Abstract
Deep brain stimulation (DBS) represents an established and internationally approved therapy for movement disorders. In the present retrospective analysis, we evaluated disease-specific longevity of dual channel impulse generators (IPG) used in different movement disorders. We correlated the battery lifetime with electrical stimulation settings, “total electrical energy delivered” (TEED), stimulation modi (monopolar, double monopolar and bipolar) and targets. Specifically, we reviewed the longevity and stimulation settings of 464 IPGs implanted between 1996 until 2011 in a single university center. Disease entities comprised Parkinson’s disease (PD, n = 257), dystonia (n = 130) and essential tremor (ET, n = 50). Further subanalyses aimed at assessing differential longevity in different subtypes of PD and dystonia. The main finding relates to longer IPG longevity in ET (thalamic DBS) and PD (subthalamic DBS) vs. dystonia (pallidal DBS; 71.9 ± 6.7 vs. 51.5 ± 2.3 vs. 37 ± 2 months). In PD the tremor-dominant type was associated with a significant shorter battery survival than in the akinetic-rigid type without tremor or the “balanced” type with tremor, bradykinesia and rigidity (38.8 ± 3.9 vs. 53.6 ± 3.4 vs. 58.8 ± 4.1 months), while there were no significant differences in longevity between the subtypes of dystonia. Frequency, amplitude, pulse widths and TEED correlated inversely with battery lifetime. Pallidal DBS in dystonia is associated with a shorter lifetime of IPGs than subthalamic or thalamic DBS for PD or ET. The present results may contribute to the rapidly evolving refinement of DBS devices. Future studies that assess energy consumption both in patients with and without IPG replacement could help to avoid potential underestimation of longevity of IPGs.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Deep brain stimulation (DBS) has become an established treatment in advanced Parkinson’s disease (PD), medically refractory dystonia and essential tremor (ET) (Deuschl et al. 2006; Kupsch et al. 2006; Blomstedt et al. 2007; Schuepbach et al. 2013). Recently DBS has also been approved for the treatment of obsessive compulsive disorder and epilepsy (Fisher and Velasco 2014; Alonso et al. 2015; Mallet et al. 2008). Moreover there is a growing interest for its use in psychiatric disorders such as depression (Mayberg et al. 2005).
Short- and long-term studies have shown that stimulation of the subthalamic nucleus (STN) and the globus pallidus internus (GPi) both improve quality of life and motor scores, dyskinesia, and ON–OFF fluctuations (Deuschl et al. 2006; Moro et al. 2002; Follett et al. 2010; Weaver et al. 2005; Schuepbach et al. 2013). Stimulation of the GPi demonstrated clinical efficacy in the treatment of different forms of dystonia, such as generalized, cervical, and segmental subtypes (Kupsch et al. 2006; Krauss 2010; Volkmann et al. 2014; Vidailhet et al. 2005). DBS of the ventral intermediate nucleus (VIM) has been identified for symptomatic treatment of ET (Blomstedt et al. 2007; Schuurman et al. 2000).
With the increased application of DBS as a treatment option for a growing number of diseases, the longevity of impulse generators (IPG) in DBS is an important clinical and economical factor. The importance of energy supply is reflected by the appearance of new manufactures (Boston Scientific, Massachusetts, USA and St. Jude, Minnesota, USA), challenging the former monopoly of Medtronic (Minnesota, USA) (Timmermann et al. 2015). IPG “end of life” (EOL) is associated with clinical worsening, and sudden DBS failure can result in medical emergencies due to a rebound of neurologic and neuropsychiatric symptoms especially in dual channel IPGs and dystonia (Vora et al. 2012; Alesch 2005; Tagliati et al. 2010; Anheim et al. 2007; Fakhar et al. 2013).
The exchange of an IPG can increase the overall morbidity due to surgical and anesthesiologic complications with a possible loss of the stability of symptom control and the potential need for extensive and time-consuming reprogramming (Allert et al. 2009; Dams et al. 2013). Furthermore, recent evidence indicates that the rate of hardware infections increases with repeated device replacements (Pepper et al. 2013; Thrane et al. 2014). Thus, information on battery lifetime and parameters that influence battery performance are indispensable.
Various tables and formulas have been published by the manufacturers of DBS devices to estimate battery lifetime (http://www.medtronic.com.hk/wcm/groups/mdtcom_sg/@mdt/@neuro/documents/documents/dbs-sys-longevity.pdf). However, these can only provide rough approximations, since they tend to overestimate battery longevity. Furthermore, formulas and tables are unable to include everyday real-life factors such as frequent parameter changes due to variations in impedances and clinical symptomatology (Fakhar et al. 2013; Montuno et al. 2013).
Surprisingly, only little is known on the longevity of dual channel IPGs. Recent evidence indicates that GPi DBS in dystonia may be associated with shorter longevity than STN stimulation in PD and VIM DBS in ET, possibly, reflecting decreased energy demands in STN-DBS in PD vs. GPi-DBS in dystonia (Rawal et al. 2014; Volkmann 2004).
The present study investigated the lifetime of the most widely used dual channel IPG during the time from 1998 to 2010 (Kinetra® 7248, Medtronic, Minnesota, USA) in chronic bilateral DBS for PD, dystonia, and ET. Furthermore, we aimed to identify the influence of different stimulation parameters, stimulation modi and the TEED. Additionally, we evaluated the effect of multiple replacements on IPG longevity in individual patients. To our knowledge our study is the first to correlate IPG longevity with disease subtypes of PD and dystonia.
Methods
This study represents a retrospective analysis of all patients treated with DBS between 01.03.1996 and 31.12.2010 at the University of Berlin Charité.
The dataset used in the analysis is based on patient files and databases, created by the University of Berlin Charité (AK), during the above mentioned time-frame, which contain inter alia information on all Charité patients treated with DBS and results from regular postoperative follow up examinations. Informed consent was obtained from all individual participants that were included in the study.
We collected demographic data, the respective indications for DBS therapy, stimulation targets and implanted IPG models. All DBS devices used in this context (Itrel, Soletra 7246, Kinetra 7248 and Activa RC/PC) were manufactured by Medtronic, the sole manufacturer at that time.
We identified the time of the first IPG implantation and of their replacements. IPG exchange was routinely planned before the complete discharge of the batteries in order to prevent an unexpected relapse of symptoms.
PD and dystonia patients were treated with continuous DBS, whereas some ET patients chose to turn off their device at night. The battery longevity was calculated and evaluated for each replaced device. Additionally, the programming adjustments including pulse frequency, voltage, pulse widths, the polarity, the number of activated electrode contacts and impedance were documented over the entire lifetime of all IPGs.
For patients with recorded impedance, TEED was calculated using the following formula: [(voltage2 × frequency × pulse width)/impedance] for both mono- and bi-polarly used IPGs (Koss et al. 2005). For controversy calculating TEED in bipolar DBS cf. Blahak et al. (Blahak et al. 2011). TEED for each device was calculated as a mean of both hemisphere. Since changes of electrical stimulation settings (ESS; frequency, impulse width and amplitude) and modus during one battery lifetime circle occurred, we documented the longest setting used in each period. For quantitative reasons the study focused on cases with the IPG type Kinetra 7428 and the movement disorders Parkinson’s disease, dystonia and ET.
Inclusion criteria comprised bilateral DBS and the UK clinical diagnostic criteria for PD (Hughes et al. 1992), dystonia and ET were classified according to Fahn et al. (1998) and Chouinard et al. (1997), respectively.
We exclusively analyzed replacements denoted as a result of battery EOL (<5 % of original battery capacity) or battery depletion. Replacements occurring for any other reasons such as infection or device malfunction were documented but not included in the analysis. Moreover, patients with more than one IPG or more than one electrode per hemisphere at the same time were excluded from our analysis.
For the statistical analysis the statistical software SPSS (Version 20.0 for Windows; SPSS Inc., Chicago, IL, USA) was used, employing the nonparametric Mann–Whitney U, Spearman rank correlation, ANOVA and Wilcoxon rank test. Statistical significance was set to p ≤ 0.05. Data are presented as mean ± SEM, unless otherwise indicated.
Results
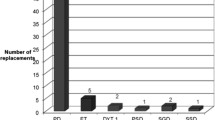
We identified 464 patients treated with DBS (259 PD, 130 dystonia, 50 ET, 7 multiple sclerosis, 1 Tourette syndrome, 3 choreatic diseases, 9 patients with otherwise classified forms of tremor, 1 depression, 1 infantile cerebral palsy, 1 progressive myoclonic epilepsy, 2 multiple system atrophy). Three hundred and nineteen IPG replacements were performed, 298 due to battery depletion, 18 due to infections and 2 for other complications. In 14 patients IPGs were not replaced during the observation period, either due to infections (n = 5) or due to a lack of effect (n = 9) (Fig. 1).
Comparison of average IPG longevity in months across diseases, subtypes and stimulation modi. Across the diseases PD, ET and dystonia IPG longevity differed significantly, while across the disease-specific subtypes there were significant differences between the PD subtypes only. IPG longevity with double monopolar stimulation is significantly shorter than with monopolar stimulation. *p ≤ 0.05, **p ≤ 0.01, ***p ≤ 0.001
Three hundred and fifty seven of the 464 patients received a dual channel Kinetra 7428. The remaining 107 patients were treated using Itrel® or Soletra® 7426 (n = 63) or Activa® (n = 44).
During the observation period 314 out of 357 patients fulfilled study criteria. In 116 (PD n = 64, dystonia n = 47, ET n = 5) out of 314 patients 172 IPG were replaced due to EOL. Eighty seven replacements occurred in PD, 80 in dystonia and 5 in ET. For demographic data see Table 1.
Longevity of the pulse generators across diagnosis, their subtypes and stimulation modi
Mean battery survival of the Kinetra 7428 differed significantly across diseases (Table 2, Mann–Whitney U test). IPG longevity in dystonia was found to be significantly shorter than in PD and ET (p < 0.001 and p = 0.002). Battery survival in PD was significantly shorter than in ET (p = 0.036).
Battery lifetime was significantly shorter in tremor-dominant PD vs. balanced (p = 0.003) vs. akinetic-rigid PD (p = 0.004, Table 2), with no significant difference between balanced type and the akinetic-rigid type (p = 0.41). Battery survival did not differ in dystonia subtypes (p = 0.46; Table 2). Battery survival was similar for mono and bipolar stimulation mode (p = 0.7), while a significantly lower battery survival was observed for the double monopolar mode (vs. monopolar mode, p = 0.002). Similar results were observed for subtypes of PD and dystonia (Supplementary Table 1).
Subgroup analysis for monopolar DBS
Since, different stimulations modi were used in our patients, we performed a subgroup analysis of patients treated with the most prevalent monopolar stimulation modus. Thus, we aimed to exclude differences in stimulation modi as the cause of statistical differences across disease entities. The subanalysis of patients that were treated with a monopolar stimulation settings showed the longest average battery lifetime for ET patients, followed by PD patients and dystonia patients (82.4 vs. 53.9 vs. 43 months; cf. Supplementary Table 1), being significant for dystonia vs. PD (p = 0.024), dystonia vs. ET (p = 0.037), but not for PD vs. ET (p = 0.067). Battery survival differed significantly also between tremor-dominant vs. the akinetic-rigid type (p = 0.045).
Electrical stimulation settings and TEED across diseases and modi
Average stimulation settings (voltage, pulse widths, frequency and TEED) are displayed in Table 3.
IPG longevity in patients with multiple replacements
In 32 of 112 patients treated with DBS more than one IPG replacement was performed. Within this group the first device exchange occurred on average after 40.9 months, the second battery was replaced after 33.7 months, the difference being significant (p = 0.02, Wilcoxon rank test).
A subset (n = 13) of this group experienced more than two replacements, for which the average battery longevity was 30.8, 24.2 and 26.8 months, for the third, fourth and fifth device, respectively (p = 0.033 second vs. third device).
Correlation of electrical stimulation settings with IPG longevity
The battery survival of IPGs (n = 159) correlated inversely with frequency (r = −0.287, p < 0.001, Fig. 2a), impulse amplitude (r = −0.309, p < 0.001, Fig. 2b), impulse widths (r = −0.438, p < 0.001, Fig. 2c) and TEED (r = 0.695, p < 0.001, Fig. 2d).
IPG longevity and correlation of IPG longevity with a frequency, b amplitude, c impulse widths and d TEED. Amplitude is separated in double monopolar (full diamonds), monopolar (light diamonds) and bipolar stimulation (open diamonds). IPG longevity correlated inverse with stimulation settings and TEED in following order: TEED > impulse width > amplitude > frequency (Spearman rank correlation)
Discussion
To our knowledge, this is the first study to investigate disease-specific longevity and battery lifetime of dual channel IPGs in DBS for movement disorders subtypes.
In the present study, the battery lifetime of dual channel IPGs (Kinetra 7428) used for bilateral DBS was shorter in dystonia (GPi) vs. PD (STN) vs. ET (VIM), complying with the results of previous studies investigating battery lifetime in single channel IPGs (Rawal et al. 2014; Allert et al. 2009). Thus, 80/87/5 IPG exchanges were performed in 86/204/34 dystonia/PD/ET patients, respectively (cf. overview on previous results, Table 4). Battery survival was shortest in dystonia and longest in ET. The long IPG survival in ET patients and the large variances between the various studies may be due to differential recommendations to switch off DBS devices at night to possibly prevent variations of stimulation efficacy. Interestingly, small differences of IPG longevity between ET and PD have been reported earlier (Rawal et al. 2014).
Furthermore, we were able to show that the IPG longevity in bilateral DBS of the STN varies significantly between the different subtypes of PD, with the tremor-dominant type being associated with the shortest battery lifetime associated with significantly higher TEED.
For dystonia subtypes we found no statistical differences in IPG lifetime, complying with previous reports, although a trend for a longer battery survival was observed in tardive dystonia patients (Blahak et al. 2011), complying with the present study in monopolarly treated patients.
Furthermore, we observed higher ESS and more frequent multiple electrode activation in pallidal DBS in dystonia. These high ESS could either be due to the need for higher initial program settings or due to a delayed improvement of symptoms in dystonia (Kupsch et al. 2011). Shorter longevity and higher ESS can also be observed in pallidal DBS for PD, presumably due the large volume of the pallidum. In contrast, ESS were lower in STN DBS than in GPi DBS, likely due to the smaller dimension of the STN and the proximity to other relevant structures in the midbrain and associated side effects (Follett et al. 2010; Matias et al. 2015; Odekerken et al. 2013; Rawal et al. 2014).
The TEED applied throughout IPG lifetime circle inversely correlated with IPG longevity, which is consistent with previous studies. Specifically, frequency, pulse width and amplitude correlated with battery survival (Fig. 2), confirming previous reports (Blahak et al. 2011; Isaias et al. 2009; Bin-Mahfoodh et al. 2003; Anheim et al. 2007; Halpern et al. 2011; Ondo et al. 2007).
Interestingly, one previous study reported an average battery survival of 24 months in dystonia patients with pallidal DBS, which contrasts the herein presented survival time of 37 months (Isaias et al. 2009). Notably, in this study a larger fraction of IPGs were used with more than one activated contact (triple or double monopolar stimulation, respectively; 5 vs. 1 and 56 vs. 32 %), with an increasing number of activated contacts during the treatment course (Isaias et al. 2009). IPG survival was shorter in DBS with double monopolar modus compared to monopolar and bipolar modus, while the former only showed minor differences. Thus, small changes in parameter settings and activation of additional electrodes influence IPG longevity (Stewart and Eljamel 2011).
In a subgroup analysis of patients requiring frequent IPG replacements, the first device had the longest battery longevity. Contesting and confirming previous reports can be found in the literature (Lumsden et al. 2012; Halpern et al. 2011; Blahak et al. 2011). The reason for this difference might be the progressive nature of the treated diseases and changes in lead impedance (Halpern et al. 2011).
Strength and limitations
The strengths of the present study comprise its big sample size, its assessment of a single device (Kinetra), its focus on bilateral DBS, and its attempts to assess the influence of disease subtypes on overall battery survival in DBS.
Both study strengths and limitations relate to the monocentric character of the study with uniform surgical technics and postoperative DBS management.
Study limitations apart from its retrospective character relate to the timing of the replacement of devices. Devices were usually replaced prior to complete depletion of a battery. Conceivably, devices may have been exchanged prematurely, and as a result, IPG longevity may have been inaccurately assessed. This limitation applies, however, similarly to all observed groups and does not necessarily reflect a bias for disease targets.
In addition, a limitation of the present study relates to a potential underestimation of longevity of IPGs. Conceivably, patients with excellent outcome and low energy consumption may benefit from longer IPG lifetime. Thus, the mean longevity of IPG might be longer than demonstrated by the present dataset.
Future studies that assess TEED both in patients with and without IPG replacement could help to answer this question.
The present data, which were obtained with an IPG device no longer available on the market, will hopefully contribute to a comparison with new IPG devices from different manufactures and might be of interest when refining rechargeable IPG devices.
Conclusion
Battery longevity of dual channel IPG varies significantly across movement disorders and different PD subtypes, but not dystonia subtypes. Short battery lifetime was observed in GPi DBS for dystonia, long battery survival in VIM–DBS for ET. Activation of multiple electrodes decreased battery survival. Stimulation parameters, especially pulse width and TEED correlated inversely with battery lifetime. The present results contribute to the rapidly evolving refinement of DBS devices.
Abbreviations
- DBS:
-
Deep brain stimulation
- EOL:
-
End of life
- ESS:
-
Electrical stimulation settings
- ET:
-
Essential tremor
- GPi:
-
Globus pallidus internus
- IPG:
-
Impulse generator
- PD:
-
Parkinson’s disease
- STN:
-
Subthalamic nucleus
- TEED:
-
Total electrical energy delivered
- VIM:
-
Ventral intermediate nucleus
References
Alesch F (2005) Sudden failure of dual channel pulse generators. Mov Disord 20(1):64–66. doi:10.1002/mds.20354 (discussion 66)
Allert N, Kirsch H, Weirich W, Karbe H (2009) Stability of symptom control after replacement of impulse generators for deep brain stimulation. J Neurosurg 110(6):1274–1277. doi:10.3171/2009.1.JNS081352
Allert N, Mehnert C, Lehrke R, Maarouf M, Sturm V (2011) Is a patient controller for Parkinson's disease patients with subthalamic nucleus deep brain stimulation reasonable? Stereotact Funct Neurosurg 89(5):305–310. doi:10.1159/000329361
Alonso P, Cuadras D, Gabriels L, Denys D, Goodman W, Greenberg BD, Jimenez-Ponce F, Kuhn J, Lenartz D, Mallet L, Nuttin B, Real E, Segalas C, Schuurman R, du Montcel ST, Menchon JM (2015) Deep brain stimulation for obsessive-compulsive disorder: a meta-analysis of treatment outcome and predictors of response. PLoS One 10(7):e0133591. doi:10.1371/journal.pone.0133591
Anheim M, Fraix V, Chabardes S, Krack P, Benabid AL, Pollak P (2007) Lifetime of Itrel II pulse generators for subthalamic nucleus stimulation in Parkinson’s disease. Mov Disord 22(16):2436–2439. doi:10.1002/mds.21726
Bin-Mahfoodh M, Hamani C, Sime E, Lozano AM (2003) Longevity of batteries in internal pulse generators used for deep brain stimulation. Stereotact Funct Neurosurg 80(1–4):56–60. doi:10.1159/000075161
Blahak C, Capelle HH, Baezner H, Kinfe TM, Hennerici MG, Krauss JK (2011) Battery lifetime in pallidal deep brain stimulation for dystonia. Eur J Neurol 18(6):872–875. doi:10.1111/j.1468-1331.2010.03290.x
Blomstedt P, Hariz GM, Hariz MI, Koskinen LO (2007) Thalamic deep brain stimulation in the treatment of essential tremor: a long-term follow-up. Br J Neurosurg 21(5):504–509. doi:10.1080/02688690701552278
Chouinard S, Louis ED, Fahn S (1997) Agreement among movement disorder specialists on the clinical diagnosis of essential tremor. Mov Disord 12(6):973–976. doi:10.1002/mds.870120621
Dams J, Siebert U, Bornschein B, Volkmann J, Deuschl G, Oertel WH, Dodel R, Reese JP (2013) Cost-effectiveness of deep brain stimulation in patients with Parkinson’s disease. Mov Disord 28(6):763–771. doi:10.1002/mds.25407
Deuschl G, Schade-Brittinger C, Krack P, Volkmann J, Schafer H, Botzel K, Daniels C, Deutschlander A, Dillmann U, Eisner W, Gruber D, Hamel W, Herzog J, Hilker R, Klebe S, Kloss M, Koy J, Krause M, Kupsch A, Lorenz D, Lorenzl S, Mehdorn HM, Moringlane JR, Oertel W, Pinsker MO, Reichmann H, Reuss A, Schneider GH, Schnitzler A, Steude U, Sturm V, Timmermann L, Tronnier V, Trottenberg T, Wojtecki L, Wolf E, Poewe W, Voges J, German Parkinson Study Group NS (2006) A randomized trial of deep-brain stimulation for Parkinson’s disease. N Engl J Med 355(9):896–908. doi:10.1056/NEJMoa060281
Fahn S, Bressman SB, Marsden CD (1998) Classification of dystonia. Adv Neurol 78:1–10
Fakhar K, Hastings E, Butson CR, Foote KD, Zeilman P, Okun MS (2013) Management of deep brain stimulator battery failure: battery estimators, charge density, and importance of clinical symptoms. PLoS One 8(3):e58665. doi:10.1371/journal.pone.0058665
Fisher RS, Velasco AL (2014) Electrical brain stimulation for epilepsy. Nat Rev Neurol 10(5):261–270. doi:10.1038/nrneurol.2014.59
Follett KA, Weaver FM, Stern M, Hur K, Harris CL, Luo P, Marks WJ Jr, Rothlind J, Sagher O, Moy C, Pahwa R, Burchiel K, Hogarth P, Lai EC, Duda JE, Holloway K, Samii A, Horn S, Bronstein JM, Stoner G, Starr PA, Simpson R, Baltuch G, De Salles A, Huang GD, Reda DJ, Group CSPS (2010) Pallidal versus subthalamic deep-brain stimulation for Parkinson’s disease. N Engl J Med 362(22):2077–2091. doi:10.1056/NEJMoa0907083
Halpern CH, McGill KR, Baltuch GH, Jaggi JL (2011) Longevity analysis of currently available deep brain stimulation devices. Stereotact Funct Neurosurg 89(1):1–5. doi:10.1159/000321710
Hughes AJ, Daniel SE, Kilford L, Lees AJ (1992) Accuracy of clinical diagnosis of idiopathic Parkinson’s disease: a clinico-pathological study of 100 cases. J Neurol Neurosurg Psychiatry 55(3):181–184
Isaias IU, Alterman RL, Tagliati M (2009) Deep brain stimulation for primary generalized dystonia: long-term outcomes. Arch Neurol 66(4):465–470. doi:10.1001/archneurol.2009.20
Koss AM, Alterman RL, Tagliati M, Shils JL (2005) Calculating total electrical energy delivered by deep brain stimulation systems. Annals Neurol 58(1):168. doi:10.1002/ana.20525 (author reply 168–169)
Krauss JK (2010) Surgical treatment of dystonia. Eur J Neurol 17(Suppl 1):97–101. doi:10.1111/j.1468-1331.2010.03059.x
Kupsch A, Benecke R, Muller J, Trottenberg T, Schneider GH, Poewe W, Eisner W, Wolters A, Muller JU, Deuschl G, Pinsker MO, Skogseid IM, Roeste GK, Vollmer-Haase J, Brentrup A, Krause M, Tronnier V, Schnitzler A, Voges J, Nikkhah G, Vesper J, Naumann M, Volkmann J, Deep-Brain Stimulation for Dystonia Study G (2006) Pallidal deep-brain stimulation in primary generalized or segmental dystonia. N Engl J Med 355(19):1978–1990. doi:10.1056/NEJMoa063618
Kupsch A, Tagliati M, Vidailhet M, Aziz T, Krack P, Moro E, Krauss JK (2011) Early postoperative management of DBS in dystonia: programming, response to stimulation, adverse events, medication changes, evaluations, and troubleshooting. Mov Disord 26(Suppl 1):S37–S53. doi:10.1002/mds.23624
Lumsden DE, Kaminska M, Tustin K, Gimeno H, Baker L, Ashkan K, Selway R, Lin JP (2012) Battery life following pallidal deep brain stimulation (DBS) in children and young people with severe primary and secondary dystonia. Child’s Nerv Syst ChNS 28(7):1091–1097. doi:10.1007/s00381-012-1728-6
Mallet L, Polosan M, Jaafari N, Baup N, Welter ML, Fontaine D, du Montcel ST, Yelnik J, Chereau I, Arbus C, Raoul S, Aouizerate B, Damier P, Chabardes S, Czernecki V, Ardouin C, Krebs MO, Bardinet E, Chaynes P, Burbaud P, Cornu P, Derost P, Bougerol T, Bataille B, Mattei V, Dormont D, Devaux B, Verin M, Houeto JL, Pollak P, Benabid AL, Agid Y, Krack P, Millet B, Pelissolo A, Group SS (2008) Subthalamic nucleus stimulation in severe obsessive-compulsive disorder. N Engl J Med 359(20):2121–2134. doi:10.1056/NEJMoa0708514
Matias CM, Mehanna R, Cooper SE, Amit A, Lempka SF, Silva D, Carlotti CG Jr, Butler RS, Machado AG (2015) Correlation among anatomic landmarks, location of subthalamic deep brain stimulation electrodes, stimulation parameters, and side effects during programming monopolar review. Neurosurgery. doi:10.1227/NEU.0000000000000645
Mayberg HS, Lozano AM, Voon V, McNeely HE, Seminowicz D, Hamani C, Schwalb JM, Kennedy SH (2005) Deep brain stimulation for treatment-resistant depression. Neuron 45(5):651–660. doi:10.1016/j.neuron.2005.02.014
Montuno MA, Kohner AB, Foote KD, Okun MS (2013) An algorithm for management of deep brain stimulation battery replacements: devising a web-based battery estimator and clinical symptom approach. Neuromodulation 16(2):147–153. doi:10.1111/j.1525-1403.2012.00457.x
Moro E, Esselink RJ, Benabid AL, Pollak P (2002) Response to levodopa in parkinsonian patients with bilateral subthalamic nucleus stimulation. Brain 125(Pt 11):2408–2417
Odekerken VJ, van Laar T, Staal MJ, Mosch A, Hoffmann CF, Nijssen PC, Beute GN, van Vugt JP, Lenders MW, Contarino MF, Mink MS, Bour LJ, van den Munckhof P, Schmand BA, de Haan RJ, Schuurman PR, de Bie RM (2013) Subthalamic nucleus versus globus pallidus bilateral deep brain stimulation for advanced Parkinson’s disease (NSTAPS study): a randomised controlled trial. Lancet Neurol 12(1):37–44. doi:10.1016/S1474-4422(12)70264-8
Ondo WG, Meilak C, Vuong KD (2007) Predictors of battery life for the Activa Soletra 7426 Neurostimulator. Parkinsonism Relat Disord 13(4):240–242. doi:10.1016/j.parkreldis.2006.11.002
Pepper J, Zrinzo L, Mirza B, Foltynie T, Limousin P, Hariz M (2013) The risk of hardware infection in deep brain stimulation surgery is greater at impulse generator replacement than at the primary procedure. Stereotact Funct Neurosurg 91(1):56–65. doi:10.1159/000343202
Rawal PV, Almeida L, Smelser LB, Huang H, Guthrie BL, Walker HC (2014) Shorter pulse generator longevity and more frequent stimulator adjustments with pallidal DBS for dystonia versus other movement disorders. Brain Stimul 7(3):345–349. doi:10.1016/j.brs.2014.01.008
Schuepbach WM, Rau J, Knudsen K, Volkmann J, Krack P, Timmermann L, Halbig TD, Hesekamp H, Navarro SM, Meier N, Falk D, Mehdorn M, Paschen S, Maarouf M, Barbe MT, Fink GR, Kupsch A, Gruber D, Schneider GH, Seigneuret E, Kistner A, Chaynes P, Ory-Magne F, Brefel Courbon C, Vesper J, Schnitzler A, Wojtecki L, Houeto JL, Bataille B, Maltete D, Damier P, Raoul S, Sixel-Doering F, Hellwig D, Gharabaghi A, Kruger R, Pinsker MO, Amtage F, Regis JM, Witjas T, Thobois S, Mertens P, Kloss M, Hartmann A, Oertel WH, Post B, Speelman H, Agid Y, Schade-Brittinger C, Deuschl G, Group ES (2013) Neurostimulation for Parkinson’s disease with early motor complications. N Engl J Med 368(7):610–622. doi:10.1056/NEJMoa1205158
Schuurman PR, Bosch DA, Bossuyt PM, Bonsel GJ, van Someren EJ, de Bie RM, Merkus MP, Speelman JD (2000) A comparison of continuous thalamic stimulation and thalamotomy for suppression of severe tremor. N Engl J Med 342(7):461–468. doi:10.1056/NEJM200002173420703
Stewart CD, Eljamel S (2011) Prediction of implantable pulse generator longevity in deep brain stimulation: limitations and possible solutions in clinical practice. Stereotact Funct Neurosurg 89(5):299–304. doi:10.1159/000329360
Tagliati M, Martin C, Alterman R (2010) Lack of motor symptoms progression in Parkinson’s disease patients with long-term bilateral subthalamic deep brain stimulation. Int J Neurosci 120(11):717–723. doi:10.3109/00207454.2010.518777
Thrane JF, Sunde NA, Bergholt B, Rosendal F (2014) Increasing infection rate in multiple implanted pulse generator changes in movement disorder patients treated with deep brain stimulation. Stereotact Funct Neurosurg 92(6):360–364. doi:10.1159/000365576
Timmermann L, Jain R, Chen L, Maarouf M, Barbe MT, Allert N, Brucke T, Kaiser I, Beirer S, Sejio F, Suarez E, Lozano B, Haegelen C, Verin M, Porta M, Servello D, Gill S, Whone A, Van Dyck N, Alesch F (2015) Multiple-source current steering in subthalamic nucleus deep brain stimulation for Parkinson’s disease (the VANTAGE study): a non-randomised, prospective, multicentre, open-label study. Lancet Neurol 14(7):693–701. doi:10.1016/S1474-4422(15)00087-3
Vidailhet M, Vercueil L, Houeto JL, Krystkowiak P, Benabid AL, Cornu P, Lagrange C, Tezenas du Montcel S, Dormont D, Grand S, Blond S, Detante O, Pillon B, Ardouin C, Agid Y, Destee A, Pollak P, French Stimulation du Pallidum Interne dans la Dystonie Study G (2005) Bilateral deep-brain stimulation of the globus pallidus in primary generalized dystonia. N Engl J Med 352(5):459–467. doi:10.1056/NEJMoa042187
Volkmann J (2004) Deep brain stimulation for the treatment of Parkinson’s disease. J Clin Neurophysiol 21(1):6–17
Volkmann J, Mueller J, Deuschl G, Kuhn AA, Krauss JK, Poewe W, Timmermann L, Falk D, Kupsch A, Kivi A, Schneider GH, Schnitzler A, Sudmeyer M, Voges J, Wolters A, Wittstock M, Muller JU, Hering S, Eisner W, Vesper J, Prokop T, Pinsker M, Schrader C, Kloss M, Kiening K, Boetzel K, Mehrkens J, Skogseid IM, Ramm-Pettersen J, Kemmler G, Bhatia KP, Vitek JL, Benecke R, dystonia DBSsgf (2014) Pallidal neurostimulation in patients with medication-refractory cervical dystonia: a randomised, sham-controlled trial. Lancet Neurol 13(9):875–884. doi:10.1016/S1474-4422(14)70143-7
Vora AK, Ward H, Foote KD, Goodman WK, Okun MS (2012) Rebound symptoms following battery depletion in the NIH OCD DBS cohort: clinical and reimbursement issues. Brain Stimul 5(4):599–604. doi:10.1016/j.brs.2011.10.004
Weaver F, Follett K, Hur K, Ippolito D, Stern M (2005) Deep brain stimulation in Parkinson disease: a metaanalysis of patient outcomes. J Neurosurg 103(6):956–967. doi:10.3171/jns.2005.103.6.0956
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This study was funded by Deutsche Forschungsgemeinschaft (Grant number GE 2629/1-1) and the German Ministry of Education and Research.
Conflict of interest
Andreas Kupsch: Consultancies: none; Advisory Boards: Medtronic USA; Honoraria for speaking from Abbvie, Allergan, Boehringer Ingelheim, Desitin, Ipsen Pharma, Lundbeck, Medtronic, Merck, Merz Pharmaceuticals, Orion, St. Jude UCB. The authors declare that they have no conflict of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
van Riesen, C., Tsironis, G., Gruber, D. et al. Disease-specific longevity of impulse generators in deep brain stimulation and review of the literature. J Neural Transm 123, 621–630 (2016). https://doi.org/10.1007/s00702-016-1562-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00702-016-1562-1