcortical structures. Subcortical mesh and volumetric outputs are generated following automatic boundary corrections. Exploratory comparative statistics were carried out with IBM’s SPSS Statistics version 22. Analyses of covariance (ANCOVA) were conducted to compare volumes of subcortical structures between study groups. Assumptions of normality, linearity and homogeneity of variances were verified. Volumes of subcortical grey matter structures were included as dependent variables, and study group allocation as the categorical independent variable. Age at the time of MRI scan was used as covariate. A p-value ≤ 0.05 was considered significant. For illustrative purposes, boxplots of volumes and plots of estimated marginal means of volumes were generated to highlight inter-group volumetric differences for each structure. A supplementary table was also generated with the estimated marginal means of volumes for each anatomical structure, standard error, between-group ANCOVA significance and p-values for Bonferroni-corrected post hoc testing.
Basal ganglia segmentation based on cortical connectivity patterns
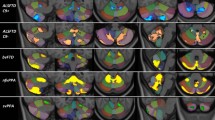
To characterise individual subcortical structures beyond their volumetric profile, parcellation was performed based on cortical connectivity patterns both for the striatum and the thalamus. The Oxford-GSK-Imanova striatal connectivity atlas was utilised for striatal segmentation. (Tziortzi et al. 2014) For thalamic parcellation, the probabilistic Oxford thalamic connectivity atlas was used, which provides connectivity probabilities to 7 cortical zones based on probabilistic diffusion tractography. (Behrens et al. 2003a, b; Johansen-Berg et al. 2005) Basal ganglia sub-regions and their corresponding cortical projections are summarised in Fig. 1. Brain-extracted individual T1-weighted structural data sets were spatially registered to the MNI152 standard space merged into 4D data sets, and density values were retrieved from the above striatal and thalamic sub-regions. Analyses of covariance (ANCOVA) were used to compare regional density alterations between FTD phenotypes correcting for age. Based on estimated marginal means, percentage grey matter density change was also calculated compared to healthy controls to characterise preferential subcortical pathology in each phenotype.
Basal ganglia segmentation based on the cortical connectivity patters. Top: Striatal parcellation based on the Oxford-GSK-Imanova atlas; yellow colour indicates striatal regions projecting to limbic regions, green: caudal motor regions, blue rostral motor regions, aquamarine: executive region, copper: parietal, red: occipital and purple: temporal cortex. Bottom: Thalamic segmentation based on the probabilistic Oxford connectivity atlas: green colour indicates thalamic regions connecting to the motor cortex, aquamarine: occipital, purple: parietal, yellow: prefrontal, copper: premotor, red: sensory and blue: temporal
Subcortical grey matter morphometry
Following brain extraction and tissue-type segmentation, grey-matter partial volume images were aligned to MNI152 standard space using affine registration. A study specific template was created, to which the grey matter images from each subject were non-linearly coregistered. A voxelwise generalized linear model and permutation-based non-parametric testing were used to highlight density alterations in a merged subcortical grey matter mask accounting for age and gender. (Winkler et al. 2014; Nichols and Holmes 2002) The Harvard-Oxford subcortical probabilistic structural atlas was used to generate a merged subcortical grey mask incorporating the left and right caudate, thalamus, accumbens, hippocampus, amygdala, putamen, and pallidum. (Frazier et al. 2005; Desikan et al. 2006).
Cortical thickness changes
The FreeSurfer image analysis suite was used to carry out cortical thickness measurements and comparative statistics. (Fischl 2012) Standard pre-processing steps were implemented, including motion corrections, averaging of the structural T1 weighted data, removal of non-brain tissue, segmentation of the subcortical white matter and deep grey matter structures, intensity normalization, tessellation of the grey matter-white matter boundary, and automated topology correction. (Bruce Fischl and Dale 2000) False Discovery Rate (FDR) correction was used to correct for multiple comparisons at p < 0.05 and group comparisons were corrected for age.
Results
Subcortical grey matter volumetrics
Basal ganglia structures exhibited significant volume reductions across the ALS-FTD spectrum. Figure 2. The age-adjusted estimated marginal means of subcortical grey matter structures and post hoc comparisons between FTD phenotypes and healthy controls are summarised in Supplementary Table 1 and Supplementary Fig. 1. Non-fluent-variant PPA patients showed significant bilateral thalamic and putaminal changes in addition to unilateral accumbens nucleus, hippocampus, and caudate atrophy. The behavioral-variant FTD cohort exhibited bilateral nucleus accumbens and hippocampal atrophy. No significant amygdala involvement was captured in any of the FTD phenotypes. While the C9-negative ALS-FTD cohort showed volume reductions in all nuclei of the basal ganglia with the exception of the amygdala, subcortical grey matter atrophy in C9-positive ALS-FTD patients was limited to the bilateral thalami, hippocampi, and right accumbens nucleus. ALS patients without cognitive or behavioral deficits showed no significant volume reductions in any of the structures.
Basal ganglia segmentation based on cortical connectivity patterns
Density analyses revealed phenotype-specific patterns of subcortical grey matter degeneration across the ALS-FTD spectrum. Table 2. Similarly to our volumetric analyses, ALSnci patients showed no density reductions. C9-positive ALS-FTD patients showed primarily density reductions in thalamic regions connected to motor and sensory cortical areas. C9-negative ALS-FTD patients exhibited striatal pathology in sub-regions projecting to rostral-motor and executive cortical regions. Figure 3. BvFTD patients showed density reductions in striatal regions connected to occipital and motor regions. The bulk of striatal and thalamic pathology in non-fluent-variant PPA patients was identified in regions projecting to motor areas. The most marked focal density changes in svPPA patients were identified in parietal projection foci. Percentage change of basal ganglia segments compared to controls grouped by FTD phenotypes is presented in Supplementary Fig. 2.
Subcortical density alterations
The morphometry-type analyses restricted to the subcortical grey matter mask revealed phenotype-specific focal density alterations in comparison to controls at p < 0.05 FWE adjusted for age. Figure 4. Relatively limited pathology was identified in hexanucleotide repeat expansion carrying ALS-FTD patients in contrast to the C9-negative ALS-FTD cohort. Behavioral-variant FTD patients exhibit strikingly symmetric anterior hippocampal and putamen pathology. In comparison to the symmetric and widespread basal ganglia pathology in non-fluent-variant FTD patients, semantic-variant patients show more restricted left sided hippocampal changes.
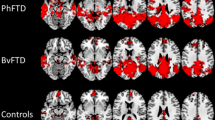
Cortical thickness changes
When individual FTD phenotypes were contrasted to healthy controls, only the two language-variant FTD cohorts showed statistically significant cortical thickness reductions using the false-discovery rate corrections. Figure 5. Cortical thickness reductions of ALSnci, bvFTD, and ALS-FTD cohorts did not reach statistical significance following FDR corrections for multiple comparisons.
Discussion
Despite the wealth of neuropsychology, histopathology and imaging studies in FTD, basal ganglia pathology is surprisingly understudied in vivo. Our findings confirm that FTD is associated with considerable subcortical grey matter degeneration. As previously demonstrated with respect to cortical signatures, specific phenotypes can be linked to distinct subcortical imaging patterns. (Omer et al. 2017; Bede et al. 2016) Our study also illustrates the advantage of using several complementary imaging modalities. While volumetric analyses may highlight overall structure vulnerability, focal changes can be mapped by region-of-interest morphometry and connectivity-based analyses provide a network-wise perspective.
One of the key findings of our study is the relatively limited basal ganglia pathology in ALS patients without cognitive or behavioral impairment. The ALScni cohort, did not exhibit statistically significant volumetric or focal density alterations compared to controls. Moreover, this cohort did not show extra-motor cortical thickness alterations either, underscoring the detection sensitivity of comprehensive neuropsychological batteries. Irrespective of their genotype, ALS-FTD cohorts showed basal-ganglia changes in subcortical grey-matter foci which project to motor regions, suggestive of concomitant cortical-subcortical degeneration. Non-fluent-variant PPA patients showed widespread and bilateral basal ganglia pathology across all the imaging modalities, compared to the much more focal and unilateral, left sided changes observed in semantic-variant PPA mirroring their respective cortical signatures. These findings support our main study hypothesis of concomitant cortico-subcortical grey matter degeneration.
The considerable radiological heterogeneity of the ALS-FTD spectrum is best explained by differing neuropathological processes. These may also account for the significant differences in progression rates, and the relatively focal changes in svPPA compared to the more widespread pathology observed in nfvPPA. While svPPA is nearly always associated with underlying TDP-43 pathology, the clinical syndrome of nfvPPA is most commonly linked to abnormal aggregates of tau. (Hodges et al. 2010; Snowden et al. 2007) The clinical syndrome of behavioral variant FTD is pathologically more heterogeneous than language variants and can be caused by underlying TDP, FUS or TAU pathology. (Perry et al. 2017) Advances in TDP-43 PET ligand development, progress in neurofilament studies and continued improvements in CSF and serum biomarker panels may help to subcategorise patients in future studies based on their underlying pathology. (Steinacker et al. 2017).
Despite the comparable symptom duration and motor disability of C9-positive and C9-negative ALS-FTD patients, their imaging profiles are strikingly divergent. The language and behavioral profile of the two ALS-FTD cohorts were similar therefore unlikely to account for the considerable radiological differences. Contrary to previous studies, we have observed significantly more widespread and severe basal ganglia changes in C9-negative ALS-FTD patients. This cohort, which tested negative for a comprehensive panel of genes implicated in both ALS and FTD, exhibited catastrophic, multifocal basal ganglia degeneration. These findings serve as a reminder that C9orf72 hexanucleotide expansions do not account for all cases of ALS-FTD and highlight the urgency for further genetic, neuropsychology and epidemiology studies in ALS-FTD.
The cortical thickness profile of the ALS-FTD spectrum is also interesting, as only the language variant phenotypes showed statistically significant reductions. Despite their considerable subcortical grey matter changes, bvFTD and ALS-FTD cohorts did not exhibit statistically significant cortical thinning, highlighting the comparative sensitivity of basal ganglia imaging in FTD and confirming the relevance of evaluating these structures. It is also noteworthy that cortical thickness alterations and voxel based morphometry reflect two different aspects of cortical grey matter integrity. While VBM-type analyses readily highlight regional density alterations in FTD, (Omer et al. 2017) cortical thickness mapping in FTD may only detect focal thinning in more restricted cortical regions.
While our patient cohorts are matched for symptom duration, similar disease-duration does not translate to comparable disease-stages across the above conditions. A ‘two-year’ disease duration in ALS often equates to late-phase disease compared to FTD where it is likely to represent early-stage disease. Consistent with the heterogeneity of disease trajectories, a considerable number of disease-specific clinical and pathological staging systems have been developed in recent years. (Balendra et al. 2014; Brettschneider et al. 2013; Piguet et al. 2011) Our cross-sectional study design precludes conclusive observations on progression rates. Follow-up studies with multi-time-point data (Bede and Hardiman 2017) are required to conclusively explore phenotype-specific patterns of propagation. (Schuster et al. 2015).
This study is not without limitations. While the sample size of controls, ALSnci and ALS-FTD cohorts is satisfactory, we acknowledge the relatively small sample size of svPPA patients. Despite sample size limitations, the participants of this study have been clinically, neuropsychologically and genetically characterised. A second limitation of the study stems from its cross-sectional design. Relatively few longitudinal and presymptomatic imaging studies have been published in FTD, (Schuster et al. 2015) and reports of longitudinal basal ganglia changes are particularly scarce. (Westeneng et al. 2015) We have intentionally not explored clinico-radiological correlations in this study and relied primarily on descriptive structural analyses. The direct correlation of psychometric variables to subcortical measures risks to overlook the contribution of white matter and cortical grey matter pathology to cognitive and behavioral deficits. (Bonelli and Cummings 2007) By limiting our analyses to structural MRI data alone we also aimed to avoid the potentially confounding effect of psychoactive medications and respiratory insufficiency on the cognitive performance of our ALS and ALS-FTD cohorts. (Verstraete et al. 2015).
Despite the above study limitations, the novelty of the study lies in the multimodal characterisation of subcortical grey matter involvement in the ALS-FTD spectrum and in the provision of a segmentation-based evaluation of basal ganglia pathology. In addition to its relevance to emerging machine-learning applications, (Bisenius et al. 2017) basal ganglia pathology is increasingly recognised as a stage-defining marker of other neurodegenerative conditions. (Brettschneider et al. 2013; Peter; Bede and Hardiman 2014) Moreover, the evaluation of subcortical grey matter structures may have pragmatic clinical advantages, such as distinguishing early-stage FTD from Alzheimer-type pathology. (Filippi et al. 2017; Davatzikos et al. 2008; McMillan et al. 2014).
Conclusions
Multimodal imaging of genetically and clinically characterised patient cohorts provides unique insights into phenotype-defining pathological profiles. Our findings suggest that the subcortical grey matter signatures of FTD phenotypes are just as unique as their cortical atrophy patterns and that inter-connected cortical-subcortical grey matter regions exhibit concomitant degeneration. The preferential involvement of specific cortical regions is mirrored in similarly selective subcortical grey matter pathology across the ALS-FTD spectrum. Our imaging findings support emerging hypotheses of connectivity based disease propagation.
References
Ahmed, R. M., Devenney, E. M., Irish, M., Ittner, A., Naismith, S., Ittner, L. M., et al. (2016). Neuronal network disintegration: common pathways linking neurodegenerative diseases. Journal of Neurology Neurosurgery, and Psychiatry, 87(11), 1234–1241. https://doi.org/10.1136/jnnp-2014-308350.
Bak, T. H., O’Donovan, D. G., Xuereb, J. H., Boniface, S., & Hodges, J. R. (2001). Selective impairment of verb processing associated with pathological changes in Brodmann areas 44 and 45 in the motor neurone disease-dementia-aphasia syndrome. Brain, 124, 103–120.
Balendra, R., Jones, A., Jivraj, N., Knights, C., Ellis, C. M., Burman, R., et al. (2014). Estimating clinical stage of amyotrophic lateral sclerosis from the ALS Functional Rating Scale. Amyotrophic Lateral Sclerosis Frontotemporal Degenerative, 15(3–4), 279–284. https://doi.org/10.3109/21678421.2014.897357.
Bede, P. (2017). Deciphering neurodegeneration: A paradigm shift from focality to connectivity. Neurology. https://doi.org/10.1212/wnl.0000000000004582.
Bede, P., Bokde, A. L., Byrne, S., Elamin, M., McLaughlin, R. L., Kenna, K., et al. (2013a). Multiparametric MRI study of ALS stratified for the C9orf72 genotype. Neurology, 81(4), 361–369. https://doi.org/10.1212/WNL.0b013e31829c5eee.
Bede, P., Elamin, M., Byrne, S., McLaughlin, R. L., Kenna, K., Vajda, A., et al. (2013b). Basal ganglia involvement in amyotrophic lateral sclerosis. Neurology, 81(24), 2107–2115. https://doi.org/10.1212/01.wnl.0000437313.80913.2c.
Bede, P., & Hardiman, O. (2014). Lessons of ALS imaging: Pitfalls and future directions — A critical review. Neuroimage: Clinical, 4(0), 436–443. https://doi.org/10.1016/j.nicl.2014.02.011.
Bede, P., & Hardiman, O. (2017). Longitudinal structural changes in ALS: a three time-point imaging study of white and gray matter degeneration. Amyotrophic Lateral Sclerosis Frontotemporal Degenerative, 1–10, https://doi.org/10.1080/21678421.2017.1407795.
Bede, P., Iyer, P. M., Finegan, E., Omer, T., & Hardiman, O. (2017). Virtual brain biopsies in amyotrophic lateral sclerosis: Diagnostic classification based on in vivo pathological patterns. Neuroimage Clinics, 15, 653–658. https://doi.org/10.1016/j.nicl.2017.06.010.
Bede, P., Iyer, P. M., Schuster, C., Elamin, M., McLaughlin, R. L., Kenna, K., et al. (2016). The selective anatomical vulnerability of ALS: ‘disease-defining’ and ‘disease-defying’ brain regions. Amyotrophic Lateral Sclerosis Frontotemporal Degenerative, 1–10, https://doi.org/10.3109/21678421.2016.1173702.
Behrens, T. E., Johansen-Berg, H., Woolrich, M. W., Smith, S. M., Wheeler-Kingshott, C. A., Boulby, P. A., et al. (2003a). Non-invasive mapping of connections between human thalamus and cortex using diffusion imaging. Nature Neuroscience, 6(7), 750–757. https://doi.org/10.1038/nn1075.
Behrens, T. E., Woolrich, M. W., Jenkinson, M., Johansen-Berg, H., Nunes, R. G., Clare, S., et al. (2003b). Characterization and propagation of uncertainty in diffusion-weighted MR imaging. Magnetic Resonance in Medicine, 50(5), 1077–1088. https://doi.org/10.1002/mrm.10609.
Bisenius, S., Mueller, K., Diehl-Schmid, J., Fassbender, K., Grimmer, T., Jessen, F., et al. (2017). Predicting primary progressive aphasias with support vector machine approaches in structural MRI data. Neuroimage Clinics, 14, 334–343. https://doi.org/10.1016/j.nicl.2017.02.003.
Bonelli, R. M., & Cummings, J. L. (2007). Frontal-subcortical circuitry and behavior. Dialogues Clinical Neuroscience, 9(2), 141–151.
Brettschneider, J., Del Tredici, K., Toledo, J. B., Robinson, J. L., Irwin, D. J., Grossman, M., et al. (2013). Stages of pTDP-43 pathology in amyotrophic lateral sclerosis. Annals Neurology.
Brooks, B. R., Miller, R. G., Swash, M., & Munsat, T. L. & World Federation of Neurology Research Group on Motor Neuron, D. (2000). El Escorial revisited: revised criteria for the diagnosis of amyotrophic lateral sclerosis. Amyotroph Lateral Sclerosis Other Motor Neuron Disorders, 1(5), 293–299.
Byrne, S., Elamin, M., Bede, P., Shatunov, A., Walsh, C., Corr, B., et al. (2012). Cognitive and clinical characteristics of patients with amyotrophic lateral sclerosis carrying a C9orf72 repeat expansion: a population-based cohort study. Lancet Neurology, 11(3), 232–240. https://doi.org/10.1016/S1474-4422(12)70014-5.
Davatzikos, C., Resnick, S. M., Wu, X., Parmpi, P., & Clark, C. M. (2008). Individual patient diagnosis of AD and FTD via high-dimensional pattern classification of MRI. Neuroimage, 41(4), 1220–1227. https://doi.org/10.1016/j.neuroimage.2008.03.050.
de Souza, L. C., Chupin, M., Bertoux, M., Lehericy, S., Dubois, B., Lamari, F., et al. (2013). Is hippocampal volume a good marker to differentiate Alzheimer’s disease from frontotemporal dementia? Journal of Alzheimers Disease, 36(1), 57–66. https://doi.org/10.3233/jad-122293.
Desikan, R. S., Segonne, F., Fischl, B., Quinn, B. T., Dickerson, B. C., Blacker, D., et al. (2006). An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage, 31(3), 968–980. https://doi.org/10.1016/j.neuroimage.2006.01.021.
Elamin, M., Bede, P., Byrne, S., Jordan, N., Gallagher, L., Wynne, B., et al. (2013). Cognitive changes predict functional decline in ALS: a population-based longitudinal study. Neurology, 80(17), 1590–1597. https://doi.org/10.1212/WNL.0b013e31828f18ac.
Elamin, M., Phukan, J., Bede, P., Jordan, N., Byrne, S., Pender, N., et al. (2011). Executive dysfunction is a negative prognostic indicator in patients with ALS without dementia. Neurology, 76(14), 1263–1269.
Filippi, M., Agosta, F., Scola, E., Canu, E., Magnani, G., Marcone, A., et al. (2013). Functional network connectivity in the behavioral variant of frontotemporal dementia. Cortex, 49(9), 2389–2401. https://doi.org/10.1016/j.cortex.2012.09.017.
Filippi, M., Basaia, S., Canu, E., Imperiale, F., Meani, A., Caso, F., et al. (2017). Brain network connectivity differs in early-onset neurodegenerative dementia. Neurology, 89(17), 1764–1772. https://doi.org/10.1212/wnl.0000000000004577.
Fischl, B. (2012). FreeSurfer. Neuroimage, 62(2), 774–781. https://doi.org/10.1016/j.neuroimage.2012.01.021.
Fischl, B., & Dale, A. M. (2000). Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proceedings of the National Academy of Sciences, 97(20), 11050–11055, https://doi.org/10.1073/pnas.200033797.
Frazier, J. A., Chiu, S., Breeze, J. L., Makris, N., Lange, N., Kennedy, D. N., et al. (2005). Structural brain magnetic resonance imaging of limbic and thalamic volumes in pediatric bipolar disorder. The American Journal of Psychiatry, 162(7), 1256–1265. https://doi.org/10.1176/appi.ajp.162.7.1256.
Grossman, M., Anderson, C., Khan, A., Avants, B., Elman, L., & McCluskey, L. (2008). Impaired action knowledge in amyotrophic lateral sclerosis. Neurology, 71(18), 1396–1401. https://doi.org/10.1212/01.wnl.0000319701.50168.8c.
Guo, C. C., Tan, R., Hodges, J. R., Hu, X., Sami, S., & Hornberger, M. (2016). Network-selective vulnerability of the human cerebellum to Alzheimer’s disease and frontotemporal dementia. Brain, 139(Pt 5), 1527–1538. https://doi.org/10.1093/brain/aww003.
Halabi, C., Halabi, A., Dean, D. L., Wang, P. N., Boxer, A. L., Trojanowski, J. Q., et al. (2013). Patterns of striatal degeneration in frontotemporal dementia. Alzheimer Disease and Associated Disorders, 27(1), 74–83. https://doi.org/10.1097/WAD.0b013e31824a7df4.
Hodges, J. R., Mitchell, J., Dawson, K., Spillantini, M. G., Xuereb, J. H., McMonagle, P., et al. (2010). Semantic dementia: demography, familial factors and survival in a consecutive series of 100 cases. Brain, 133, 300–306. doi:https://doi.org/10.1093/Brain/Awp248.
Jiskoot, L. C., Dopper, E. G., Heijer, T., Timman, R., van Minkelen, R., van Swieten, J. C., et al. (2016). Presymptomatic cognitive decline in familial frontotemporal dementia: A longitudinal study. Neurology, 87(4), 384–391. https://doi.org/10.1212/wnl.0000000000002895.
Johansen-Berg, H., Behrens, T. E., Sillery, E., Ciccarelli, O., Thompson, A. J., Smith, S. M., et al. (2005). Functional-anatomical validation and individual variation of diffusion tractography-based segmentation of the human thalamus. Cerebral Cortex, 15(1), 31–39. https://doi.org/10.1093/cercor/bhh105.
Jucker, M., & Walker, L. C. (2013). Self-propagation of pathogenic protein aggregates in neurodegenerative diseases. Nature, 501(7465), 45–51. https://doi.org/10.1038/nature12481.
Lagarde, J., Valabregue, R., Corvol, J. C., Garcin, B., Volle, E., Le Ber, I., et al. (2015). Why do patients with neurodegenerative frontal syndrome fail to answer: ‘In what way are an orange and a banana alike?’ Brain, 138(Pt 2), 456–471. https://doi.org/10.1093/brain/awu359.
Lee, S. E., Sias, A. C., Mandelli, M. L., Brown, J. A., Brown, A. B., Khazenzon, A. M., et al. (2017). Network degeneration and dysfunction in presymptomatic C9ORF72 expansion carriers. Neuroimage Clinics, 14, 286–297. https://doi.org/10.1016/j.nicl.2016.12.006.
Lu, P. H., Lee, G. J., Shapira, J., Jimenez, E., Mather, M. J., Thompson, P. M., et al. (2014). Regional differences in white matter breakdown between frontotemporal dementia and early-onset Alzheimer’s disease. Journal of Alzheimers Disease, 39(2), 261–269. https://doi.org/10.3233/jad-131481.
Ludolph, A., Drory, V., Hardiman, O., Nakano, I., Ravits, J., Robberecht, W., et al. (2015). A revision of the El Escorial criteria – 2015. Amyotrophic Lateral Sclerosis Frontotemporal Degenerative, 16(5–6), 291–292. https://doi.org/10.3109/21678421.2015.1049183.
Luis, E., Ortiz, A., Eudave, L., Ortega-Cubero, S., Borroni, B., van der Zee, J., et al. (2016). Neuroimaging Correlates of Frontotemporal Dementia Associated with SQSTM1 Mutations. Journal of Alzheimers Disease, 53(1), 303–313. https://doi.org/10.3233/jad-160006.
Macfarlane, M. D., Jakabek, D., Walterfang, M., Vestberg, S., Velakoulis, D., Wilkes, F. A., et al. (2015). Striatal Atrophy in the Behavioural Variant of Frontotemporal Dementia: Correlation with Diagnosis, Negative Symptoms and Disease Severity. PLoS One, 10(6), e0129692. https://doi.org/10.1371/journal.pone.0129692.
Machts, J., Loewe, K., Kaufmann, J., Jakubiczka, S., Abdulla, S., Petri, S., et al. (2015). Basal ganglia pathology in ALS is associated with neuropsychological deficits. Neurology, 85(15), 1301–1309. https://doi.org/10.1212/wnl.0000000000002017.
McMillan, C. T., Avants, B. B., Cook, P., Ungar, L., Trojanowski, J. Q., & Grossman, M. (2014). The power of neuroimaging biomarkers for screening frontotemporal dementia. Human Brain Mapping, 35(9), 4827–4840. https://doi.org/10.1002/hbm.22515.
Meyer, S., Mueller, K., Stuke, K., Bisenius, S., Diehl-Schmid, J., Jessen, F., et al. (2017). Predicting behavioral variant frontotemporal dementia with pattern classification in multi-center structural MRI data. Neuroimage Clinics, 14, 656–662. https://doi.org/10.1016/j.nicl.2017.02.001.
Moller, C., Dieleman, N., van der Flier, W. M., Versteeg, A., Pijnenburg, Y., Scheltens, P., et al. (2015). More atrophy of deep gray matter structures in frontotemporal dementia compared to Alzheimer’s disease. Journal of Alzheimers Disease, 44(2), 635–647. https://doi.org/10.3233/jad-141230.
Nichols, T. E., & Holmes, A. P. (2002). Nonparametric permutation tests for functional neuroimaging: a primer with examples. Human Brain Mapping, 15(1), 1–25.
O’Callaghan, C., Bertoux, M., & Hornberger, M. (2013). Beyond and below the cortex: the contribution of striatal dysfunction to cognition and behaviour in neurodegeneration. Journal of Neurology, Neurosurgery, and Psychiatry. https://doi.org/10.1136/jnnp-2012-304558.
Omer, T., Finegan, E., Hutchinson, S., Doherty, M., Vajda, A., McLaughlin, R. L., et al. (2017). Neuroimaging patterns along the ALS-FTD spectrum: a multiparametric imaging study. Amyotrophic Lateral Sclerosis Frontotemporal Degenerative, 1–13, https://doi.org/10.1080/21678421.2017.1332077.
Patenaude, B., Smith, S. M., Kennedy, D. N., & Jenkinson, M. (2011). A Bayesian model of shape and appearance for subcortical brain segmentation. Neuroimage, 56(3), 907–922.
Perry, D. C., Brown, J. A., Possin, K. L., Datta, S., Trujillo, A., Radke, A., et al. (2017). Clinicopathological correlations in behavioural variant frontotemporal dementia. Brain, 140(12), 3329–3345. https://doi.org/10.1093/brain/awx254.
Phukan, J., Elamin, M., Bede, P., Jordan, N., Gallagher, L., Byrne, S., et al. (2012). The syndrome of cognitive impairment in amyotrophic lateral sclerosis: a population-based study. Journal of Neurology, Neurosurgery, and Psychiatry, 83(1), 102–108.
Piguet, O., Hornberger, M., Mioshi, E., & Hodges, J. R. (2011). Behavioural-variant frontotemporal dementia: diagnosis, clinical staging, and management. Lancet Neurology, 10(2), 162–172. https://doi.org/10.1016/S1474-4422(10)70299-4.
Premi, E., Garibotto, V., Gazzina, S., Formenti, A., Archetti, S., Gasparotti, R., et al. (2014). Subcortical and Deep Cortical Atrophy in Frontotemporal Dementia due to Granulin Mutations. Dementia and Geriatric Cognitive Disorders Extra, 4(1), 95–102. https://doi.org/10.1159/000355428.
Rascovsky, K., Hodges, J. R., Knopman, D., Mendez, M. F., Kramer, J. H., Neuhaus, J., et al. (2011). Sensitivity of revised diagnostic criteria for the behavioural variant of frontotemporal dementia. Brain, 134, 2456–2477. https://doi.org/10.1093/Brain/Awr179.
Rohrer, J. D., Nicholas, J. M., Cash, D. M., van Swieten, J., Dopper, E., Jiskoot, L., et al. (2015). Presymptomatic cognitive and neuroanatomical changes in genetic frontotemporal dementia in the Genetic Frontotemporal dementia Initiative (GENFI) study: a cross-sectional analysis. Lancet Neurology, 14(3), 253–262. https://doi.org/10.1016/s1474-4422(14)70324-2.
Schuster, C., Elamin, M., Hardiman, O., & Bede, P. (2015). Presymptomatic and longitudinal neuroimaging in neurodegeneration–from snapshots to motion picture: a systematic review. Journal of Neurology, Neurosurgery, and Psychiatry, 86(10), 1089–1096. https://doi.org/10.1136/jnnp-2014-309888.
Schuster, C., Hardiman, O., & Bede, P. (2017). Survival prediction in Amyotrophic lateral sclerosis based on MRI measures and clinical characteristics. BMC Neurology, 17(1), 73. https://doi.org/10.1186/s12883-017-0854-x.
Snowden, J., Neary, D., & Mann, D. (2007). Frontotemporal lobar degeneration: clinical and pathological relationships. Acta Neuropathologica, 114(1), 31–38. https://doi.org/10.1007/s00401-007-0236-3.
Steinacker, P., Semler, E., Anderl-Straub, S., Diehl-Schmid, J., Schroeter, M. L., Uttner, I., et al. (2017). Neurofilament as a blood marker for diagnosis and monitoring of primary progressive aphasias. Neurology, 88(10), 961–969. https://doi.org/10.1212/wnl.0000000000003688.
Tziortzi, A. C., Haber, S. N., Searle, G. E., Tsoumpas, C., Long, C. J., Shotbolt, P., et al. (2014). Connectivity-based functional analysis of dopamine release in the striatum using diffusion-weighted MRI and positron emission tomography. Cerebral Cortex, 24(5), 1165–1177. https://doi.org/10.1093/cercor/bhs397.
Verstraete, E., Turner, M. R., Grosskreutz, J., Filippi, M., & Benatar, M. (2015). Mind the gap: The mismatch between clinical and imaging metrics in ALS. Amyotrophic Lateral Sclerosis Frontotemporal Degenerative, 16(7–8), 524–529. https://doi.org/10.3109/21678421.2015.1051989.
Verstraete, E., Veldink, J. H., van den Berg, L. H., & van den Heuvel, M. P. (2013). Structural brain network imaging shows expanding disconnection of the motor system in amyotrophic lateral sclerosis. Human Brain Mapping. https://doi.org/10.1002/hbm.22258.
Westeneng, H. J., Verstraete, E., Walhout, R., Schmidt, R., Hendrikse, J., Veldink, J. H., et al. (2015). Subcortical structures in amyotrophic lateral sclerosis. Neurobiology Aging, 36(2), 1075–1082. https://doi.org/10.1016/j.neurobiolaging.2014.09.002.
Whitwell, J. L., Jack, C. R. Jr., Boeve, B. F., Senjem, M. L., Baker, M., Rademakers, R., et al. (2009). Voxel-based morphometry patterns of atrophy in FTLD with mutations in MAPT or PGRN. Neurology, 72(9), 813–820. https://doi.org/10.1212/01.wnl.0000343851.46573.67.
Whitwell, J. L., Weigand, S. D., Boeve, B. F., Senjem, M. L., Gunter, J. L., DeJesus-Hernandez, M., et al. (2012). Neuroimaging signatures of frontotemporal dementia genetics: C9ORF72, tau, progranulin and sporadics. Brain, 135(Pt 3), 794–806. https://doi.org/10.1093/brain/aws001.
Winkler, A. M., Ridgway, G. R., Webster, M. A., Smith, S. M., & Nichols, T. E. (2014). Permutation inference for the general linear model. Neuroimage, 92, 381–397. https://doi.org/10.1016/j.neuroimage.2014.01.060.
Zhou, J., Greicius, M. D., Gennatas, E. D., Growdon, M. E., Jang, J. Y., Rabinovici, G. D., et al. (2010). Divergent network connectivity changes in behavioural variant frontotemporal dementia and Alzheimer’s disease. Brain, 133(Pt 5), 1352–1367. https://doi.org/10.1093/brain/awq075.
Acknowledgements
We gratefully acknowledge the kindness and generosity of our patients and their caregivers. This work was supported by the Health Research Board (HRB – Ireland; HRB EIA-2017-019), the Irish Institute of Clinical Neuroscience IICN − Novartis Ireland Research Grant, the Iris O’Brien Foundation, the Perrigo Clinician-Scientist Research Fellowship, and the Research Motor Neuron (RMN-Ireland) Foundation.
Funding
This study was funded by the Health Research Board (HRB – Ireland; HRB EIA-2017-019), the Irish Institute of Clinical Neuroscience IICN − Novartis Ireland Research (IICN – 2016), the Iris O’Brien Foundation, the Perrigo Clinician-Scientist Research Fellowship, and the Research Motor Neuron (RMN-Ireland) Foundation.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Peter Bede declares that he has no conflict of interest. Taha Omer declares that he has no conflict of interest. Eoin Finegan declares that he has no conflict of interest. Rangariroyashe H. Chipika declares that she has no conflict of interest. Parameswaran M. Iyer declares that he has no conflict of interest. Mark A. Doherty declares that he has no conflict of interest. Alice Vajda declares that she has no conflict of interest. Niall Pender declares that he has no conflict of interest. Russell L. McLaughlin declares that he has no conflict of interest. Siobhan Hutchinson declares that she has no conflict of interest. Orla Hardiman has received speaking honoraria from Janssen Cilag, Biogen Idec, Sanofi Aventis and Merck-Serono, she has been a member of advisory panels for Biogen Idec, Allergen, Cytokinetics, Ono Pharmaceuticals and Sanofi Aventis.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional (Ethics (Medical Research) Committee - Beaumont Hospital, Dublin, Ireland) and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
This article does not contain any studies with animals performed by any of the authors.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Electronic supplementary material
Below is the link to the electronic supplementary material.


11682_2018_9837_MOESM3_ESM.jpg
The grey matter density profile of individual FTD phenotypes in connectivity-based striatal and thalamic sub-regions (JPG 440 KB)
Rights and permissions
About this article
Cite this article
Bede, P., Omer, T., Finegan, E. et al. Connectivity-based characterisation of subcortical grey matter pathology in frontotemporal dementia and ALS: a multimodal neuroimaging study. Brain Imaging and Behavior 12, 1696–1707 (2018). https://doi.org/10.1007/s11682-018-9837-9
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11682-018-9837-9