Abstract
While deep brain stimulation (DBS) treatment is relatively rare in children, it may have a role in dystonia to reduce motor symptoms and disability. Pediatric DBS studies are sparse and limited by small sample size, and thus, outcomes are poorly understood. Thus, we performed a systematic review of the literature including studies of DBS for pediatric (age < 21) dystonia. Patient demographics, disease causes and characteristics, motor scores, and disability scores were recorded at baseline and at last post-operative follow-up. We identified 19 studies reporting DBS outcomes in 76 children with dystonia. Age at surgery was 13.8 ± 3.9 (mean ± SD) years, and 58% of individuals were male. Post-operative follow-up duration was 2.8 ± 2.8 years. Sixty-eight percent of patients had primary dystonia (PD), of whom 56% had a pathological mutation in DYT1 (DYT1+). Across all patients, regardless of dystonia type, 43.8 ± 36% improvement was seen in Burke-Fahn-Marsden Dystonia Rating Scale (BFMDRS) motor (-M) scores after DBS, while 43.7 ± 31% improvement was observed in BFMDRS disability (-D) scores. Patients with PD were more likely to experience ≥ 50% improvement (56%) in BFMDRS-M scores compared to patients with secondary causes of dystonia (21%, p = 0.004). DYT1+ patients were more likely to achieve ≥ 50% improvement (65%) in BFMDRS-D than DTY1− individuals (29%, p = 0.02), although there was no difference in BFMDRS-M ≥ 50% improvement rates between DYT1+ (66%) or DYT1− (43%) children (p = 0.11). While DBS is less common in pediatric patients, individuals with severe dystonia may receive worthwhile benefit with neuromodulation treatment.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Deep brain stimulation (DBS) is a powerful neurosurgical technique that has been used in the treatment of a myriad of pathologies including Parkinson’s disease [21], tremor [3], obsessive-compulsive disorder [5], depression [35], Tourette syndrome [43], pain [44], obesity [10], disorders of consciousness [16], vocal tremor [18], and traumatic brain injury [45], among others. Over the last several decades, the capabilities of DBS have expanded rapidly with technological innovations, surgeon experience, and scientific discoveries. Despite these advances, pediatric-specific randomized controlled trials and large case series using DBS have not yet been reported for any indication. Precise diagnosis of dystonia etiology (genetic or otherwise) remains a challenge; there is a lack of consensus on treatment approach, and there are significant barriers to performing robust multi-institutional studies.
Dystonia is a debilitating neurological condition with prevalence of 15 to 30 cases per 1,000,000 people [42]. There are many causes of dystonia—including primary causes, most often due to genetic mutation [7, 28]. Primary generalized dystonia (PD) can present at any age, with those patients diagnosed earlier in life (before age 26) likely to experience more severe disease [42]. Although medications remain first-line therapy for symptom amelioration, the options are limited, and evaluating response to therapy is further challenged by developmental, behavioral, and speech delays that are often comorbid in the pediatric patient population. Intrathecal baclofen and botulinum toxin injections can be useful for treating dystonia, but outcomes are variable, particularly with regard to upper extremity symptoms [8, 32, 39]. Furthermore, dystonia is often progressive and not curative through pharmacological therapies, increasing the complexity of treating these patients. Thus, surgical approaches to treating pediatric dystonia may offer the only disease-altering course of treatment.
Secondary causes of dystonia are highly variable and consist of many pathologies. DBS has been widely reported in children with movement disorders [29], with primary causes of dystonia being the most commonly treated [2], although a broad range of pathologies have been treated with DBS. However, DBS treatment guidelines for pediatric patients with dystonia are not well-established. Similarly, even among patients carrying the same genetic mutation causing primary dystonia, symptom severity and response to medications is highly variable. Primary cases of dystonia have largely been diagnosed by genetic testing and have been most frequently treated with DBS compared to secondary etiologies of dystonia [31]. However, there is no consensus on specific recommendations regarding when, if at all, DBS should be performed and for patients with various causes of dystonia.
The specific challenges involved in treating children with these complex disorders are numerous. While grading systems of symptom and movement severity, such as the Burke-Fahn-Marsden Dystonia Rating Scale (BFMDS), have been used, there are no standardized recommendations for thresholds that must be met to warrant surgical intervention [4]. Development of future grading systems for pediatric dystonia should make every effort to limit observer bias and prioritize objectivity as many comorbid conditions make clinical evaluation difficult. Thus, improved diagnostic and treatment paradigms for these patients are needed, and DBS represents an attractive option for pediatric patients with dystonia. In general, studies of DBS for dystonia in children are sparse and limited by small sample size, and thus, indications and outcomes are poorly understood. There is also significant variability in outcomes for patients with primary vs. secondary causes of dystonia, further delineating the need for improved disease classification and response to DBS. However, patients that respond to DBS experience a significant improvement in his/her quality of life despite being unable to correct the underlying causes of disease. It may therefore be useful to systematically summarize the limited data that are currently available in the literature, to help identify questions requiring further study. Here, we present the first systematic review of DBS outcomes in children with dystonia.
Materials and methods
Literature search
PubMed was searched in March 2018 according to PRISMA guidelines for systematic reviews (Fig. 1). The search terms included “(“dystonia” OR “movement disorder” AND “deep brain stimulation” AND (“children” or “pediatric” or “adolescent”)).” This query resulted in 263 articles. Inclusion criteria included (1) peer-reviewed publications from 1980 through March 2018 and (2) studies containing one or more pediatric patient (≤ 21 years of age) treated with deep brain stimulation for dystonia. Exclusion criteria included (1) non-English articles, (2) review or surgical technique articles, and (3) articles in which individual pediatric patient data could not be extracted. Titles and abstracts were then reviewed for inclusion. Review articles were intentionally excluded from our analysis so that only quantitative outcomes studies, rather than qualitative information, could be aggregated. In addition, this article is the first to aggregate DBS surgical outcomes data in pediatric patients with movement disorders, although there have been a number of articles reporting qualitative information describing the utility of DBS. If exclusion was not confirmed based on the title and abstract, the full text was reviewed. In total, 19 articles met all criteria and were reviewed in their entirety (Table 1). This study was registered with PROSPERO, the international prospective register of systematic reviews (https://www.crd.york.ac.uk/prospero/).
Data extraction
The following information was extracted from the included studies by M.A.M. and confirmed independently by A.T.H. and D.J.E.: (1) age at onset (< 18 years); (2) age at surgery (< 21 years); (3) duration of illness before surgery; (4) sex; (5) etiology of dystonia, including genetic testing; (6) anatomical target for DBS; (7) pre- and post-operative Burke-Fahn-Marsden Dystonia Rating Scale (BFMDRS) motor (BFMDRS-M) and (BFMDRS-D) disability scores; and (8) length of follow-up after surgery. Only pediatric data that could be disaggregated from adult data was included in this study. All patients were diagnosed before age 18 and were thus considered pediatric cases. Variables were selected based on the availability within individual texts suitable for quantitative outcomes analysis. Individual patient data are provided in Supplemental Materials, online.
Statistics
Chi-square was used to relate etiology of dystonia to < 50% vs ≥ 50% improvement in BFMDRS-M and BFMDRS-D after surgery. We chose to report the data as greater than 50% improvement (using the chi-squared test) since there are no standardized indications for operative intervention based on BFMDRS scores. Furthermore, based on the synthesis of our review of the literature, many consider greater than 50% improvement to be clinically significant. Univariate logistic regression was used to relate age, gender, or duration of symptoms to improvement in BFMDRS-M and BFMDRS-D. Unpaired, two-way, Student’s t test was performed to relate percent change in BFMDRS-M and BFMDRS-D to etiology of dystonia. These statistics were performed for summary purposes only, as insufficient data were available in the literature for formal meta-analysis.
Results
Demographics
We performed a systematic review of DBS outcomes in the treatment of dystonia in children. All included studies and individual patient characteristics are listed in Supplemental Materials, online. Overall, we identified 19 studies including 76 patients (58% male) reporting DBS outcomes for dystonia in children. The mean age at surgery was 13.8 ± 3.9 (mean ± SD) years. Duration of time between onset of symptoms and surgery was 6.4 ± 3.5 years, and post-operative follow-up was 2.8 ± 2.8 years, with 78% of patients having more than 1 year of follow-up. All studies were retrospective, and no prospective studies or trials were reported. Summary data are listed in Table 1.
Dystonia etiology
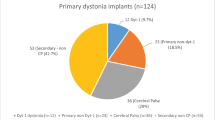
Primary generalized dystonia (PD) was the most common form of dystonia reported (68%) while other causes of dystonia—including secondary generalized and focal dystonia—were reported in 32% of children. Causes of PD included mutations in torsin family 1 member A (DYT1+), the most common genetic cause of dystonia [47], or patients with PD and no known pathological mutation in DYT1 (DTY1−). Due to the limited number of reports and inconsistencies in reporting body part–specific distribution patterns of dystonia, we are unable to complete this subtype analysis on this current cohort of patients. Causes of secondary dystonia included cerebral palsy of unknown etiology (n = 7), primal focal/segmental dystonia (n = 5), secondary generalized dystonia of unknown etiology (n = 2), dystonic cerebral palsy due to hyperbilirubinemia (n = 2), dystonic cerebral palsy due to hypoxic-ischemic encephalopathy (n = 2), dystonia due to traumatic brain injury (n = 1), basal ganglia calcifications (n = 1), dystonic cerebral palsy due to meningitis (n = 1), intracerebral hemorrhage (n = 1), Hallervorden-Spatz disease (n = 1, secondary due to iron accumulation), axonal brain injury (n = 1), encephalopathy (n = 1), and kernicterus (n = 1). Since there was such heterogeneity reported in the literature on secondary causes and quantitative outcomes information (i.e., Burke-Fahn-Marsden Dystonia Rating Scale) was sparsely reported, we chose to treat all secondary causes as one category. The symptoms most reported in the literature as indications for DBS included dystonic or “stiff” movement as well as “abnormal movements.” However, these data were sparsely reported in cases where the diagnosis was not PD. Further classifying qualitative symptom improvement in pediatric patients with dystonia may be difficult due to comorbid developmental delay. Thus, our focus here was to provide a quantitative assessment of motor and disability scores that could later be expanded as additional information becomes available.
DBS treatment
Ninety-one percent of individuals were implanted with a bilateral globus pallidus interna (GPi) target, one (1.3%) patient received a unilateral GPi implant, one (1.3%) individual received bilateral GPi plus subthalamic nucleus (STN), and five (6.6%) children received a unilateral GPi implant and a contralateral GPi lesion. Thus, we only considered surgical interventions targeting the GPi. Across all patients with data available, BFMDRS-M scores improved by 43.8 ± 36% (mean ± SD) after surgery, with 45% of individuals achieving ≥ 50% improvement, while BFMDRS-D scores improved by 43.7 ± 31% post-operatively, with 47% of children achieving ≥ 50% improvement. Patients with PD (56%) were more likely to experience > 50% improvement in BFMDRS-M scores after surgery compared to patients with other causes of dystonia (21%, p = 0.004, chi-square), as shown in Fig. 1. Improvement in BFMDRS-D scores after surgery could not be compared in primary vs. secondary dystonia, as post-operative scores were only reported in two patients with secondary dystonia. Among individuals with PD, there was no difference in the likelihood of achieving ≥ 50% improvement in BFMDRS-M scores between patients with DYT1+ (66%) versus DYT1− (52%) disease (p = 0.11, chi-square), although DYT1+ patients were more likely (65%) to achieve ≥ 50% improvement in BFMDRS-D than DTY1− individuals (29%, p = 0.02, chi-square), as shown in Figs. 2 and 3. Additional genetic causes of dystonia could not be investigated here due to limited reporting in the literature. Age, gender, duration of symptoms, and length of follow-up were not found to be predictive of BFMDRS-M or BFMDRS-D outcomes (p > 0.05 for each, logistic regression). Study limitations are listed in Table 2.
Discussion
We performed the first systematic review of DBS outcomes in the treatment of pediatric patients with movement disorders, including primary generalized dystonia and secondary causes of dystonia. Our results suggest that DBS can be an efficacious treatment in patients with dystonia, but patients with PD may be more likely to experience greater improvement in motor symptoms than those with secondary dystonia. Among PD patients, those who are DYT1+ may be more likely to experience an improvement in disability scores compared to patients who are DYT1−. Thus, it is possible that a known genetic etiology of dystonia portends a more favorable DBS outcome. While our analysis is limited by a small number of reported cases in the literature, this report serves as a proof-of-principle that DBS may be effective in children with dystonia. Furthermore, we are the first, to our knowledge, to aggregate quantitative outcomes data for DBS treatment of pediatric dystonia. However, larger prospective studies and/or a centralized patient registry, such as the Pediatric International Deep Brain Stimulation Registry Project (PEDiDBS) [30], is needed to evaluate outcomes in greater detail.
All patients were described as poorly responsive or refractory to medical therapy for dystonia and failed medication trials of anticholinergic drugs, benzodiazepine derivatives, botulinum toxin injections, neuroleptics, oral baclofen, and/or intrathecal baclofen. Overall, patients experienced symptoms for 6.4 ± 3.5 years prior to DBS treatment. The number and order of medications attempted prior to DBS placement were not reported in any of the studies included in our review. Comparisons of pre-operative and post-operative medication regimens were scarce, though several studies reported substantial decrease in the amount and variety of daily maintenance medications after DBS placement [25, 46, 51].
The primary scoring system used to assess pre-operative severity as well as post-operative improvement in patients with dystonia is the BFMDRS, as described by Albanese et al. [1]. This rating scale takes into account both motor and overall disability measures and has been demonstrated to be a relatively reliable tool for estimating severity of disease, but it is not the only method to quantify movement disorder outcomes [14]. However, the BFMDRS is not pediatric-specific, thereby limiting its use in clinically evaluating pediatric patients with dystonia. Thus, while some level of functional outcome can be captured using BFMDRS, it is worth considering whether additional scoring systems should be developed to better reflect the specific features seen in pediatric cases of dystonia.
Previous reviews on DBS in children have focused on general indications [9, 27], surgical technique [46], qualitative assessment of movement disorder [2, 11, 31], and the value of genetic testing in patient selection for DBS [20]. Prior pediatric-specific reviews of DBS treatment for movement disorders have focused on single-center experiences, surgical techniques specific to children, and descriptions of illustrative cases [2, 9, 11, 20, 27, 31, 46]. However, no quantitative analysis of pediatric-specific outcomes after DBS has been performed, to our knowledge. Given the surprisingly low number of articles that met our inclusion criteria, additional quantitative studies in pediatric DBS need to be performed. Previous qualitative reviews have described that DYT1+ patients may be more likely to respond to DBS treatment than individuals with heterogeneous causes of secondary dystonia [2, 31], which is supported by our present findings. Thus, the quantitative results presented here are mostly in agreement with prior qualitative reviews on this topic.
Although DYT1+ patients experienced more favorable outcomes, DYT1 is not the only genetic cause of primary dystonia. Complex and myoclonic dystonias can result from mutations in PKAN, among other monogenic causes. However, genetic testing for many of these disorders is not routinely used and reported in the literature. Various groups have reported remarkably diverse genetic etiologies underlying physical manifestations of dystonia in children and adults [12, 17, 34, 50]. While surgical management of these patients may improve functional outcomes, molecular and systems-based analysis of known and unknown genetic drivers of movement disorders are needed to better understand disease pathophysiology. Importantly, as gene-sequencing and “personalized medicine” approaches may become more mainstream, more selective genetic criteria may be elucidated to select for patients who would most likely benefit from DBS. While the field is not currently primed for this level of granularity, it is worth considering the potential genomics may play in guiding the treatment of dystonia and other neurological disorders. The potential to identify younger patients with dystonia likely to benefit from surgery, based on genetic criteria, may be worthwhile.
Secondary causes of pediatric dystonia are highly variable and reflected in the current aggregation of the literature. While there are clear pathophysiological mechanisms responsible for driving PGD, such as mutations in DYT1, secondary causes of dystonia can occur without antecedent genetic causes. Thus, it could be surmised that amelioration of associated dystonia symptoms in patients with secondary dystonia may improve quality of life. However, secondary dystonia is associated with many genetic conditions that are not mediated by DYT1, such as inborn errors of metabolism, vitamin deficiencies, and neurodevelopmental conditions. Therefore, treatment strategies have been focused on reversing the underlying cause of the dystonia symptoms. Our data imply that use of DBS may provide some symptomatic benefit for these patients, despite the inability to permanently reverse the underlying cause of the disease. However, additional studies are needed to delineate the degree to which DBS can ameliorate dystonic symptoms due to secondary causes.
Standardized surgical indications and treatment of pediatric dystonia would represent a major leap forward for the field. While additional well-controlled, multi-center studies are needed to delineate optimal treatment practices, preliminary data suggests that pediatric patients with varying forms of dystonia could benefit from DBS. We hope this review serves as a springboard for discussion of expanding the use of DBS in pediatric dystonia, providing an unmet therapeutic need to this patient population.
Limitations and conclusions
Our study has several important limitations to consider. All studies examined were retrospective, mostly mixed population, and with variable follow-up have been reported, limiting data quality (Table 2). Furthermore, the studies are all small and many are not restricted to the pediatric population. All data here was extracted from mixed-population studies and was presented here only when the pediatric-specific information could be disaggregated. Publication bias may also lead to selective reporting of more favorable outcomes limiting accurate assessment of outcomes in this patient population. Finally, univariate statistics are provided here for summary purposes only, as insufficient data are available for formal meta-analysis in this field. Nonetheless, our study is the first, to our knowledge, to systematically examine the available literature of DBS for pediatric dystonia, providing quantitative outcomes data. We conclude that DBS may be effective in improving motor symptoms and degree of disability in this disorder. Outcomes may be more favorable in PD than secondary dystonia, and among PD patients, in DYT+ patients compared to those who are DYT−. Larger, prospective studies will be important to further evaluate long-term outcome rates and predictors in this field. We hope our review serves as a useful summary of how future data should be presented in order to aggregate data across multiple studies and centers.
References
Albanese A, Sorbo FD, Comella C, Jinnah HA, Mink JW, Post B, Vidailhet M, Volkmann J, Warner TT, Leentjens AF, Martinez-Martin P, Stebbins GT, Goetz CG, Schrag A (2013) Dystonia rating scales: critique and recommendations. Mov Disord 28(7):874–883. https://doi.org/10.1002/mds.25579
Alterman RL, Tagliati M (2007) Deep brain stimulation for torsion dystonia in children. Childs Nerv Syst 23(9):1033–1040. https://doi.org/10.1007/s00381-007-0382-x
Artusi CA, Farooqi A, Romagnolo A, Marsili L, Balestrino R, Sokol LL, Wang LL, Zibetti M, Duker AP, Mandybur GT, Lopiano L, Merola A (2018) Deep brain stimulation in uncommon tremor disorders: indications, targets, and programming. J Neurol 265:2473–2493. https://doi.org/10.1007/s00415-018-8823-x
Asakawa T, Sugiyama K, Nozaki T, Sameshima T, Kobayashi S, Wang L, Hong Z, Chen SJ, Li CD, Ding D, Namba H (2018) Current behavioral assessments of movement disorders in children. CNS Neurosci Ther 24:863–875. https://doi.org/10.1111/cns.13036
Ashkan K, Rogers P, Bergman H, Ughratdar I (2017) Insights into the mechanisms of deep brain stimulation. Nat Rev Neurol 13(9):548–554. https://doi.org/10.1038/nrneurol.2017.105
Cersosimo MG, Raina GB, Piedimonte F, Antico J, Graff P, Micheli FE (2008) Pallidal surgery for the treatment of primary generalized dystonia: long-term follow-up. Clin Neurol Neurosurg 110(2):145–150. https://doi.org/10.1016/j.clineuro.2007.10.003
Cif L, Coubes P (2017) Historical developments in children’s deep brain stimulation. Eur J Paediatr Neurol 21(1):109–117. https://doi.org/10.1016/j.ejpn.2016.08.010
Dickey MP, Rice M, Kinnett DG, Lambert R, Donauer S, Gerber MA, Staat MA (2013) Infectious complications of intrathecal baclofen pump devices in a pediatric population. Pediatr Infect Dis J 32(7):715–722. https://doi.org/10.1097/INF.0b013e318287f02a
DiFrancesco MF, Halpern CH, Hurtig HH, Baltuch GH, Heuer GG (2012) Pediatric indications for deep brain stimulation. Childs Nerv Syst 28(10):1701–1714. https://doi.org/10.1007/s00381-012-1861-2
Dupre DA, Tomycz N, Oh MY, Whiting D (2015) Deep brain stimulation for obesity: past, present, and future targets. Neurosurg Focus 38(6):E7. https://doi.org/10.3171/2015.3.Focus1542
Fehlings D, Brown L, Harvey A, Himmelmann K, Lin JP, Macintosh A, Mink JW, Monbaliu E, Rice J, Silver J, Switzer L, Walters I (2018) Pharmacological and neurosurgical interventions for managing dystonia in cerebral palsy: a systematic review. Dev Med Child Neurol 60(4):356–366. https://doi.org/10.1111/dmcn.13652
Fuchs T, Saunders-Pullman R, Masuho I, Luciano MS, Raymond D, Factor S, Lang AE, Liang TW, Trosch RM, White S, Ainehsazan E, Herve D, Sharma N, Ehrlich ME, Martemyanov KA, Bressman SB, Ozelius LJ (2013) Mutations in GNAL cause primary torsion dystonia. Nat Genet 45(1):88–92. https://doi.org/10.1038/ng.2496
Ghosh PS, Machado AG, Deogaonkar M, Ghosh D (2012) Deep brain stimulation in children with dystonia: experience from a tertiary care center. Pediatr Neurosurg 48(3):146–151. https://doi.org/10.1159/000345830
Gimeno H, Tustin K, Selway R, Lin JP (2012) Beyond the Burke-Fahn-Marsden Dystonia Rating Scale: deep brain stimulation in childhood secondary dystonia. Eur J Paediatr Neurol 16(5):501–508. https://doi.org/10.1016/j.ejpn.2011.12.014
Goto S, Yamada K, Shimazu H, Murase N, Matsuzaki K, Tamura T, Nagahiro S, Kuratsu J, Kaji R (2006) Impact of bilateral pallidal stimulation on DYT1-generalized dystonia in Japanese patients. Mov Disord 21(10):1785–1787. https://doi.org/10.1002/mds.21021
Gummadavelli A, Kundishora AJ, Willie JT, Andrews JP, Gerrard JL, Spencer DD, Blumenfeld H (2015) Neurostimulation to improve level of consciousness in patients with epilepsy. Neurosurg Focus 38(6):E10. https://doi.org/10.3171/2015.3.Focus1535
Heinzen EL, Swoboda KJ, Hitomi Y, Gurrieri F, Nicole S, de Vries B, Tiziano FD, Fontaine B, Walley NM, Heavin S, Panagiotakaki E, Fiori S, Abiusi E, Di Pietro L, Sweney MT, Newcomb TM, Viollet L, Huff C, Jorde LB, Reyna SP, Murphy KJ, Shianna KV, Gumbs CE, Little L, Silver K, Ptacek LJ, Haan J, Ferrari MD, Bye AM, Herkes GK, Whitelaw CM, Webb D, Lynch BJ, Uldall P, King MD, Scheffer IE, Neri G, Arzimanoglou A, van den Maagdenberg AM, Sisodiya SM, Mikati MA, Goldstein DB (2012) De novo mutations in ATP1A3 cause alternating hemiplegia of childhood. Nat Genet 44(9):1030–1034. https://doi.org/10.1038/ng.2358
Ho AL, Erickson-Direnzo E, Pendharkar AV, Sung CK, Halpern CH (2015) Deep brain stimulation for vocal tremor: a comprehensive, multidisciplinary methodology. Neurosurg Focus 38(6):E6. https://doi.org/10.3171/2015.3.Focus1537
Jin ST, Lee MK, Ghang JY, Jeon SM (2012) Deep brain stimulation of the globus pallidus in a 7-year-old girl with DYT1 generalized dystonia. J Korean Neurosurg Soc 52(3):261–263. https://doi.org/10.3340/jkns.2012.52.3.261
Jinnah HA, Alterman R, Klein C, Krauss JK, Moro E, Vidailhet M, Raike R (2017) Deep brain stimulation for dystonia: a novel perspective on the value of genetic testing. J Neural Transm (Vienna, Austria : 1996) 124(4):417–430. https://doi.org/10.1007/s00702-016-1656-9
Jourdain VA, Schechtmann G, Di Paolo T (2014) Subthalamotomy in the treatment of Parkinson’s disease: clinical aspects and mechanisms of action. J Neurosurg 120(1):140–151. https://doi.org/10.3171/2013.10.Jns13332
Keen JR, Przekop A, Olaya JE, Zouros A, Hsu FP (2014) Deep brain stimulation for the treatment of childhood dystonic cerebral palsy. J Neurosurg Pediatr 14(6):585–593. https://doi.org/10.3171/2014.8.Peds141
Krause M, Fogel W, Kloss M, Rasche D, Volkmann J, Tronnier V (2004) Pallidal stimulation for dystonia. Neurosurgery 55(6):1361–1368 discussion 1368-1370
Krause P, Bruggemann N, Volzmann S, Horn A, Kupsch A, Schneider GH, Lohmann K, Kuhn A (2015) Long-term effect on dystonia after pallidal deep brain stimulation (DBS) in three members of a family with a THAP1 mutation. J Neurol 262(12):2739–2744. https://doi.org/10.1007/s00415-015-7908-z
Krause P, Lauritsch K, Lipp A, Horn A, Weschke B, Kupsch A, Kiening KL, Schneider GH, Kuhn AA (2016) Long-term results of deep brain stimulation in a cohort of eight children with isolated dystonia. J Neurol 263(11):2319–2326. https://doi.org/10.1007/s00415-016-8253-6
Kupsch A, Klaffke S, Kuhn AA, Meissner W, Arnold G, Schneider GH, Maier-Hauff K, Trottenberg T (2003) The effects of frequency in pallidal deep brain stimulation for primary dystonia. J Neurol 250(10):1201–1205. https://doi.org/10.1007/s00415-003-0179-0
Lipsman N, Ellis M, Lozano AM (2010) Current and future indications for deep brain stimulation in pediatric populations. Neurosurg Focus 29(2):E2. https://doi.org/10.3171/2010.5.Focus1095
Lumsden DE, Kaminska M, Ashkan K, Selway R, Lin JP (2017) Deep brain stimulation for childhood dystonia: is ‘where’ as important as in ‘whom’? Eur J Paediatr Neurol 21(1):176–184. https://doi.org/10.1016/j.ejpn.2016.10.002
Lumsden DE, Kaminska M, Gimeno H, Tustin K, Baker L, Perides S, Ashkan K, Selway R, Lin JP (2013) Proportion of life lived with dystonia inversely correlates with response to pallidal deep brain stimulation in both primary and secondary childhood dystonia. Dev Med Child Neurol 55(6):567–574. https://doi.org/10.1111/dmcn.12117
Marks W, Bailey L, Sanger TD (2017) PEDiDBS: the pediatric international deep brain stimulation registry project. Eur J Paediatr Neurol 21(1):218–222. https://doi.org/10.1016/j.ejpn.2016.06.002
Marks WA, Honeycutt J, Acosta F, Reed M (2009) Deep brain stimulation for pediatric movement disorders. Semin Pediatr Neurol 16(2):90–98. https://doi.org/10.1016/j.spen.2009.04.001
Marsh WA, Monroe DM, Brin MF, Gallagher CJ (2014) Systematic review and meta-analysis of the duration of clinical effect of onabotulinumtoxinA in cervical dystonia. BMC Neurol 14:91. https://doi.org/10.1186/1471-2377-14-91
Mehrkens JH, Borggraefe I, Feddersen B, Heinen F, Botzel K (2010) Early globus pallidus internus stimulation in pediatric patients with generalized primary dystonia: long-term efficacy and safety. J Child Neurol 25(11):1355–1361. https://doi.org/10.1177/0883073810365369
Meyer E, Carss KJ, Rankin J, Nichols JM, Grozeva D, Joseph AP, Mencacci NE, Papandreou A, Ng J, Barral S, Ngoh A, Ben-Pazi H, Willemsen MA, Arkadir D, Barnicoat A, Bergman H, Bhate S, Boys A, Darin N, Foulds N, Gutowski N, Hills A, Houlden H, Hurst JA, Israel Z, Kaminska M, Limousin P, Lumsden D, McKee S, Misra S, Mohammed SS, Nakou V, Nicolai J, Nilsson M, Pall H, Peall KJ, Peters GB, Prabhakar P, Reuter MS, Rump P, Segel R, Sinnema M, Smith M, Turnpenny P, White SM, Wieczorek D, Wiethoff S, Wilson BT, Winter G, Wragg C, Pope S, Heales SJ, Morrogh D, Pittman A, Carr LJ, Perez-Duenas B, Lin JP, Reis A, Gahl WA, Toro C, Bhatia KP, Wood NW, Kamsteeg EJ, Chong WK, Gissen P, Topf M, Dale RC, Chubb JR, Raymond FL, Kurian MA (2017) Mutations in the histone methyltransferase gene KMT2B cause complex early-onset dystonia. Nat Genet 49(2):223–237. https://doi.org/10.1038/ng.3740
Mi K (2016) Use of deep brain stimulation for major affective disorders. Exp Ther Med 12(4):2371–2376. https://doi.org/10.3892/etm.2016.3622
Miyagi Y, Koike Y (2013) Tolerance of early pallidal stimulation in pediatric generalized dystonia. J Neurosurg Pediatr 12(5):476–482. https://doi.org/10.3171/2013.8.Peds12578
Olaya JE, Christian E, Ferman D, Luc Q, Krieger MD, Sanger TD, Liker MA (2013) Deep brain stimulation in children and young adults with secondary dystonia: the Children’s Hospital Los Angeles experience. Neurosurg Focus 35(5):E7. https://doi.org/10.3171/2013.8.Focus13300
Oterdoom DLM, van Egmond ME, Ascencao LC, van Dijk JMC, Saryyeva A, Beudel M, Runge J, de Koning TJ, Abdallat M, Eggink H, Tijssen MAJ, Krauss JK (2018) Reversal of status dystonicus after relocation of pallidal electrodes in DYT6 generalized dystonia. Tremor Other Hyperkinet Mov (NY) 8:530. https://doi.org/10.7916/d82f90dx
Pan IW, Kuo GM, Luerssen TG, Lam SK (2015) Impact of antibiotic prophylaxis for intrathecal baclofen pump surgery in pediatric patients. Neurosurg Focus 39(6):E10. https://doi.org/10.3171/2015.9.Focus15385
Parr JR, Green AL, Joint C, Andrew M, Gregory RP, Scott RB, McShane MA, Aziz TZ (2007) Deep brain stimulation in childhood: an effective treatment for early onset idiopathic generalised dystonia. Arch Dis Child 92(8):708–711. https://doi.org/10.1136/adc.2006.095380
Petrossian MT, Paul LR, Multhaupt-Buell TJ, Eckhardt C, Hayes MT, Duhaime AC, Eskandar EN, Sharma N (2013) Pallidal deep brain stimulation for dystonia: a case series. J Neurosurg Pediatr 12(6):582–587. https://doi.org/10.3171/2013.8.Peds13134
Phukan J, Albanese A, Gasser T, Warner T (2011) Primary dystonia and dystonia-plus syndromes: clinical characteristics, diagnosis, and pathogenesis. Lancet Neurol 10(12):1074–1085. https://doi.org/10.1016/s1474-4422(11)70232-0
Rotsides J, Mammis A (2013) The use of deep brain stimulation in Tourette’s syndrome. Neurosurg Focus 35(5):E4. https://doi.org/10.3171/2013.8.Focus13292
Russo JF, Sheth SA (2015) Deep brain stimulation of the dorsal anterior cingulate cortex for the treatment of chronic neuropathic pain. Neurosurg Focus 38(6):E11. https://doi.org/10.3171/2015.3.Focus1543
Shin SS, Dixon CE, Okonkwo DO, Richardson RM (2014) Neurostimulation for traumatic brain injury. J Neurosurg 121(5):1219–1231. https://doi.org/10.3171/2014.7.Jns131826
Starr PA, Turner RS, Rau G, Lindsey N, Heath S, Volz M, Ostrem JL, Marks WJ Jr (2004) Microelectrode-guided implantation of deep brain stimulators into the globus pallidus internus for dystonia: techniques, electrode locations, and outcomes. Neurosurg Focus 17(1):E4
Tanabe LM, Kim CE, Alagem N, Dauer WT (2009) Primary dystonia: molecules and mechanisms. Nat Rev Neurol 5(11):598–609. https://doi.org/10.1038/nrneurol.2009.160
Tronnier VM, Fogel W (2000) Pallidal stimulation for generalized dystonia. Report of three cases. J Neurosurg 92(3):453–456. https://doi.org/10.3171/jns.2000.92.3.0453
Vidailhet M, Vercueil L, Houeto JL, Krystkowiak P, Benabid AL, Cornu P, Lagrange C, Tezenas du Montcel S, Dormont D, Grand S, Blond S, Detante O, Pillon B, Ardouin C, Agid Y, Destee A, Pollak P (2005) Bilateral deep-brain stimulation of the globus pallidus in primary generalized dystonia. N Engl J Med 352(5):459–467. https://doi.org/10.1056/NEJMoa042187
Wortmann SB, Vaz FM, Gardeitchik T, Vissers LE, Renkema GH, Schuurs-Hoeijmakers JH, Kulik W, Lammens M, Christin C, Kluijtmans LA, Rodenburg RJ, Nijtmans LG, Grunewald A, Klein C, Gerhold JM, Kozicz T, van Hasselt PM, Harakalova M, Kloosterman W, Baric I, Pronicka E, Ucar SK, Naess K, Singhal KK, Krumina Z, Gilissen C, van Bokhoven H, Veltman JA, Smeitink JA, Lefeber DJ, Spelbrink JN, Wevers RA, Morava E, de Brouwer AP (2012) Mutations in the phospholipid remodeling gene SERAC1 impair mitochondrial function and intracellular cholesterol trafficking and cause dystonia and deafness. Nat Genet 44(7):797–802. https://doi.org/10.1038/ng.2325
Zorzi G, Marras C, Nardocci N, Franzini A, Chiapparini L, Maccagnano E, Angelini L, Caldiroli D, Broggi G (2005) Stimulation of the globus pallidus internus for childhood-onset dystonia. Mov Disord 20(9):1194–1200. https://doi.org/10.1002/mds.20510
Funding
A.T.H. is supported by the Vanderbilt University Medical Scientist Training Program (T32GM007347). D.J.E. receives support from the National Institutes of Health (NIH, R00 NS097618).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare that they have no conflict of interest.
Ethical approval
No medical records were accessed in completing this article, and only previously approved, published studies were included in our analysis. Thus, institutional review board approval was required.
Informed consent
Only published studies were used for our analysis, and no patient medical records were accessed in completing this article. Thus, patients’ consent was not required.
Electronic supplementary material
ESM 1
(DOCX 65 kb)
Rights and permissions
About this article
Cite this article
Hale, A.T., Monsour, M.A., Rolston, J.D. et al. Deep brain stimulation in pediatric dystonia: a systematic review. Neurosurg Rev 43, 873–880 (2020). https://doi.org/10.1007/s10143-018-1047-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10143-018-1047-9