Abstract
Pallidal deep brain stimulation (DBS) is an established treatment for patients with severe isolated dystonia. However, clinical evidence for the long-term use of DBS in children is limited and controlled trials have not yet been conducted. Here, we provide the long-term results of up to 13 years of pallidal DBS in eight pediatric patients with generalized idiopathic or hereditary isolated dystonia (five males, mean age at surgery 12.5 ± 3.5 years), as assessed by retrospective video rating. Video rating was performed at three time points: pre-operative, 1-year short-term follow-up (1y-FU) and long-term last FU (LT-FU, up to 13 years). Symptom severity and disability were assessed using the Burke–Fahn–Marsden Dystonia Rating Scale (BFMDRS). Disability scores were obtained from clinical charts and during the last FU. The mean improvement in BFMDRS motor score was 54.4 ± 8.9 % at 1y-FU and 42.9 ± 11.6 % at LT-FU; the disability scores improved by 59.8 ± 10.3 and 63.3 ± 7.8 %, respectively. Electrode dislocation was noted in one patient and implantable pulse generator dislocation in another, both requiring surgical intervention; no further serious adverse events occurred. Our study presents the first blinded video rating assessment of the short- and long-term effects of pallidal DBS in children with idiopathic or hereditary isolated dystonia. Results confirm that pallidal DBS is a safe and efficacious long-term treatment in children, with overall motor improvement similar to that described in controlled trials in adults.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Idiopathic or hereditary isolated generalized dystonia (ID) is a movement disorder that particularly affects children and young adults. It is characterized by the exclusive clinical feature of dystonia, without presentation of other neurological abnormalities apart from tremor [2, 12]. Approximately, 40–60 % of all cases of early-onset ID are caused by a CAG deletion in the TOR1A-gene of the DYT1 locus [25, 28]. The first signs of generalized ID often occur at the age of about 8–12 years, with the initial presentation of focal symptoms in one limb. Subsequent generalization leaves most patients severely handicapped, with long-term complications such as contractures and fixed musculoskeletal deformities [28]. Moreover, most affected individuals experience social as well as educational withdrawal, resulting in a significant reduction of quality of life [1, 23]. Medical therapeutic approaches for ID need to be explored adequately, but are often unsatisfactory with respect to tolerability and efficacy [27].
Deep brain stimulation (DBS) is an established and relatively safe treatment option for patients with medically intractable dystonia [10]. Over the past two decades, pallidal DBS has become part of the standard care for dystonia because of its adjustability and reversibility, with robust clinical improvements that have been proven in large sham-controlled studies in adults [6, 10, 11, 15, 16, 20, 32, 34]. However, these studies did not focus on early surgical treatment in children, even though ID due to TOR1A-gene mutation particularly affects young children. Early effective treatment might offer the opportunity to prevent lifelong disability, dependency and social withdrawal of children and future adults with dystonia [1, 3, 23]. Several case series have demonstrated the efficacy of DBS in children, but long-term results as well as blinded clinical evaluations of therapeutic effects are sparse [1, 3, 9, 13, 21–24, 26, 31]. Here, we present a cohort of eight children treated with pallidal DBS for up to 13 years that was assessed by blinded video rating by a movement disorder specialist.
Methods
Patients and surgery
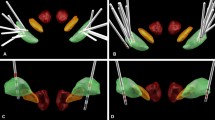
Eight consecutive children [five males, mean (±standard deviation) age at surgery of 12.5 ± 3.5 years, range 7–17 years] with severely disabling, medically intractable idiopathic or hereditary isolated early-onset generalized dystonia, who had been treated at the Charité, University Medicine Berlin since 2000, were included in our study. Six patients tested positive for the TOR1A-gene mutation, one patient presented with a heterozygote DYT16 mutation of unknown pathogenicity and one patient with ID tested negative for TOR1A- and THAP-gene mutations (for more demographic data see Table 1). None of the patients had any structural brain abnormalities in individual MRI. Apart from Patient 7, all patients underwent bilateral DBS in the globus pallidus internus (GPi) at the Charité, University Medicine Berlin by the same neurosurgeon (GHS) and were followed by the same movement disorder specialists (AAK and PK); Patient 7 was implanted with bilateral pallidal DBS at the University Hospital Heidelberg and was transferred to Charité for immediate postoperative management, and follow-up. DBS surgery was conducted under general anesthesia. Electrodes were targeted at the posteroventrolateral portion of the GPi, with intended coordinates located 20–21 mm lateral to the midline, 2–6 mm below and 2 mm anterior to the midcommissural point. Intraoperative macrostimulation was used to test for side effects. The permanent quadripolar macroelectrodes were implanted, using model 3389 (Patients 3, 4, 5, 8) or 3387 (Patients 1, 2, 6) (Medtronic Neurological Division, Minneapolis, MN, USA) and connected to the implantable pulse generator (IPG; two Kinetra, five Activa PC). For Patient 7, the Brio system (St. Jude medical) was used with a quadripolar electrode using model 6147. Postoperative CT (Patients 4, 6) or MR (Patients 1–3, 5, 8) imaging confirmed the correct placement of the macroelectrode in six out of seven patients. A representative example of electrode position in GPi in one of the patients is shown in Fig. 1a. Electrode displacement, where the electrodes were located too lateral in the external pallidum together with limited clinical improvement in Patient 3 led to the decision to undergo replacement surgery 2 years after the initial surgery. Replacement was performed by targeting the more medial and posterior portion of the GPi using the Vercise system (Boston Scientific). The initial and corrected electrode positions in the GPi in this patient are shown in Fig. 1b. Perioperative antibiotic treatment was administered in all patients.
a Localization of DBS electrodes in the postoperative MRI of Patient 1 in the coronal section (left panel) and at the level of active contacts for axial section (right panel). Arrows point to the DBS electrode artifacts in the internal pallidum. b Three-(top) and two-(bottom) dimensional localization results in the same patient, performed based on postoperative imaging before (red) and after (green) revision surgery. Anatomical structures as defined in MNI 152 2009b nonlinear space by the DISTAL atlas [37]: internal (green) and external (blue) pallidum, subthalamic nucleus (orange) and red nucleus (red). The 2009b T2-weighted MNI template is shown in the background. Axial slices are cut at the average height of the second lowermost contact of both pre- and post-revision electrodes which correspond to the planning target
Outcome measures and clinical evaluation
Dystonia severity and disability were assessed using the Burke–Fahn–Marsden Dystonia Rating Scale (BFMDRS) [7]. Individual motor symptoms were rated from the video recordings at preoperative baseline (BL), postoperatively at 1-year follow-up (1y-FU; mean 12.38 ± 1.9 months; mean ± SE, range 7–20 months) and at the last long-term follow-up (LT-FU; 58.5 ± 18.0 months, range 20–156 months). One of the experts (AL), who was not involved in the treatment of the children, rated the videos in a random order blinded to stimulation conditions. These scores are referred to as “blinded rating”, although the rating is not entirely blinded on a time scale because of child development. This limitation was counteracted by random presentation of videos from BL and the 1y-FU and LT-FU visits. A second video rating was performed by a group of movement disorder experts (AL, AAK, PK), who agreed on a score (non-blindedR). Disability scores were recorded from archival charts for BL as well as 1y-FU and obtained in all patients during LT-FU.
To determine whether the response to DBS varied anatomically among the patients, their respective BFMDRS motor scores were divided into the three subscores: craniocervical (section A–D), trunk (section F) and extremities (sections E and G).
Statistical analysis
The BFMDRS motor scores were normally distributed and compared between time points by means of a repeated measures ANOVA using SPSS (IBM SPSS Statistics for Windows, version 20, IBM Corp., Armonk, NY, USA). Post hoc testing between time points was performed using paired Student’s t test. Percentage improvement in BFMDRS subscores was tested as difference from no change. BFMDRS disability scores were not normally distributed and non-parametric statistics (Friedman test and post hoc Wilcoxon test) were used. All data are given as mean ± standard error (SE), unless otherwise stated. A p value <0.05 was considered to be significant.
Results
Clinical improvement
All patients were severely affected by dystonia and experienced improvement of motor symptoms at a relatively short latency within the first week after onset of DBS. At 1y-FU, seven out of eight patients were classified as responders with a clinically meaningful >20 % improvement in BFMDRS severity score, as assessed by the blinded video rating. The mean improvement in BFMDRS was 54.4 ± 8.9 % (range 4.6–78.1 %) for the motor score and 59.8 ± 10.3 % (range 0–86.7 %) for the disability score. At LT-FU compared to BL values, the mean motor improvement was 42.9 ± 11.6 % (range 21–97 %, with worsening in case 3), while the mean disability improvement was 63.3 ± 7.8 % (range 35.7–100 %). All patients were able to withdraw from antidystonic medication at LT-FU. Figure 2a, b presents the mean data and individual BFMDRS motor scores for each patient. Individual functional long-term improvement is given in Table 2.
a Mean percentage improvement in BFMDRS motor and disability score at 1-year follow-up (1y-FU) and the last long-term FU (LT-FU) in blinded rating. b Blinded rating of individual motor scores for 1y-FU and LT-FU in all patients. c Mean percentage change in motor subscores for the craniocervical region, trunk and extremities from the blinded rating scores at 1y-FU and LT-FU. Note the significant improvement of dystonia in all subscores at 1y-FU (p < 0.01), but only for limb dystonia at LT-FU (*p < 0.001)
At LT-FU, three patients showed an overall increase in dystonic symptoms of more than 5 points on the BFMDRS: Patient 3 developed new symptoms of cervical and truncal dystonia, Patient 5 developed craniocervical dystonia, and Patient 6 developed truncal involvement over a time course of 2–4 years after surgery. For a detailed analysis of the DBS response in different body regions, the motor subscores for the craniocervical region, trunk and extremities were calculated from the blinded rating scores (n = 7; Patient 3 was excluded because of limb involvement only at baseline). A similar percentage improvement in different body regions was revealed at 1 y-FU (64–69 %; p < 0.01), but at LT-FU a stable and significant DBS response was only revealed for limb dystonia (p < 0.001) (see Fig. 2c).
Similarly, the non-blinded rating confirmed a significant motor improvement that reached 57.9 ± 7.6 % at 1y-FU and 52.8 ± 10.5 % at LT-FU. Comparison of blinded and non-blinded rating scores revealed a trend for larger improvement for non-blinded assessment at LT-FU (~10 %) (p = 0.057).
No statistically significant correlation between DBS improvements and age at onset, age at surgery, disease duration, disease duration/age at surgery ratio or DYT-TOR1a status was found.
At LT-FU, patients were treated with a frequency of 159 ± 37 Hz (range 130–210 Hz), a pulse width of 86 ± 30 µs (range 87–210 µs) and a stimulation amplitude of 1.3 ± 0.3 V (0.9–5.9 V). These settings were similar to parameters used at 1y-FU, but more complex stimulation settings have been used in some patients with double contacts or an interleaving mode that increases the energy delivered.
Adverse events
The main reason for surgical intervention after successful implantation was replacement of the IPG after battery expiry, necessitating ten replacements in four patients. The replacement interval was 34.1 ± 3.3 months. Patient 2 needed revision of the IPG due to dislocation 11 years after the initial electrode implantation. Patient 3 underwent bilateral electrode revision 3 years after the initial pallidal DBS. Stimulation-induced dysarthria limited further increase of stimulation amplitude in two patients, and bradykinesia was induced by DBS in one severely affected patient with high stimulation amplitudes. None of the remaining patients experienced any further complication or serious adverse effect due to DBS. Patient 1 underwent several orthopedic surgeries due to severe contractures and musculoskeletal deformities resulting from longer disease duration before DBS surgery.
Discussion
Here, we present the short- and long-term effects of bilateral pallidal DBS in a cohort of eight children with idiopathic or hereditary generalized isolated dystonia, over an observation time of up to 13 years. The mean motor improvement of ~54 % at 1y-FU blinded assessment is similar to that reported in sham-controlled trials in adults [20, 32]. This is important to note as previous open-label case series have suggested a larger motor improvement in children than in adults when assessing short-term FU [10]. Similarly, other case series on pediatric patients revealed motor improvement of 77 % in DYT1+ patients [5] or up to 94 % in larger open-label consecutive series [13]. In our cohort, the motor response was quite variable from 4.6 to 97 %. Higher variability in DBS results was also noted in Zorzi et al. [36]. It has to be considered that an essential prerequisite for effective DBS is correct electrode placement. In our cohort, one patient (Patient 3) who was initially classified as a non-responder showed clinically meaningful improvement of dystonia only after electrode replacement. The prognostic factors for favorable DBS outcome in childhood dystonia are still under debate and include shorter disease duration, younger age at surgery, lower baseline motor scores, DYT-TOR1A-positive status, absence of fixed skeletal deformities and a larger pallidal volume [4, 14–17, 19, 30, 32]. In our patient cohort, no statistically significant correlation between DBS improvements and these clinical parameters was identified, possibly due to the relatively small cohort. However, fixed orthopedic contractures were a limiting factor for successful DBS response in two of our patients with longer disease duration. These children had to undergo orthopedic surgeries, but nevertheless reached a clinically important motor improvement of ~50 % that was stable over 10 years (Patient 1). Faster and better DBS effects in dystonia have been associated with mobile dystonia compared to tonic posturing [19, 27, 33]. Similarly, in our cohort mobile dystonia started to improve within days after initiation of DBS and patients with prominent mobile dystonia (Patients 5, 6, 7) reached a motor improvement of >70 % at 1y-FU. To evaluate which prognostic factors are most important, taking into account the neurophysiological parameters of motor plasticity to evaluate the differential impact of reorganization of motor networks, genotype, clinical phenotype and orthopedic aspects, it will be important to collaborate on an international level by setting up large patient databases and multicenter trials (first attempts have recently been reported by Koy et al.) [18].
The complication rate and adverse events over the long follow-up period were rather low in our patients. Serious adverse events occurred in two patients: electrode misplacement of initial leads and IPG dislocation. Stimulation-induced dysarthria was the most frequently observed stimulation-induced side effect. Postoperative infection has been described as the most prevalent complication in some series, with incidence rates of 5–33 % and higher rates being reported among children [17, 35]. However, in our series no device-related infection occurred. Most of the children were older than 10 years at surgery and all suffered from hereditary or idiopathic isolated dystonia, which is in line with the clinical observation that higher infection rates occur especially in children younger than 10 years with secondary dystonia [1]. Nevertheless, one has to consider that the median IPG replacement interval was 34 months in our cohort, which is in line with previous reports [15, 29] but holds a risk for each patient as an extra intervention. Therefore, newly available rechargeable IPGs with smaller size and curved edges might be favorable in pediatric patients.
Quality of life has not been assessed in our cohort apart from the disability scale of the BFMDRS that was significantly reduced in all patients at LT-FU. Moreover, it should be noted that all patients were able to completely withdraw from antidystonic medication. Improvement in motor symptoms was paralleled by a higher functional independence in all children, allowing at least partial social reintegration including attendance of regular school or higher education and more time spent with their peers.
Our study describes the first long-term follow-up in pediatric patients, with effective DBS even after >10 years of continuous stimulation (Patients 1 and 2, with 52 and 41 % motor improvement, respectively). Long-term improvement remained more stable in limb dystonia, highlighting the importance of the body region involved in dystonia, which is similar to observations in adults [32]. A particular strength of our study is the blinded assessment of DBS effects to reduce rater bias, which at the same time may be an explanation for the smaller mean motor improvement in our pediatric patients compared to previous studies. Further limitations of our study include variable disease duration and electrode displacement that occurred in one patient. Taken together, these factors might have reduced the overall outcome. Nevertheless, these results provide a realistic picture of motor improvements in a cohort of consecutive pediatric patients from a single center. Thus, our data support DBS as a valuable long-term treatment in children with early-onset generalized dystonia. As data on DBS in children is still rare, and patient numbers in single centers are often small, a joint effort from different centers to form large cohorts should allow for stronger conclusions in the future.
References
Air EL, Ostrem JL, Sanger TD, Starr PA (2011) Deep brain stimulation in children: experience and technical pearls. J Neurosurg Pediatr 8:566–574
Albanese A, Bhatia K, Bressman SB, Delong MR, Fahn S, Fung VS, Hallett M, Jankovic J, Jinnah HA, Klein C, Lang AE, Mink JW, Teller JK (2013) Phenomenology and classification of dystonia: a consensus update. Mov Disord Off J Mov Disord Soc 28:863–873
Alterman RL, Tagliati M (2007) Deep brain stimulation for torsion dystonia in children. Child’s Nerv Sys ChNS Off J Int Soc Pediatr Neurosurg 23:1033–1040
Andrews C, Aviles-Olmos I, Hariz M, Foltynie T (2010) Which patients with dystonia benefit from deep brain stimulation? A metaregression of individual patient outcomes. J Neurol Neurosurg Psychiatry 81:1383–1389
Borggraefe I, Mehrkens JH, Telegravciska M, Berweck S, Botzel K, Heinen F (2010) Bilateral pallidal stimulation in children and adolescents with primary generalized dystonia–report of six patients and literature-based analysis of predictive outcomes variables. Brain Dev 32:223–228
Bruggemann N, Kuhn A, Schneider SA, Kamm C, Wolters A, Krause P, Moro E, Steigerwald F, Wittstock M, Tronnier V, Lozano AM, Hamani C, Poon YY, Zittel S, Wachter T, Deuschl G, Kruger R, Kupsch A, Munchau A, Lohmann K, Volkmann J, Klein C (2015) Short- and long-term outcome of chronic pallidal neurostimulation in monogenic isolated dystonia. Neurology 84:895–903
Burke RE, Fahn S, Marsden CD, Bressman SB, Moskowitz C, Friedman J (1985) Validity and reliability of a rating scale for the primary torsion dystonias. Neurology 35:73–77
Camargos S, Scholz S, Simón-Sánchez J, Paisán-Ruiz C, Lewis P, Hernandez D, Ding J, Gibbs JR, Cookson MR, Bras J, Guerreiro R, Oliveira CR, Lees A, Hardy J, Cardoso F, Singleton AB (2008) DYT16, a novel young-onset dystonia-parkinsonism disorder: identification of a segregating mutation in the stress-response protein PRKRA. Lancet Neurol 7:207–215
Cif L, Vasques X, Gonzalez V, Ravel P, Biolsi B, Collod-Beroud G, Tuffery-Giraud S, Elfertit H, Claustres M, Coubes P (2010) Long-term follow-up of DYT1 dystonia patients treated by deep brain stimulation: an open-label study. Mov Disord Off J Mov Disord Soc 25:289–299
Coubes P, Cif L, El Fertit H, Hemm S, Vayssiere N, Serrat S, Picot MC, Tuffery S, Claustres M, Echenne B, Frerebeau P (2004) Electrical stimulation of the globus pallidus internus in patients with primary generalized dystonia: long-term results. J Neurosurg 101:189–194
Coubes P, Roubertie A, Vayssiere N, Hemm S, Echenne B (2000) Treatment of DYT1-generalised dystonia by stimulation of the internal globus pallidus. Lancet 355:2220–2221
Fahn SBS, Marsden CD (1998) Classification of dystonia. Adv Neurol 78:1–10
Haridas A, Tagliati M, Osborn I, Isaias I, Gologorsky Y, Bressman SB, Weisz D, Alterman RL (2011) Pallidal deep brain stimulation for primary dystonia in children. Neurosurgery 68:738–743 (discussion 743)
Holloway KL, Baron MS, Brown R, Cifu DX, Carne W, Ramakrishnan V (2006) Deep brain stimulation for dystonia: a meta-analysis. Neuromodulation J Int Neuromodulation Soc 9:253–261
Isaias IU, Alterman RL, Tagliati M (2009) Deep brain stimulation for primary generalized dystonia: long-term outcomes. Arch Neurol 66:465–470
Isaias IU, Alterman RL, Tagliati M (2008) Outcome predictors of pallidal stimulation in patients with primary dystonia: the role of disease duration. Brain J Neurol 131:1895–1902
Isaias IU, Volkmann J, Kupsch A, Burgunder JM, Ostrem JL, Alterman RL, Mehdorn HM, Schonecker T, Krauss JK, Starr P, Reese R, Kuhn AA, Schupbach WM, Tagliati M (2011) Factors predicting protracted improvement after pallidal DBS for primary dystonia: the role of age and disease duration. J Neurol 258:1469–1476
Koy A, Timmermann L (2016) Deep brain stimulation in cerebral palsy: challenges and opportunities. Eur J Paediatr Neurol. doi:10.1016/j.ejpn.2016.05.015
Krauss JK, Loher TJ, Weigel R, Capelle HH, Weber S, Burgunder JM (2003) Chronic stimulation of the globus pallidus internus for treatment of non-dYT1 generalized dystonia and choreoathetosis: 2-year follow up. J Neurosurg 98:785–792
Kupsch A, Benecke R, Muller J, Trottenberg T, Schneider GH, Poewe W, Eisner W, Wolters A, Muller JU, Deuschl G, Pinsker MO, Skogseid IM, Roeste GK, Vollmer-Haase J, Brentrup A, Krause M, Tronnier V, Schnitzler A, Voges J, Nikkhah G, Vesper J, Naumann M, Volkmann J, Deep-Brain Stimulation for Dystonia Study G (2006) Pallidal deep-brain stimulation in primary generalized or segmental dystonia. N Engl J Med 355:1978–1990
Lipsman N, Ellis M, Lozano AM (2010) Current and future indications for deep brain stimulation in pediatric populations. Neurosurg Focus 29:E2
Lumsden DE, Kaminska M, Gimeno H, Tustin K, Baker L, Perides S, Ashkan K, Selway R, Lin JP (2013) Proportion of life lived with dystonia inversely correlates with response to pallidal deep brain stimulation in both primary and secondary childhood dystonia. Dev Med Child Neurol 55:567–574
Marks WA, Honeycutt J, Acosta F, Reed M (2009) Deep brain stimulation for pediatric movement disorders. Semin Pediatr Neurol 16:90–98
Mehrkens JH, Borggraefe I, Feddersen B, Heinen F, Botzel K (2010) Early globus pallidus internus stimulation in pediatric patients with generalized primary dystonia: long-term efficacy and safety. J Child Neurol 25:1355–1361
Ozelius LJ, Hewett JW, Page CE, Bressman SB, Kramer PL, Shalish C, de Leon D, Brin MF, Raymond D, Corey DP, Fahn S, Risch NJ, Buckler AJ, Gusella JF, Breakefield XO (1997) The early-onset torsion dystonia gene (DYT1) encodes an ATP-binding protein. Nat Genet 17:40–48
Parr JR, Green AL, Joint C, Andrew M, Gregory RP, Scott RB, McShane MA, Aziz TZ (2007) Deep brain stimulation in childhood: an effective treatment for early onset idiopathic generalised dystonia. Arch Dis Child 92:708–711
Tagliati M, Shils J, Sun C, Alterman R (2004) Deep brain stimulation for dystonia. Expert Rev Med Devices 1:33–41
Uc EY, Rodnitzky RL (2003) Childhood dystonia. Semin Pediatr Neurol 10:52–61
van Riesen C, Tsironis G, Gruber D, Klostermann F, Krause P, Schneider GH, Kupsch A (2016) Disease-specific longevity of impulse generators in deep brain stimulation and review of the literature. J Neural Transm 123:621–630
Vasques X, Cif L, Gonzalez V, Nicholson C, Coubes P (2009) Factors predicting improvement in primary generalized dystonia treated by pallidal deep brain stimulation. Mov Disord Off J Mov Disord Soc 24:846–853
Vidailhet M, Vercueil L, Houeto JL, Krystkowiak P, Lagrange C, Yelnik J, Bardinet E, Benabid A-L, Navarro S, Dormont D, Grand S, Blond S, Ardouin C, Pillon B, Dujardin K, Hahn-Barma V, Agid Y, Destée A, Pollak P (2007) Bilateral, pallidal, deep-brain stimulation in primary generalised dystonia: a prospective 3 year follow-up study. Lancet Neurol 6:223–229
Vidailhet M, Vercueil L, Houeto JL, Krystkowiak P, Benabid AL, Cornu P, Lagrange C, Tezenas du Montcel S, Dormont D, Grand S, Blond S, Detante O, Pillon B, Ardouin C, Agid Y, Destee A, Pollak P, French Stimulation du Pallidum Interne dans la Dystonie Study G (2005) Bilateral deep-brain stimulation of the globus pallidus in primary generalized dystonia. N Engl J Med 352:459–467
Volkmann J, Benecke R (2002) Deep brain stimulation for dystonia: patient selection and evaluation. Mov Disord Off J Mov Disord Soc 17(Suppl 3):S112–S115
Volkmann J, Mueller J, Deuschl G, Kühn AA, Krauss JK, Poewe W, Timmermann L, Falk D, Kupsch A, Kivi A, Schneider G-H, Schnitzler A, Südmeyer M, Voges J, Wolters A, Wittstock M, Müller J-U, Hering S, Eisner W, Vesper J, Prokop T, Pinsker M, Schrader C, Kloss M, Kiening K, Boetzel K, Mehrkens J, Skogseid IM, Ramm-Pettersen J, Kemmler G, Bhatia KP, Vitek JL, Benecke R (2014) Pallidal neurostimulation in patients with medication-refractory cervical dystonia: a randomised, sham-controlled trial. Lancet Neurol 13:875–884
Yianni J, Nandi D, Shad A, Bain P, Gregory R, Aziz T (2004) Increased risk of lead fracture and migration in dystonia compared with other movement disorders following deep brain stimulation. J Clin Neurosci Off J Neurosurg Soc Australas 11:243–245
Zorzi G, Marras C, Nardocci N, Franzini A, Chiapparini L, Maccagnano E, Angelini L, Caldiroli D, Broggi G (2005) Stimulation of the globus pallidus internus for childhood-onset dystonia. Mov Disord Off J Mov Disord Soc 20:1194–1200
Ewert S, Horn A (2016) Three-dimensional definition of two prominent deep brain stimulation targets based on a multimodal high-definition MNI template. bioRxiv 062851. doi:10.1101/062851
Acknowledgments
AAK, PK and GHS were supported by a grant from the German Research foundation (DFG), KFO247. KL was supported by a grant from the German Research Foundation (DFG, LO 1555/3-2). AK received Grants from the German Research Council and the German Ministry of Education and Research.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
AA. Kühn was funded by the German Research Foundation (DFG KFO247) and received speakers honoraria or consultancies from Medtronic, Boston Scientific and St. Jude Medical, and travel Grants from Ipsen Pharma and Merz. P. Krause was funded by the German Research Foundation (DFG KFO247). K. Lauritsch: none. A. Horn was funded by the German Research Foundation (DFG KFO247) and received grants from the junior clinical scientist program and the Max-Rubner-Price of the Charité. A. Lipp: None. B. Weschke received a grant from the EC within the Collaborative Project “EPISTOP” of the Seventh Framework Program. A. Kupsch received grants from the German Research Council and the German Ministry of Education and Research, belongs to the Advisory Board of ´Medtronic USA´ and received honoraria from Allergan, Boehringer Ingelheim, Ipsen Pharma, Lundbeck, Medtronic, Merck, Merz Pharmaceuticals, Orion, St. Jude, UCB. G.H. Schneider was supported by a grant from the German Research Foundation (DFG), KFO247. K. Kiening: none.
Funding sources for study
The study was supported by the German Research Foundation (DFG) KFO 247.
Ethical standard
The study was approved by the local ethics committee of the Charité, University Medicine Berlin.
Informed consent
All patients gave written informed consent.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Video legend: Segment 1: Patient 2 with generalized dystonia with predominant phasic movements of the head as well as right upper extremity and dystonic gait disorder with bending of the trunk, inward rotation and involuntary flexion of the legs while walking. At no time did she experience speech and/or swallowing problems. Segment 2: After 21 months of pallidal DBS, phasic involuntary movements of the head and right arm were almost completely suppressed, along with significant improvement of cervical and truncal dystonia as well as relevant reduction of dystonia of the upper extremities and dystonic gait. Segment 3: DBS effects remaining stable for more than 13 years, with sustained relief of disability and cessation of the phasic involuntary head and upper limb movements. Mild residual dystonia of the limbs results in minor limitations of fine motor skills and gait. (WMV 43560 kb)
Rights and permissions
About this article
Cite this article
Krause, P., Lauritsch, K., Lipp, A. et al. Long-term results of deep brain stimulation in a cohort of eight children with isolated dystonia. J Neurol 263, 2319–2326 (2016). https://doi.org/10.1007/s00415-016-8253-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-016-8253-6