Abstract
Because the progression of multiple system atrophy (MSA) is usually rapid and there still is no effective cause-related therapy, early and accurate diagnosis is important for the proper management of patients as well as the development of neuroprotective agents. However, despite the progression in the field of MSA research in the past few years, the diagnosis of MSA in clinical practice still relies largely on clinical features and there are limitations in terms of sensitivity and specificity, especially in the early course of the disease. Furthermore, recent pathological, clinical, and neuroimaging studies have shown that (1) MSA can present with a wider range of clinical and pathological features than previously thought, including features considered atypical for MSA; thus, MSA can be misdiagnosed as other diseases, and conversely, disorders with other etiologies and pathologies can be clinically misdiagnosed as MSA; and (2) several investigations may help to improve the diagnosis of MSA in clinical practice. These aspects should be taken into consideration when revising the current diagnostic criteria. This is especially true given that disease-modifying treatments for MSA are under investigation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Multiple system atrophy (MSA) is an adult-onset sporadic neurodegenerative disorder characterized by any combination of parkinsonism, cerebellar ataxia, and autonomic failure [1]. Because the progression of MSA is usually rapid and relentless with a mean survival of 6–10 years [2, 3] and there still is no effective disease-modifying therapy, early and accurate diagnosis is crucial not only for the optimal management of patients but also for the development of therapeutic strategies. Despite the progress in the field of MSA research in the past few years, the diagnosis of MSA in clinical practice still relies largely on clinical symptoms and signs. However, recent pathological and clinical studies have reported the following findings: (1) patients with MSA can present with a wider range of clinical features and have more variable disease courses than previously thought, including features considered atypical for MSA; (2) pathological changes vary more than previously described in terms of the regions involved as well as the degrees of neurodegeneration, and (3) neuroimaging and electrophysiological tests used for the diagnosis of MSA in clinical practice can be misleading [4–11]. Thus, disorders with other etiologies and pathologies can be clinically misdiagnosed as MSA, and conversely, MSA can be misdiagnosed as other diseases.
To this end, we review recent advances regarding the clinical, pathological, and neuroimaging features of MSA regarding the current diagnostic criteria [12] and discuss the problems and difficulties that hamper the diagnosis and differential diagnosis of MSA.
Current diagnostic criteria and their problems
Clinical diagnostic criteria
As in other neurodegenerative disorders, a definite diagnosis of MSA is based on a postmortem pathological confirmation. However, in clinical practice, a definite diagnosis cannot be reached for obvious reasons, and clinicians have to rely on the suggested clinical diagnostic criteria which include clinical features and, when needed, neuroimaging features [12]. Clinical features for a diagnosis of MSA consist of autonomic failure in combination with motor symptoms. Autonomic failure in MSA includes cardiovascular dysfunction, genitourinary dysfunction, thermoregulatory and sudomotor dysfunction, fecal incontinence and constipation, and sleep-disordered breathing [1, 13], among which orthostatic hypotension or urinary symptoms are required for the diagnosis. Motor symptoms include poorly levodopa-responsive parkinsonism or cerebellar ataxia. A diagnosis of probable MSA is made when a patient has urinary incontinence or an orthostatic decrease in blood pressure within 3 min of standing by at least 30 mmHg systolic or 15 mmHg diastolic in addition to motor symptoms. If the autonomic dysfunction of the patient does not meet this requirement, a diagnosis of possible MSA is made, but only when there is at least one of the “additional” clinical or neuroimaging features (Table 1) because without it, the specificity of the diagnosis will decrease, and MSA will be overdiagnosed.
However, current clinical diagnostic criteria for MSA have several limitations. First, it is predominantly focused on the motor manifestations of the disease. However, both poorly levodopa-responsive parkinsonism and cerebellar ataxia are not rare conditions and actually occur more commonly in disorders other than MSA [14, 15]. Furthermore, although rare, even the combination of both can occur in other disorders [5, 16]. Second, autonomic failure, especially genitourinary dysfunction is not specific to MSA. It can occur in other neurodegenerative disorders and even in otherwise healthy individuals in their 50 and 60 s, which is a common onset age of MSA [2, 17, 18]. Indeed, it is estimated that the prevalence of urinary incontinence is 20–40 % in community-dwelling middle aged women and 7–24 % in men [19–21]. With this high prevalence in the general population, one can assume that a patient with parkinsonism or cerebellar ataxia with pathology other than MSA can coincidently develop urinary incontinence with a pathophysiology unrelated to motor symptoms, which will lead to overdiagnosis of MSA. Furthermore, current clinical diagnostic criteria do not consider the types of urinary incontinence such that the diagnosis of MSA can be made in a patient with any type of urinary incontinence despite the fact that urge incontinence and stress incontinence appear to have different pathophysiologies [22]. Another thing to mention is that there is no item for the female equivalent of male impotence, although reduced genital sensitivity has been suggested [13, 23]. Third, orthostatic hypotension can also be seen in many conditions other than MSA. The current guideline recommends that other causes of orthostatic hypotension should be excluded [12]. However, sometimes it is not easy to tell whether orthostatic hypotension in a patient is due to MSA or due to other causes. For example, one study showed that approximately 20 % of Parkinson Disease (PD) patients developed symptomatic orthostatic hypotension [24]. Furthermore, orthostatic hypotension can be observed even in healthy individuals. A study on community-dwelling elderly showed that 6.4 % of the otherwise healthy individuals had orthostatic hypotension with a decrease in systolic blood pressure greater than 20 mmHg, and 1.6 % had a decrease in systolic blood pressure greater than 30 mmHg [25]. Given that MSA is a rare disorder, this low prevalence of orthostatic hypotension in the general population can affect the specificity of the clinical diagnosis of MSA. Determining the presence of orthostatic hypotension is another problem. Sometimes a patient with a positive orthostatic hypotension test shows no orthostatic hypotension in a test performed several hours or days later and vice versa, which complicates the diagnosis. The meaning of ‘significant orthostatic blood pressure decline that does not meet the level required in probable MSA’ for the diagnosis of possible MSA is also not clear without specifying the level of decrease in blood pressure. Fourth, both autonomic failure and motor symptoms are needed for the diagnosis, but it does not develop simultaneously in many patients. Some MSA patients develop only autonomic failure early in the course of the disease and are misdiagnosed as primary autonomic failure for years, whereas some other patients develop autonomic failure after as long as 15 years after disease onset delaying the correct diagnosis [9, 26].
Pathological diagnostic criteria
The histopathology of MSA encompasses four major features: (1) selective neuronal loss and axonal degeneration mainly involving the nigrostriatal and pontocerebellar systems; (2) four types of cellular α-synuclein (αSyn) immunoreactive inclusions [glial cytoplasmic inclusions(GCIs) within oligodendrocytes, less frequent glial nuclear inclusions (GNIs), neuronal cytoplasmic inclusions (NCIs), neuronal nuclear inclusions (NNIs)] [27]; (3) astroglial cytoplasmic inclusions and threads of similar composition, and (4) myelin pallor and accompanying gliosis [28]. Because there are no specific markers for the clinical diagnosis of MSA, the definite diagnosis rests on the results of neuropathological examination. The histological hallmark is the presence of cytoplasmic αSyn positive GCIs within oligodendroglial cells, which is required for the postmortem diagnosis of definite MSA [4, 29]. However, pathological diagnostic criteria also bear some limitations.
There is evidence that PD and the parkinsonian variant of MSA (MSA-P) overlap at multiple levels [30]. Both disorders are characterized by deposition of abnormally phosphorylated fibrillar αSyn within the CNS suggesting shared pathophysiological mechanisms [31]. Whereas αSyn aggregates in MSA predominantly involve the cytoplasm of oligodendrocytes, in PD brains these aggregates (Lewy bodies and neurites) are found in neurons and axons [32, 33]. While the type, composition, and cellular distribution of the aggregates are clearly different [4, 33], there is still important pathological overlap [34]. In both disorders, neurodegeneration is associated with the Lewy body and GCI burden as well as an increase of soluble αSyn in substantia nigra and striatum [30], whereas no insoluble αSyn was found in MSA, a divergence from other synucleinopathies [35]. Apart from the overlap in degeneration of the dopaminergic nigrostriatal system, involvement of the autonomic nervous system including the dorsal motor vagal nucleus, autonomic parts of the spinal cord, and the peripheral autonomic nervous system (e.g. the cardiac and enteric systems) is common in both MSA-P and PD [34, 36–38].
Intriguingly, brainstem Lewy bodies—a classical hallmark of PD—were also reported in MSA [37, 39, 40], and, vice versa, GCI pathology occurred in familial PD cases with rapid disease progression [32, 41] whereas a study of 59 Japanese MSA cases did not find any concomitant Lewy pathology [42] that could reflect genetic or environmental differences to European patients. In the brain of an elderly patient diagnosed with PD, in addition to widespread Lewy pathology and moderate cell loss in substantia nigra, GCIs were found in multiple brain areas, suggesting a combination of PD and early stage of MSA that had not progressed to striatal involvement [43]. This association, termed “transitional variant,” is of unknown clinical and pathological significance [44].
These findings suggest a continuum of changes rather than strictly divided entities [45], but the molecular basis and pathogenesis of these co-existing pathologies remain to be elucidated. Recently, in a British family with autosomal-dominant inheritance related to a G51D SNCA mutation, sharing features of both PD and MSA were reported [46], and in a Finish patient with a novel SNCA mutation A53E, PD-type pathology was associated with severe atypical MSA [47], both providing a possible link between the two disorders. These morphological similarities provide further evidence that αSyn aggregates are able to interfere with physiological processes thereby triggering neurodegenerative processes irrespective of the underlying inclusion pathology.
Diagnosis of MSA can be readily made without difficulty in some patients. However, for the reasons mentioned above, in some patients, it can be overdiagnosed, and in other patients, the diagnosis can be missed or delayed [33]. To this end, to improve the clinical diagnosis, current diagnostic criteria provides additional, supporting, and non-supporting features for the diagnosis (Tables 1, 2). Notwithstanding, the accuracy, especially the sensitivity of a clinical diagnosis of MSA, is still not high enough at 88.2 % when diagnosed by movement disorder specialists and 64.3 % when diagnosed by general neurologists [48, 49]. Furthermore, although many new pathological, clinical, and neuroimaging features which can improve the diagnosis of MSA have been described in recent studies, they are not considered in the current diagnostic criteria mostly because those studies were published after the publication of the current diagnostic criteria.
Investigations for improved diagnosis of MSA
In addition to MRI, single photon emission computed tomography (SPECT), and positron emission tomography (PET), which are included in the current diagnostic criteria, several investigations may help to improve the diagnosis of MSA in clinical practice [50]. However, the usefulness of these investigations and how much the findings from these investigations improve the diagnosis should be scrutinized before they are recommended to clinicians. There are also neuroimaging studies using more investigational protocols which need specialized techniques or use of novel ligands which are not widely available. Several studies have analyzed samples from patients including plasma and cerebrospinal fluid which have revealed the distinguishing features of MSA. However, these methods will not be considered in this review because they are not readily available in clinical practice yet, and more studies are needed for those methods to be reliably applied to clinical practice.
MRI
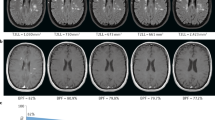
Atrophy of the putamen, middle cerebellar peduncle, pons, or cerebellum on an MRI is included as an additional feature for the diagnosis of possible MSA in the current diagnostic criteria. In addition, several studies have shown that lateral slit-like hyperintense putaminal rim and putaminal hypointensity occur more often in MSA patients than in controls and PD patients on conventional brain MRIs [7, 51–55]. Slit-like hyperintense putaminal rim has been related to the enlargement of the intertissue space between the putamen and the external capsule and the tissue rarefaction associated with neuronal loss and gliosis [56, 57]. However, several studies have shown that the slit-like hyperintense rim is present in normal subjects on 3.0T MRIs [7, 58–60], and even on 1.5T MRIs [61], (Fig. 1), which is thought to be a truncation artifact or age-related disproportionate ferritin deposit between the lateral margin area and the remainder of the putamen. Thus, it is suggested that the discontinuity or irregular disruption of rim is a more reliable marker for MSA than the rim itself [7, 61]. Putaminal hypointensity is observed especially in the posterolateral region along with putaminal atrophy and may reflect diffuse ferritin and Fe3+ deposits [57]. It is more readily observed with imaging sequences sensitive to the susceptibility effect of mineral deposits. However, a recent study using 3.0T MRI showed that age-matched patients with PD and controls also presented putaminal hypointensity, which might have resulted from the increased susceptibility effect of 3.0T MRI [58]. A cruciform hyperintensity within the pons, referred to as a ‘hot-cross bun’ sign, was observed in 63 % of patients with MSA and 80 % of patients with MSA-cerebellar type (MSA-C) [62, 63]. However, this finding is not specific to MSA and can be observed in other diseases with pontocerebellar degeneration including spinocerebellar ataxia (SCA), variant Creutzfeldt-Jakob disease, vasculitis-associated parkinsonism, cerebrotendinous xanthomatosis, and even after bilateral pontine infarction [64–67] (Fig. 2)
‘Hot cross bun’ sign (arrows) in a patient with SCA17 (a) and a patients with bilateral pontine infarction (b) (Reproduced from Roh et al. [67] with kind permission of Journal of Movement Disorders)
Functional brain imaging
Abnormalities in 18F-2-fluoro-dexosy-d-glucose (FDG) PET and SPECT are included as an additional feature for the diagnosis of possible MSA in the current diagnostic criteria (Table 1). Previous studies have shown that FDG PET and perfusion SPECT could discriminate MSA-P from PD and MSA-C from other degenerative cerebellar ataxias by visual inspection [52]. However, the discrimination of MSA is not robust due to significant overlaps, and it needs to be evaluated whether these methods can reliably be used in patients with early MSA. Presynaptic dopaminergic imaging shows presynaptic nigrostriatal degeneration even in uncertain cases; however, it cannot discriminate MSA from other parkinsonian syndromes including PD and progressive supranuclear palsy (PSP) [68, 69]. Furthermore, some patients with MSA-C show normal dopamine transporter imaging [70].
Imaging of cardiac sympathetic innervation
123I-metaiodobenzylguanidine (MIBG) is an analog of guanethidine, an adrenergic blocking agent, which is taken up and stored by sympathetic nerve endings through a mechanism similar to that of noradrenaline. Therefore, MIBG myocardial scintigraphy can assess the postganglionic presynaptic cardiac sympathetic nerve endings. In contrast to PD where cardiac MIBG uptake is reduced reflecting the degeneration of sympathetic axons in the heart, cardiac MIBG uptake was preserved in MSA in many studies, and it is claimed that MIBG myocardial scintigraphy can differentiated MSA from PD in the early stage of the disease [71, 72]. However, there is a significant overlap in MIBG uptake between PD and MSA, and some studies have shown reduced uptake in MSA [6, 73, 74]. Given the overlap and much higher prevalence of PD over MSA, its diagnostic value is limited.
Transcranial sonography
Transcranial B-mode sonography (TCS) is a relatively inexpensive, radiation-free, easily applicable technique to visualize parenchymal structures such as the substantia nigra and lenticular nucleus. Whereas approximately 90 % of PD patients present enlarged echogenicity of the substantia nigra, though not related to the disease duration or severity, 10–30 % of patients with atypical parkinsonism show an abnormality thus differentiating MSA from PD [52, 75, 76]. In addition, 70–90 % of patients with MSA or PSP present hyperechogenicity of the lenticular nucleus while it is observed in 23 % of PD patients [77, 78]. Based on these findings, it has been claimed that TCS can be used to discriminate between PD and atypical parkinsonism, and a recent study has demonstrated that this method shows accuracies comparable to FDG-PET for the differential diagnosis of other neurodegenerative parkinsonisms [79]. However, the major drawback of TCS in the diagnosis of MSA is its inability to differentiate MSA from other atypical parkinsonisms. Furthermore, there is a poor temporal window in about 10–15 % of the subjects, and the technique depends somewhat on the examiner’s experience.
Sphincter electromyography
Anal sphincter electromyography (EMG) is a useful method for detecting the neurogenic change of the anal sphincter muscle, which reflects the degeneration of Onuf’s nucleus. Anal sphincter EMG has been reported to be useful in the differential diagnosis between MSA and PD [80] and was included as a diagnostic investigation in the 1st consensus criteria [81]. However, further studies have shown that anal sphincter EMG does not distinguish MSA from PD, and a negative test could not exclude a diagnosis of MSA, especially in the early stage of the disease [8, 82]. Thus, anal sphincter EMG was not included as a diagnostic investigation in the 2nd consensus criteria.
Quantitative autonomic function tests
Although various autonomic dysfunctions develop in patients with MSA, only orthostatic hypotension and urinary symptoms are included in the diagnostic criteria. However, as mentioned earlier, these autonomic symptoms can occur in other neurodegenerative disorders including PD, which hampers the diagnosis of MSA. In this regard, several standard quantitative autonomic tests including Valsalva maneuver, tilt table test, quantitative sudomotor axon reflex test, and thermoregulatory sweat test (TST) have been used to discriminate MSA from PD, and the results showed that none of these tests distinguished between MSA and PD, alone or in combination [83, 84]. However, recently, it has been claimed that TST combined with the composite autonomic scoring scale for laboratory quantification of generalized autonomic failure can be used for the differential diagnosis of MSA [6, 85].
Video oculography
The detection of subclinical cerebellar dysfunction in patients with parkinsonism would help the differential diagnosis of MSA. In this regard, clinical examination of extraocular movements with laboratory recordings may provide a useful adjunct. One study has shown that patients with MSA are characterized by excessive square wave jerk, hypometric saccades, impaired suppression of the vestibule-ocular reflex, and spontaneous nystagmus as well as positional downbeat nystagmus (pDBN) [86]. Another study has documented that the presence of perverted head-shaking nystagmus and pDBN may be a clue for the diagnosis of MSA [87]. However, no other studies have examined the usefulness of the examination of extraocular movement in the differential diagnosis of MSA, and thus, further studies are needed.
Boundary issues in the diagnosis of MSA
As discussed earlier, recent pathological and clinical studies have shown that MSA can have a wider range of presentations than previously thought. This makes the accurate diagnosis of MSA more difficult and expands the list of differential diagnoses. Among these, several issues need to be discussed in detail regarding the current diagnostic criteria.
Neuropathological Issues
Overlapping and distinguishing features between MSA-P and PD are shown in Table 3.
Despite a rare co-occurrence of MSA with PSP, a four-repeat tauopathy morphologically featured by tufted astrocytes, tau-positive neuronal and oligodendroglial inclusions [88], four cases showing both pathologies were published [89–91]. The cerebellar phenotype of PSP Richardson’s syndrome [92, 93] and genetic or secondary late-onset ataxias may be labeled as MSA-C, because of similar disease presentation [94], but they show different neuropathologies.
Neuropathological examination of a male aged 74 years with a clinical diagnosis of “probable MSA-C” showed MSA-C with pronounced β-amyloid pathology in the frontal lobe and mild hippocampal tau pathology [95], but concomitant AD-like pathologies in MSA are less frequent than those in age-matched controls [39]. Recently, two patients aged 71 and 72 years were reported to show combined MSA and mild AD (Braak neuritic stages III and IV), with abundant αSyn-positive GCIs and NCIs and co-occurrence of αSyn and tau pathology in hippocampus and entorhinal cortex. Immunoreactivity for p62, a ubiquitin–proteasome system-related protein, and UBB+1, a mutant form of ubiquitin and marker for proteasomal dysfunction, was found in most tangles, but only in few αSyn-positive inclusions, suggesting that the proteasomal pathways differ between αSyn and p-tau-bearing neurons [96].
Sporadic adult-onset ataxia of unknown etiology
Sporadic adult-onset ataxia of unknown etiology (SAOA), also called idiopathic adult-onset cerebellar ataxia, constitutes the most common progressive ataxias in adults. SAOA starts at the age of 50–55 years which is slightly lower than that of 55–60 years in MSA [97, 98]. The diagnosis of SAOA is made after excluding known causes of ataxias [14]. Probably, it is a heterogeneous group of diseases with various etiologies including genetic, inflammatory, immunologic, and metabolic factors. MSA-C can be indistinguishable from SAOA in the early stage when the patient has only cerebellar symptoms. Given that SAOA has much more benign course [98, 99], an early accurate diagnosis has important clinical consequences. However, in a patient with recently developed progressive ataxia, one can rarely predict whether the patient will remain in SAOA or will later develop autonomic failure and evolve to MSA. Sometimes, the presence of mild parkinsonism helps the diagnosis, but this is not usual. In this scenario, early MSA can be misdiagnosed as SAOA. Indeed, one study has shown that 24 % of patients diagnosed with SAOA evolved to MSA in 5 years [99]. Conversely, a patient with SAOA can be misdiagnosed as having MSA when the patient develops urinary dysfunction or orthostatic hypotension due to other causes.
SCAs and other genetic disorders
SCA refers to a group of autosomal dominant genetic disorders characterized by progressive neurodegeneration of the cerebellum and its efferent and afferent connections. The presence of a family history of cerebellar ataxia usually helps the diagnosis of SCA; however, a significant proportion of patients with SCA do not have a family history with the frequency of patients with the SCA mutation being 9–22 % among patients with apparently sporadic cerebellar ataxia [100–102]. Both MSA-C and SCA present with cerebellar ataxia; thus, when a patient with apparently sporadic SCA develops autonomic failure in addition to cerebellar ataxia, this patient can be misdiagnosed with MSA-C. Furthermore, some SCAs including SCA 2, 3, 6, and 17, and dentatorubropallidoluysian atrophy develop parkinsonism with nigrostriatal degeneration evidenced by abnormal dopamine transporter imaging [103, 104], sometimes even without cerebellar dysfunctions, which can be misdiagnosed as MSA-P when accompanied by autonomic failure. In agreement with this, a recent study showed that mutations in the SCA genes were found in 7.3 % of the patients who met the clinical diagnostic criteria for MSA [5], suggesting that genetic testing for SCAs should be included in the diagnostic workup for MSA. Of note is that there are reports on Lewy bodies or αSyn-positive GCIs in patients with SCA [105–107], suggesting a relationship between SCA mutations and α–synucleinopathies.
There are other genetic disorders which can clinically mimic MSA. Among them are fragile X tremor/ataxia syndrome, Friedreich ataxia, Perry syndrome, hereditary spastic paraplegia, mitochondrial disorders, and other autosomal recessive cerebellar ataxias [108, 109]. SNCA multiplications also can lead to pathological and clinical features of MSA as well as PD and dementia with Lewy bodies (DLB) [41].
Familial MSA
Multiple system atrophyis considered a sporadic disorder, and a family history of ataxia or parkinsonism is defined as a non-supporting feature in the current diagnostic criteria. However, familial aggregation of parkinsonism has been reported in MSA [110, 111], and there are reports on autopsy-proven familial MSA [11, 112, 113]. Based upon these, a recent Japanese study showed that COQ2 is a causative gene as well as a risk gene for familial and sporadic MSA [112]. However, this finding has not been replicated in other ethnic groups, and the role of COQ2 in the pathogenesis of MSA is still questionable [114–116].
MSA and dementia
Despite mild cognitive dysfunction being repeatedly described in patients with MSA [117–119], dementia is defined as a non-supporting feature in the current diagnostic criteria. However, dementia as well as mild cognitive dysfunction has been reported in patients with autopsy-proven MSA [39, 120], and recent reports have shown that dementia occurs in up to 31 % of MSA patients [10], which indicates that the diagnosis of MSA cannot be excluded by the presence of dementia. The structural correlates of cognitive decline in MSA are still unclear, because a recent clinicopathological study of nine MSA cases each with and without cognitive impairment found no essential qualitative and quantitative differences in MSA-specific αSyn, GCI density and distribution, or secondary pathological conditions such as concomitant Alzheimer-related pathology, cerebral amyloid angiopathy or cerebrovascular disease between the two groups [121]. In a clinicopathological study of 44 MSA patients, four (aged 65–72 years) with mild memory disturbances and frontal executive dysfunctions scored Braak neuritic stage III or IV with variable amounts of cortical amyloid plaques. One demented woman aged 82 years showed MSA-P with fully developed AD (Braak stage V; NIA-AA ABC score 3/3/3) and severe amyloid deposits in the whole cortex, while the brain of a demented male aged 55 years with MSA-C showed no AD-related or any other concomitant cerebral lesions. The GCI load in striatum and the proportion of cases with subcortical small vessel disease did not significantly differ between MSA cases with and without dementia [39]. In view of limited data on the molecular basis of cognitive and behavioral disorders in MSA, currently related to slowly progressive striatofrontal deafferentation [10, 122] or other subcorticocortical lesions which occasionally may spread to the cortex, further studies on morphological substrates of the increasingly observed cognitive impairment in MSA are warranted.
The presence of dementia in MSA, especially in MSA-P, may pose a problem in the diagnosis of DLB. MSA-P and DLB are different diseases with different pathologies; however, both diseases share poorly levodopa-responsive parkinsonism and autonomic failure [123]. According to the current diagnostic criteria, the most obvious feature distinguishing DLB from MSA-P is the presence of dementia with fluctuating cognition and visual hallucinations. Given that dementia occurs in patients with MSA and that even visual hallucination occurs in a small proportion of patients with MSA [18, 124, 125], it will be difficult to make a diagnosis between DLB and MSA-P when a patient presents with parkinsonism, dementia, and autonomic dysfunction without cerebellar symptoms. Furthermore, there is overlap in the cognitive profiles of DLB and MSA although more profound in DLB [124]. Fluctuating cognition may help the diagnosis because it appears to be absent in MSA [123]. However, as it has been suggested, this feature may have been overlooked in MSA [10]. Further studies are needed to improve the accuracy of the differential diagnosis between these two diseases.
MSA with normal dopamine transporter imaging
Neuropathology of MSA involves both presynaptic nigrostriatal and postsynaptic striatal neurons in patients with parkinsonism and even in patients with MSA-C without overt parkinsonism. Dopamine transporter imaging readily shows presynaptic nigrostriatal degeneration in these patients. However, recently, there have been two reports on normal dopamine transporter imaging after 3 and 10 years of parkinsonism, respectively, in autopsy-proven MSA-P patients [126, 127]. The authors suggested that there might have been postsynaptic-only striatal pathology at the time of the imaging, and the presynaptic pathology developed later. These findings are different from that of scan without evidence of dopaminergic deficiency (SWEDD) in PD, in that MSA was pathologically proven in these patients. Given that dopamine transporter imaging is performed in less than 20 % of patients with MSA [18] and now it is becoming more available, it is possible that more MSA cases with normal dopamine transporter imaging will be found.
Prolonged survival in MSA
Multiple system atrophy is a rapidly and relentlessly progressing disorder with a mean survival of 6–10 years. However, recent reports have shown that 2–3 % of MSA patients have a prolonged survival of 15 years or more [9, 26]. Most, if not all, of these patients had similar disease courses with a slow progression of parkinsonism resembling PD in the first 10 years of disease and a subsequent rapid deterioration after the development of autonomic failure. Patients like this may well be diagnosed with PD before they develop autonomic failure, which shows the difficulty in making an accurate diagnosis of MSA. Of note, many of these patients develop motor fluctuations and levodopa-induced generalized choreic dyskinesias, which may lead to deep brain stimulation which should not be done in patients with MSA [128]. These rare cases of MSA-P with slow progression and prolonged survival were considered as “benign” forms [9], whereas another case with prolonged clinical course of 18 years showed extensive distribution of GCIs in CNS [129]. A non-motor variant of pathologically confirmed MSA showed no overt parkinsonism nor cerebellar symptoms [130].
‘Minimal change’ MSA
Rare cases of “minimal change” MSA-P showing GCIs and degeneration almost restricted to substantia nigra and putamen, thus representing “pure” striatonigral degeneration [62, 131–135], suggest that GCI formation is an early event and may be responsible for some of the clinical symptoms. One patient with preclinical MSA-C showed widespread GCIs, whereas NCIs and NNIs restricted to pontine basis, cerebellar vermis, and inferior olivary nuclei were associated with neuronal loss, suggesting a common link between these two lesions in early stages of the disease [136]. This case showing only mild clinical symptoms was recently suggested to represent “early MSA”, rather than “minimal change” MSA [137]. Coexistence of sporadic Creutzfeldt-Jakob disease with “minimal change” MSA was recently reported in a Spanish woman aged 64 years [138].
Postmortem detection of MSA pathology in neurologically normal individuals (“prodromal/preclinical MSA”) is extremely rare [132, 134]. They showed GCIs which were limited to the pons and inferior olivary nuclei, whereas neuronal loss was restricted to substantia nigra. The presence of GCIs may represent an age-related phenomenon, not necessarily progressing to over neuronal disease. These rare cases could be classified as “incidental MSA”, similar to incidental Lewy body disease [139].
Conclusion
Multiple system atrophy is a rapid progressive disorder with no effective treatment, and its accurate diagnosis is important for the proper management of patients, especially in the early course of the disease when the disease is not fully developed yet. However, the current clinical diagnostic criteria have some limitations regarding early diagnosis, and it does not include recent clinical and laboratory findings which may improve the diagnosis. Similarly, the current pathological diagnostic criteria for MSA do not capture the recent findings in neuropathology. These aspects should be taken into consideration when revising the current diagnostic criteria (Table 4). This is especially true given that disease-modifying treatments for MSA are under investigation, and including patients with relevant pathology in an early phase of the disease as much as possible is of utmost importance.
References
Stefanova N, Bücke P, Duerr S, Wenning GK (2009) Multiple system atrophy: an update. Lancet Neurol 8:1172–1178
Kim HJ, Jeon BS, Lee JY, Yun JY (2011) Survival of Korean patients with multiple system atrophy. Mov Disord 26:909–912
Wenning GK, Geser F, Krismer F, Seppi K, Duerr S, Boesch S, Köllensperger M, Goebel G, Pfeiffer KP, Barone P (2013) The natural history of multiple system atrophy: a prospective European cohort study. Lancet Neurol 12:264–274
Jellinger KA (2014) Neuropathology of multiple system atrophy: New thoughts about pathogenesis. Mov Disord 29:1720–1741
Kim H-J, Jeon BS, Shin J, Lee W-W, Park H, Jung YJ, Ehm G (2014) Should genetic testing for SCAs be included in the diagnostic workup for MSA? Neurology 83:1733–1738
Kimpinski K, Iodice V, Burton DD, Camilleri M, Mullan BP, Lipp A, Sandroni P, Gehrking TL, Sletten DM, Ahlskog J (2012) The role of autonomic testing in the differentiation of Parkinson’s disease from multiple system atrophy. J Neurol Sci 317:92–96
Lee J-Y, Yun JY, Shin C-W, Kim H-J, Jeon BS (2010) Putaminal abnormality on 3-T magnetic resonance imaging in early parkinsonism-predominant multiple system atrophy. J Neurol 257:2065–2070
Linder J, Libelius R, Nordh E, Holmberg B, Stenlund H, Forsgren L (2012) Anal sphincter electromyography in patients with newly diagnosed idiopathic parkinsonism. Acta Neurol Scand 126:248–255
Petrovic IN, Ling H, Asi Y, Ahmed Z, Kukkle PL, Hazrati LN, Lang AE, Revesz T, Holton JL, Lees AJ (2012) Multiple system atrophy–parkinsonism with slow progression and prolonged survival: a diagnostic catch. Mov Disord 27:1186–1190
Stankovic I, Krismer F, Jesic A, Antonini A, Benke T, Brown RG, Burn DJ, Holton JL, Kaufmann H, Kostic VS (2014) Cognitive impairment in multiple system atrophy: a position statement by the neuropsychology task force of the MDS multiple system atrophy (MODIMSA) study group. Mov Disord 29:857–867
Wüllner U, Schmitt I, Kammal M, Kretzschmar HA, Neumann M (2009) Definite multiple system atrophy in a German family. J Neurol Neurosurg Psychiatry 80:449–450
Gilman S, Wenning GK, Low PA, Brooks DJ, Mathias CJ, Trojanowski JQ, Wood NW, Colosimo C, Durr A, Fowler CJ (2008) Second consensus statement on the diagnosis of multiple system atrophy. Neurology 71:670–676
Colosimo C (2011) Nonmotor presentations of multiple system atrophy. Nat Rev Neurol 7:295–298
Klockgether T (2010) Sporadic ataxia with adult onset: classification and diagnostic criteria. Lancet Neurol 9:94–104
Stamelou M, Hoeglinger GU (2013) Atypical parkinsonism: an update. Curr Opin Neurol 26:401–405
Kamm C, Healy DG, Quinn NP, Wüllner U, Moller JC, Schols L, Geser F, Burk K, Børglum AD, Pellecchia MT (2005) The fragile X tremor ataxia syndrome in the differential diagnosis of multiple system atrophy: data from the EMSA Study Group. Brain 128:1855–1860
Gatto E, Rodríguez-Violante M, Consentino C, Chana-Cuevas P, Miranda M, Gallin E, Etcheverry JL, Nuñez Y, Parisi V, Persi G (2014) Pan-American Consortium of Multiple System Atrophy (PANMSA). A Pan-American multicentre cohort study of multiple system Atrophy. J Parkinsons Dis. doi:10.3233/JPD-140434
Köllensperger M, Geser F, Ndayisaba JP, Boesch S, Seppi K, Ostergaard K, Dupont E, Cardozo A, Tolosa E, Abele M (2010) Presentation, diagnosis, and management of multiple system atrophy in Europe: final analysis of the European multiple system atrophy registry. Mov Disord 25:2604–2612
Goode PS, Burgio KL, Redden DT, Markland A, Richter HE, Sawyer P, Allman RM (2008) Population based study of incidence and predictors of urinary incontinence in black and white older adults. J Urol 179:1449–1454
Hannestad YS, Rortveit G, Sandvik H, Hunskaar S (2000) A community-based epidemiological survey of female urinary incontinence: the Norwegian EPINCONT Study. J Clin Epidemiol 53:1150–1157
Smart C (2014) Male urinary incontinence and the urinary sheath. Br J Nurs 23:S20–S25
Norton P, Brubaker L (2006) Urinary incontinence in women. Lancet 367:57–67
Oertel WH, Wächter T, Quinn NP, Ulm G, Brandstädter D (2003) Reduced genital sensitivity in female patients with multiple system atrophy of parkinsonian type. Mov Disord 18:430–432
Senard J, Rai S, Lapeyre-Mestre M, Brefel C, Rascol O, Rascol A, Montastruc J (1997) Prevalence of orthostatic hypotension in Parkinson’s disease. J Neurol Neurosurg Psychiatry 63:584–589
Mader SL, Josephson KR, Rubenstein LZ (1987) Low prevalence of postural hypotension among community-dwelling elderly. JAMA 258:1511–1514
Kim HJ, Jeon BS (2012) Multiple system atrophy with prolonged survival. Mov Disord 27:1837–1840
Jellinger KA, Lantos PL (2010) Papp-Lantos inclusions and the pathogenesis of multiple system atrophy: an update. Acta Neuropathol 119:657–667
Jellinger KA (2014) Neuropathology. In: Wenning GK, Fanciulli A (eds) Multiple System Atrophy. Springer-Verlag, Vienna, pp 17–55
Trojanowski J, Revesz T (2007) Proposed neuropathological criteria for the post mortem diagnosis of multiple system atrophy. Neuropathol Appl Neurobiol 33:615–620
Dickson DW, Liu W, Hardy J, Farrer M, Mehta N, Uitti R, Mark M, Zimmerman T, Golbe L, Sage J, Sima A, D’Amato C, Albin R, Gilman S, Yen SH (1999) Widespread alterations of alpha-synuclein in multiple system atrophy. Am J Pathol 155(4):1241–1251
Jellinger KA (2014) Aetiopathogenesis. In: Wenning GK, Fanciulli A (eds) Multiple System Atrophy. Springer-Verlag, Vienna, pp 57–81
Houlden H, Singleton AB (2012) The genetics and neuropathology of Parkinson’s disease. Acta Neuropathol 124:325–338
Osaki Y, Ben-Shlomo Y, Lees AJ, Wenning GK, Quinn NP (2009) A validation exercise on the new consensus criteria for multiple system atrophy. Mov Disord 24:2272–2276
Halliday GM, Holton JL, Revesz T, Dickson DW (2011) Neuropathology underlying clinical variability in patients with synucleinopathies. Acta Neuropathol 122:187–204
Campbell BC, McLean CA, Culvenor JG, Gai WP, Blumbergs PC, Jakala P, Beyreuther K, Masters CL, Li QX (2001) The solubility of alpha-synuclein in multiple system atrophy differs from that of dementia with Lewy bodies and Parkinson’s disease. J Neurochem 76(1):87–96
Armstrong RA, Lantos PL, Cairns NJ (2007) Spatial topography of the neurofibrillary tangles in cortical and subcortical regions in progressive supranuclear palsy. Parkinsonism Relat Disord 13:50–54
Ozawa T (2007) Morphological substrate of autonomic failure and neurohormonal dysfunction in multiple system atrophy: impact on determining phenotype spectrum. Acta Neuropathol 114:201–211
Pouclet H, Lebouvier T, Coron E, Rouaud T, Flamant M, Toulgoat F, Roy M, Vavasseur F, Bruley des Varannes S, Neunlist M (2012) Analysis of colonic alpha-synuclein pathology in multiple system atrophy. Parkinsonism Relat Disord 18:893–895
Jellinger KA (2007) More frequent Lewy bodies but less frequent Alzheimer-type lesions in multiple system atrophy as compared to age-matched control brains. Acta Neuropathol 114:299–303
Wenning G, Ben-Shlomo Y, Magalhaes M, Daniel S, Quinn N (1995) Clinicopathological study of 35 cases of multiple system atrophy. J Neurol Neurosurg Psychiatry 58:160–166
Fuchs J, Nilsson C, Kachergus J, Munz M, Larsson EM, Schüle B, Langston JW, Middleton FA, Ross O, Hulihan M (2007) Phenotypic variation in a large Swedish pedigree due to SNCA duplication and triplication. Neurology 68:916–922
Ozawa T, Tada M, Kakita A, Onodera O, Ishihara T, Morita T, Shimohata T, Wakabayashi K, Takahashi H, Nishizawa M (2010) The phenotype spectrum of Japanese multiple system atrophy. J Neurol Neurosurg Psychiatry 81:1253–1255
Mochizuki A, Komatsuzaki Y, Shoji S (2002) Association of Lewy bodies and glial cytoplasmic inclusions in the brain of Parkinson’s disease. Acta Neuropathol 104:534–537
Tison F, Wenning GK, Danie S, Quinn NP (1995) Multiple system atrophy with Lewy bodies. Rev Neurol (Paris) 151:398–403
Wenning GK, Jellinger KA (2005) The role of α-synuclein in the pathogenesis of multiple system atrophy. Acta Neuropathol 109:129–140
Kiely AP, Asi YT, Kara E, Limousin P, Ling H, Lewis P, Proukakis C, Quinn N, Lees AJ, Hardy J (2013) α-Synucleinopathy associated with G51D SNCA mutation: a link between Parkinson’s disease and multiple system atrophy? Acta Neuropathol 125:753–769
Pasanen P, Myllykangas L, Siitonen M, Raunio A, Kaakkola S, Lyytinen J, Tienari PJ, Pöyhönen M, Paetau A (2014) A novel α-synuclein mutation A53E associated with atypical multiple system atrophy and Parkinson’s disease-type pathology. Neurobiol Aging 35:2180 (e2181–2185)
Hughes AJ, Daniel SE, Ben-Shlomo Y, Lees AJ (2002) The accuracy of diagnosis of parkinsonian syndromes in a specialist movement disorder service. Brain 125:861–870
Joutsa J, Gardberg M, Röyttä M, Kaasinen V (2014) Diagnostic accuracy of parkinsonism syndromes by general neurologists. Parkinsonism Relat Disord 20:840–844
Fanciulli A, Wenning GK (2015) Multiple-system atrophy. N Engl J Med 372:249–263
Arabia G, Morelli M, Paglionico S, Novellino F, Salsone M, Giofrè L, Torchia G, Nicoletti G, Messina D, Condino F (2010) An magnetic resonance imaging T2*-weighted sequence at short echo time to detect putaminal hypointensity in Parkinsonisms. Mov Disord 25:2728–2734
Brooks DJ, Seppi K (2009) Proposed neuroimaging criteria for the diagnosis of multiple system atrophy. Mov Disord 24:949–964
Ito S, Shirai W, Hattori T (2007) Evaluating posterolateral linearization of the putaminal margin with magnetic resonance imaging to diagnose the Parkinson variant of multiple system atrophy. Mov Disord 22:578–581
Meijer FJ, Aerts MB, Abdo WF, Prokop M, Borm GF, Esselink RA, Goraj B, Bloem BR (2012) Contribution of routine brain MRI to the differential diagnosis of parkinsonism: a 3-year prospective follow-up study. J Neurol 259:929–935
Wang Y, Butros SR, Shuai X, Dai Y, Chen C, Liu M, Haacke E, Hu J, Xu H (2012) Different iron-deposition patterns of multiple system atrophy with predominant parkinsonism and idiopathetic Parkinson diseases demonstrated by phase-corrected susceptibility-weighted imaging. AJNR 33:266–273
Konagaya M, Matsuoka Y, Goto Y, Yoshida M, Hashizume Y (1999) Pathological correlate of the slitlike changes on MRI at the putaminal margin in multiple system atrophy. J Neurol 246:142–143
Matsusue E, Fujii S, Kanasaki Y, Sugihara S, Miyata H, Ohama E, Ogawa T (2008) Putaminal lesion in multiple system atrophy: postmortem MR-pathological correlations. Neuroradiology 50:559–567
Feng J-y, Huang B, Yang W-Q, Zhang Y-h, Wang L-m, Wang L-j, Zhong X-l (2014) The putaminal abnormalities on 3.0 T magnetic resonance imaging: can they separate parkinsonism-predominant multiple system atrophy from Parkinson’s disease? Acta Radiol. doi:10.1177/0284185114524090
Fujii S, Matsusue E, Kinoshita T, Sugihara S, Ohama E, Ogawa T (2007) Hyperintense putaminal rim at 3T reflects fewer ferritin deposits in the lateral marginal area of the putamen. AJNR 28:777–781
Lee W-H, Lee C-C, Shyu W-C, Chong P-N, Lin S-Z (2005) Hyperintense putaminal rim sign is not a hallmark of multiple system atrophy at 3T. AJNR 26:2238–2242
Tha KK, Terae S, Tsukahara A, Soma H, Morita R, Yabe I, Ito YM, Sasaki H, Shirato H (2012) Hyperintense putaminal rim at 1.5 T: prevalence in normal subjects and distinguishing features from multiple system atrophy. BMC Neurol 12:39
Horimoto Y, Aiba I, Yasuda T, Ohkawa Y, Katayama T, Yokokawa Y, Goto A, Ito Y (2002) Longitudinal MRI study of multiple system atrophy—when do the findings appear, and what is the course? J Neurol 249:847–854
Watanabe H, Saito Y, Terao S, Ando T, Kachi T, Mukai E, Aiba I, Abe Y, Tamakoshi A, Doyu M (2002) Progression and prognosis in multiple system atrophy an analysis of 230 Japanese patients. Brain 125:1070–1083
Jain RS, Sannegowda RB, Agrawal A, Hemrajani D, Jain R, Mathur T (2013) ‘Hot cross bun’sign in a case of cerebrotendinous xanthomatosis: a rare neuroimaging observation. BMJ Case Rep. doi:10.1136/bcr-2012-006641
Lee YC, Liu CS, Wu HM, Wang PS, Chang MH, Soong BW (2009) The ‘hot cross bun’sign in the patients with spinocerebellar ataxia. Eur J Neurol 16:513–516
Muqit M, D Mort KM, Shakir R (2001) “Hot cross bun” sign in a patient with parkinsonism secondary to presumed vasculitis. J Neurol Neurosurg Psychiatry 71:565
Rho SY, H-s Jang, Kim YH (2013) Hot Cross Bun Sign Following Bilateral Pontine Infarction: a Case Report. J Mov Disord 6:37–39
Kim YJ, Ichise M, Ballinger JR, Vines D, Erami SS, Tatschida T, Lang AE (2002) Combination of dopamine transporter and D2 receptor SPECT in the diagnostic evaluation of PD, MSA, and PSP. Mov Disord 17:303–312
Perju-Dumbrava LD, Kovacs GG, Pirker S, Jellinger K, Hoffmann M, Asenbaum S, Pirker W (2012) Dopamine transporter imaging in autopsy-confirmed Parkinson’s disease and multiple system atrophy. Mov Disord 27:65–71
Muñoz E, Iranzo A, Rauek S, Lomeña F, Gallego J, Ros D, Santamaría J, Tolosa E (2011) Subclinical nigrostriatal dopaminergic denervation in the cerebellar subtype of multiple system atrophy (MSA-C). J Neurol 258:2248–2253
King AE, Mintz J, Royall DR (2011) Meta-analysis of 123I-MIBG cardiac scintigraphy for the diagnosis of Lewy body–related disorders. Mov Disord 26:1218–1224
Orimo S, Suzuki M, Inaba A, Mizusawa H (2012) 123I-MIBG myocardial scintigraphy for differentiating Parkinson’s disease from other neurodegenerative parkinsonism: a systematic review and meta-analysis. Parkinsonism Relat Disord 18:494–500
Chung EJ, Lee WY, Yoon WT, Kim BJ, Lee GH (2009) MIBG scintigraphy for differentiating Parkinson’s disease with autonomic dysfunction from Parkinsonism-predominant multiple system atrophy. Mov Disord 24:1650–1655
Nagayama H, Ueda M, Yamazaki M, Nishiyama Y, Hamamoto M, Katayama Y (2010) Abnormal cardiac [123I]-meta-iodobenzylguanidine uptake in multiple system atrophy. Mov Disord 25:1744–1747
Behnke S, Berg D, Naumann M, Becker G (2005) Differentiation of Parkinson’s disease and atypical parkinsonian syndromes by transcranial ultrasound. J Neurol Neurosurg Psychiatry 76:423–425
Walter U, Niehaus L, Probst T, Benecke R, Meyer B, Dressler D (2003) Brain parenchyma sonography discriminates Parkinson’s disease and atypical parkinsonian syndromes. Neurology 60:74–77
Vlaar AM, Bouwmans A, Mess WH, Tromp SC, Weber WE (2009) Transcranial duplex in the differential diagnosis of parkinsonian syndromes. J Neurol 256:530–538
Walter U, Školoudík D (2014) Transcranial sonography (TCS) of brain parenchyma in movement disorders: quality standards, diagnostic applications and novel technologies. Ultraschall Med 35:322–331
Hellwig S, Reinhard M, Amtage F, Guschlbauer B, Buchert R, Tüscher O, Weiller C, Niesen W, Meyer P (2014) Transcranial sonography and [18F] fluorodeoxyglucose positron emission tomography for the differential diagnosis of parkinsonism: a head-to-head comparison. Eur J Neurol 21:860–866
Vodušek DB (2001) Sphincter EMG and differential diagnosis of multiple system atrophy. Mov Disord 16:600–607
Gilman S, Low P, Quinn N, Albanese A, Ben-Shlomo Y, Fowler C, Kaufmann H, Klockgether T, Lang A, Lantos P (1998) Consensus statement on the diagnosis of multiple system atrophy. Clin Auton Res 8:359–362
Yamamoto T, Sakakibara R, Uchiyama T, Liu Z, Ito T, Awa Y, Yamamoto K, Kinou M, Yamanishi T, Hattori T (2005) When is Onuf’s nucleus involved in multiple system atrophy? A sphincter electromyography study. J Neurol Neurosurg Psychiatry 76:1645–1648
Reimann M, Schmidt C, Herting B, Prieur S, Junghanns S, Schweitzer K, Globas C, Schoels L, Reichmann H, Berg D (2010) Comprehensive autonomic assessment does not differentiate between Parkinson’s disease, multiple system atrophy and progressive supranuclear palsy. J Neural Transm 117:69–76
Schmidt C, Herting B, Prieur S, Junghanns S, Schweitzer K, Globas C, Schöls L, Reichmann H, Berg D, Ziemssen T (2009) Valsalva manoeuvre in patients with different Parkinsonian disorders. J Neural Transm 116:875–880
Iodice V, Lipp A, Ahlskog JE, Sandroni P, Fealey RD, Parisi JE, Matsumoto JY, Benarroch EE, Kimpinski K, Singer W (2012) Autopsy confirmed multiple system atrophy cases: mayo experience and role of autonomic function tests. J Neurol Neurosurg Psychiatry 83:453–459
Anderson T, Luxon L, Quinn N, Daniel S, David Marsden C, Bronstein A (2008) Oculomotor function in multiple system atrophy: clinical and laboratory features in 30 patients. Mov Disord 23:977–984
Lee JY, Lee WW, Kim JS, Kim HJ, Kim JK, Jeon BS (2009) Perverted head-shaking and positional downbeat nystagmus in patients with multiple system atrophy. Mov Disord 24:1290–1295
Dickson DW, Hauw J, Agid Y, Litvan I (2011) Progressive supranuclear palsy and corticobasal degeneration. In: Dickson DW, Weller RO (eds) Neurodegeneration: The Molecular Pathology of Dementia and Movement Disorders, 2nd edn. Blackwell Publishing Ltd, Oxford, pp 135–155
Silveira-Moriyama L, González AM, O’Sullivan SS, Williams DR, Massey L, Parkkinen L, Ahmed Z, de Silva R, Chacón JR, Revesz T (2009) Concomitant progressive supranuclear palsy and multiple system atrophy: more than a simple twist of fate? Neurosci Lett 467:208–211
Silveira-Moriyama L, Holton JL, Kingsbury A, Ayling H, Petrie A, Sterlacci W, Poewe W, Maier H, Lees AJ, Revesz T (2009) Regional differences in the severity of Lewy body pathology across the olfactory cortex. Neurosci Lett 453:77–80
Uchikado H, DelleDonne A, Uitti R, Dickson DW (2006) Coexistence of PSP and MSA: a case report and review of the literature. Acta Neuropathol 111:186–192
Iwasaki Y, Mori K, Ito M, Tatsumi S, Mimuro M, Yoshida M (2013) An autopsied case of progressive supranuclear palsy presenting with cerebellar ataxia and severe cerebellar involvement. Neuropathology 33:561–567
Williams DR, de Silva R, Paviour DC, Pittman A, Watt HC, Kilford L, Holton JL, Revesz T, Lees AJ (2005) Characteristics of two distinct clinical phenotypes in pathologically proven progressive supranuclear palsy: Richardson’s syndrome and PSP-parkinsonism. Brain 128:1247–1258
Wenning GK, Colosimo C, Geser F, Poewe W (2004) Multiple system atrophy. Lancet Neurol 3:93–103
Bujan B, Hofer MJ, Oertel WH, Pagenstecher A, Bürk K (2012) Multiple system atrophy of the cerebellar type (MSA-C) with concomitant beta-amyloid and tau pathology. Clin Neuropathol 32:286–290
Terni B, Rey MJ, Boluda S, Torrejón-Escribano B, Sabate MP, Calopa M, van Leeuwen FW, Ferrer I (2007) Mutant ubiquitin and p62 immunoreactivity in cases of combined multiple system atrophy and Alzheimer’s disease. Acta Neuropathol 113:403–416
Klockgether T, Schroth G, Diener H, Dichgans J (1990) Idiopathic cerebellar ataxia of late onset: natural history and MRI morphology. J Neurol Neurosurg Psychiatry 53:297–305
Tsuji S, Onodera O, Goto J, Nishizawa M (2008) Sporadic ataxias in Japan–a population-based epidemiological study. Cerebellum 7:189–197
Gilman S, Little R, Johanns J, Heumann M, Kluin K, Junck L, Koeppe R, An H (2000) Evolution of sporadic olivopontocerebellar atrophy into multiple system atrophy. Neurology 55:527–532
Abele M, Bürk K, Schöls L, Schwartz S, Besenthal I, Dichgans J, Zühlke C, Riess O, Klockgether T (2002) The aetiology of sporadic adult-onset ataxia. Brain 125:961–968
Futamura N, Matsumura R, Fujimoto Y, Horikawa H, Suzumura A, Takayanagi T (1998) CAG repeat expansions in patients with sporadic cerebellar ataxia. Acta Neurol Scand 98:55–59
Schöls L, Szymanski S, Peters S, Przuntek H, Epplen JT, Hardt C, Riess O (2000) Genetic background of apparently idiopathic sporadic cerebellar ataxia. Hum Genet 107:132–137
Kim JM, Hong S, Kim GP, Choi YJ, Kim YK, Park SS, Kim SE, Jeon BS (2007) Importance of low-range CAG expansion and CAA interruption in SCA2 Parkinsonism. Arch Neurol 64:1510–1518
Kim JY, Kim SY, Kim JM, Kim YK, Yoon KY, Lee BC, Kim JS, Paek SH, Park SS, Kim SE (2009) Spinocerebellar ataxia type 17 mutation as a causative and susceptibility gene in parkinsonism. Neurology 72:1385–1389
Factor SA, Qian J, Lava NS, Hubbard JD, Payami H (2005) False-positive SCA8 gene test in a patient with pathologically proven multiple system atrophy. Ann Neurol 57:462–463
Nirenberg MJ, Libien J, Vonsattel JP, Fahn S (2007) Multiple system atrophy in a patient with the spinocerebellar ataxia 3 gene mutation. Mov Disord 22:251–253
Takao M, Aoyama M, Ishikawa K, Sakiyama Y, Yomono H, Saito Y, Kurisaki H, Mihara B, Murayama S (2011) Spinocerebellar ataxia type 2 is associated with Parkinsonism and Lewy body pathology. BMJ Case Rep. doi:10.1136/bcr.1101.2011.3685
Mehta AR, Fox SH, Tarnopolsky M, Yoon G (2011) Mitochondrial mimicry of multiple system atrophy of the cerebellar subtype. Mov Disord 26:753–755
Stamelou M, Quinn NP, Bhatia KP (2013) “Atypical” atypical parkinsonism: new genetic conditions presenting with features of progressive supranuclear palsy, corticobasal degeneration, or multiple system atrophy—A diagnostic guide. Mov Disord 28:1184–1199
Fujioka S, Ogaki K, Tacik PM, Uitti RJ, Ross OA, Wszolek ZK (2014) Update on novel familial forms of Parkinson’s disease and multiple system atrophy. Parkinsonism Relat Disord 20:S29–S34
Vidal J-S, Vidailhet M, Derkinderen P, Tzourio C, Alpérovitch A (2010) Familial aggregation in atypical Parkinson’s disease: a case control study in multiple system atrophy and progressive supranuclear palsy. J Neurol 257:1388–1393
MSA Research Collaboration (2013) Mutations in COQ2 in familial and sporadic multiple-system atrophy. N Engl J Med 369:233–244
Hara K, Momose Y, Tokiguchi S, Shimohata M, Terajima K, Onodera O, Kakita A, Yamada M, Takahashi H, Hirasawa M (2007) Multiplex families with multiple system atrophy. Arch Neurol 64:545–551
Jeon BS, Farrer MJ, Bortnick SF (2014) Mutant COQ2 in multiple-system atrophy. N Engl J Med 371:80
Schottlaender L, Houlden H (2014) Mutant COQ2 in multiple-system atrophy. N Engl J Med 371:81
Sharma M, Wenning GK, Kruger R (2014) Mutant COQ2 in multiple-system atrophy. N Engl J Med 371:80–81
Bak T, Caine D, Hearn V, Hodges J (2006) Visuospatial functions in atypical parkinsonian syndromes. J Neurol Neurosurg Psychiatry 77:454–456
Bürk K, Daum I, Rüb U (2006) Cognitive function in multiple system atrophy of the cerebellar type. Mov Disord 21:772–776
Wenning G, Tison F, Ben Shlomo Y, Daniel S, Quinn N (1997) Multiple system atrophy: a review of 203 pathologically proven cases. Mov Disord 12:133–147
Wenning G, Ben-Shlomo Y, Hughes A, Daniel S, Lees A, Quinn N (2000) What clinical features are most useful to distinguish definite multiple system atrophy from Parkinson’s disease? J Neurol Neurosurg Psychiatry 68:434–440
Asi YT, Ling H, Ahmed Z, Lees AJ, Revesz T, Holton JL (2014) Neuropathological features of multiple system atrophy with cognitive impairment. Mov Disord 29:884–888
Kawai Y, Suenaga M, Takeda A, Ito M, Watanabe H, Tanaka F, Kato K, Fukatsu H, Naganawa S, Kato T (2008) Cognitive impairments in multiple system atrophy MSA-C vs MSA-P. Neurology 70:1390–1396
McKeith I, Dickson D, Lowe J, Emre M, O’brien J, Feldman H, Cummings J, Duda J, Lippa C, Perry E (2005) Diagnosis and management of dementia with Lewy bodies third report of the DLB consortium. Neurology 65:1863–1872
Kao AW, Racine CA, Quitania LC, Kramer JH, Christine CW, Miller BL (2009) Cognitive and neuropsychiatric profile of the synucleinopathies: Parkinson’s disease, dementia with Lewy bodies and multiple system atrophy. Alzheimer Dis Assoc Disord 23:365–370
Williams DR, Lees AJ (2005) Visual hallucinations in the diagnosis of idiopathic Parkinson’s disease: a retrospective autopsy study. Lancet Neurol 4:605–610
Kolenc M, Popović M, Grmek M, Pirtošek Z, Trošt M (2012) A case of multiple system atrophy with normal dopamine transporter imaging. J Neurol 259:2729–2731
McKinley J, O’Connell M, Farrell M, Lynch T (2014) Normal dopamine transporter imaging does not exclude multiple system atrophy. Parkinsonism Relat Disord 20:933–934
Kim H-J, Jeon BS, Lee J-Y, Yun JY, Kim YE, Paek SH (2012) Young-onset multiple system atrophy. J Neurol Sci 319:168–170
Masui K, Nakata Y, Fujii N, Iwaki T (2012) Extensive distribution of glial cytoplasmic inclusions in an autopsied case of multiple system atrophy with a prolonged 18-year clinical course. Neuropathology 32:69–76
Gaig C, Iranzo A, Tolosa E, Vilaseca I, Rey M, Santamaria J (2008) Pathological description of a non-motor variant of multiple system atrophy. J Neurol Neurosurg Psychiatry 79:1399–1400
Berciano J, Valldeoriola F, Ferrer I, Rumià J, Pascual J, Marín C, Rey MJ, Tolosa E (2002) Presynaptic parkinsonism in multiple system atrophy mimicking Parkinson’s disease: a clinicopathological case study. Mov Disord 17:812–816
Fujishiro H, Ahn T-B, Frigerio R, DelleDonne A, Josephs KA, Parisi JE, Ahlskog JE, Dickson DW (2008) Glial cytoplasmic inclusions in neurologically normal elderly: prodromal multiple system atrophy? Acta Neuropathol 116:269–275
Kon T, Mori F, Tanji K, Miki Y, Wakabayashi K (2013) An autopsy case of preclinical multiple system atrophy (MSA-C). Neuropathology 33:667–672
Parkkinen L, Hartikainen P, Alafuzoff I (2006) Abundant glial alpha-synuclein pathology in a case without overt clinical symptoms. Clin Neuropathol 26:276–283
Wenning G, Shlomo YB, Magalhaes M, Danie S, Quinn N (1994) Clinical features and natural history of multiple system atrophy An analysis of 100 cases. Brain 117:835–845
Wakabayashi K, Mori F, Nishie M, Oyama Y, Kurihara A, Yoshimoto M, Kuroda N (2005) An autopsy case of early (“minimal change”) olivopontocerebellar atrophy (multiple system atrophy-cerebellar). Acta Neuropathol 110:185–190
Ahmed Z, Asi Y, Sailer A, Lees A, Houlden H, Revesz T, Holton J (2012) The neuropathology, pathophysiology and genetics of multiple system atrophy. Neuropathol Appl Neurobiol 38:4–24
Rodriguez-Diehl R, Rey MJ, Gironell A, Martinez-Saez E, Ferrer I, Sánchez-Valle R, Jagüe J, Nos C, Gelpi E (2012) “Preclinical” MSA in definite Creutzfeldt-Jakob disease. Neuropathology 32:158–163
DelleDonne A, Klos KJ, Fujishiro H, Ahmed Z, Parisi JE, Josephs KA, Frigerio R, Burnett M, Wszolek ZK, Uitti RJ (2008) Incidental Lewy body disease and preclinical Parkinson disease. Arch Neurol 65:1074–1080
Acknowledgments
This study was supported by a grant of the Korea Health technology R&D Project, Ministry of Health & Welfare, Republic of Korea (A101273).
Conflicts of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kim, HJ., Jeon, B.S. & Jellinger, K.A. Diagnosis and differential diagnosis of MSA: boundary issues. J Neurol 262, 1801–1813 (2015). https://doi.org/10.1007/s00415-015-7654-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-015-7654-2