Abstract
Background
Multiple system atrophy (MSA) is an adult onset, fatal neurodegenerative disease. However, no reliable biomarker is currently available to guide clinical diagnosis and help to determine the prognosis. Thus, a comprehensive meta-analysis is warranted to determine effective biomarkers for MSA and provide useful guidance for clinical diagnosis.
Methods
A comprehensive literature search was made of the PubMed, Embase, Cochrane and Web of Science databases for relevant clinical trial articles for 1984–2019. Two review authors examined the full-text records, respectively, and determined which studies met the inclusion criteria. We estimated the mean difference, standard deviation and 95% confidence intervals.
Results
A total of 28 studies and 11 biomarkers were included in our analysis. Several biomarkers were found to be useful to distinguish MSA patients from healthy controls, including the reduction of phosphorylated tau, α-synuclein (α-syn), 42-amino-acid form of Aβ and total tau (t-tau), the elevation of neurofilament light-chain protein (NFL) in cerebrospinal fluid, the elevation of uric acid and reduction of homocysteine and coenzyme Q10 in plasma. Importantly, α-syn, NFL and t-tau could be used to distinguish MSA from Parkinson’s disease (PD), indicating that these three biomarkers could be useful biomarkers in MSA diagnosis.
Conclusion
The findings of our meta-analysis demonstrated diagnostic biomarkers for MSA. Moreover, three biomarkers could be used in differential diagnosis of MSA and PD. The results could be helpful for the early diagnosis of MSA and the accuracy of MSA diagnosis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Multiple system atrophy (MSA) is an adult-onset fatal neurodegenerative disease, which is characterized by autonomic failure, pyramidal features, Parkinsonian (MSA-P) and cerebellar features (MSA-C) [1, 2]. In Western countries, more than 70% of MSA patients show the MSA-P variant [1], while in China, there might be no significant difference between the number of MSA-P and MSA-C patients [3]. Although MSA is primarily a sporadic disease, familial MSA has also been reported [4]. The mean survival from the onset of symptoms is 6–10 years; however, only symptomatic therapy is currently available [5].
Misfolded α-synuclein (α-syn) is deposited in the glial cytoplasmic inclusions of oligodendroglial cells in patients with MSA, and in the neuronal soma and throughout axons in patients with Parkinson’s disease (PD), indicating that MSA, together with PD belongs to a group of neurodegenerative diseases, synucleinopathies [6, 7]. Due to the overlapping clinical presentation among synucleinopathies, it can be difficult to distinguish MSA from PD in early disease. However, no reliable biomarker is currently available to guide clinical diagnosis and help to determine the prognosis.
In recent years, several biomarkers, the 42-amino-acid form of Aβ (Aβ42), total tau (t-tau), phosphorylated tau (p-tau), neurofilament light-chain protein (NFL), YKL-40 (chitinase-3-like protein 1, CHI3L1), FMS-like tyrosine kinase ligand (FLT3), and α-syn in the cerebrospinal fluid (CSF), have been identified and assessed in multiple studies for their diagnostic value of MSA [8,9,10,11]. Additionally, some studies have focused on blood levels of homocysteine (HCY), C-reactive protein (CRP), uric acid (UA) and coenzyme Q10 (CoQ10) in MSA patients [12, 13]; however, these studies remain controversial.
In this study, we undertook the first systematic review with meta-analysis of studies measuring CSF and peripheral blood levels of different biomarkers in patients with MSA compared with PD and healthy controls (HCs), hoping to facilitate the early identification of patients at early or even presymptomatic stages.
Methods
The meta-analyses performed in this study followed the guidelines recommended by the PRISMA statement (Preferred Reporting Items for Systematic Reviews and meta-analysis) [14].
Search strategy
Two investigators, Xiang and Cong, performed a systematic review of clinical trial articles on PubMed, Embase, Cochrane and Web of Science during 1984–2019. Our search terms were “Multiple system atrophy and (α-synuclein or amyloid precursor protein or β-amyloid42 or ubiquitin–proteasome system or neuroinflammation or YKL-40 or interferon γ or oxidative stress or axonal degeneration or tau or phospho-tau proteins or neurofilament or neuron-specific enolase or glial degeneration or myelin basic protein or glial fibrillary acidic protein or neurotransmitter or dopamine or serotonin or norepinephrine or hypocretin or growth hormone insulin or insulin-like growth factor or proteomic or microRNA or amino acid or coenzyme Q10 or homocysteine acid or CRP or uric acid or cytokine)”.
Study inclusion and exclusion criteria
We included the original observational studies that reported the biomarkers of MSA and contained HCs or PD controls. The exclusion criteria are as follows: (1) biomarkers of MSA that were measured in animal models; (2) samples from postmortem body; (3) data in vitro; (4) without PD or HCs; (5) with other serious disease or caused by serious complications; (6) without necessary data; (7) total number of studies for a biomarker was less than two; (8) the samples were collected before patients were diagnosed with MSA; and (9) the samples overlapped with other studies.
Data extraction and article quality analysis
Two investigators extracted and verified the data from articles included in the meta-analysis. If disagreements could not be resolved through careful discussion by two investigators, a consensus was achieved by the involvement of a third reviewer (Prof. Cong Shuyan). The main extracted data included the author and publication year of each article, the size of each group, the mean concentration of biomarkers and standard deviation (SD) of each group to generate the effective significance (ES). Additionally, we extracted data about age, gender, country, duration of disease, mean Hoehn and Yahr scale (H and Y scale), mean Unified Parkinson’s Disease Rating Scale (UPDRS) score, mean International Cooperative Ataxia Rating Scale (ICARS), mean Mini-mental State Examination (MMSE), follow-up time, body mass index (BMI) and assay type for potential moderator analysis. Two authors (Cong and Xiang) independently assessed study quality and risk of bias using the scheme suggested by the Cochrane collaboration (“Tool to assess risk of bias in cohort studies”) to evaluate quality of the included literature and assess the credibility of the conclusions.
Statistical analysis
All meta-analyses were done using Review Manager 5.3 (The Nordic Cochrane Centre, The Cochrane Collaboration 2012, Portland, OR, USA) software. The mean ± SD is used to describe each index in experimental and control groups. The ES was mainly generated by sample size, mean concentration and SD. We also used the sample size and P value to generate ES if the data for mean concentration and SD were not available. The mean difference (MD) was used to compare and analyze the numerical data with the same units. According to the Cochrane Handbook, I2 is used to reflect the heterogeneity of the included studies into three categories of 0.25, 0.50 and 0.75, indicating mild, moderate and high levels of heterogeneity, respectively [15]. We used a random-effect model when P < 0.05 and I2 > 50%, and used a fixed-effect model when P > 0.05 and I2 < 50% [16]. The results of the combination of the experimental and the control groups were expressed by MD and 95% CI (95% confidence interval). Significance was set as P < 0.05. Forest maps were used to describe the ES and 95% CI included in the study. Egger’s test was applied to reflect literature publication bias, and the hypothesis test used Chi-square values and P < 0.05 was deemed to indicate a publication bias. We evaluated the stability of the synthesis results by sensitivity analysis.
Results
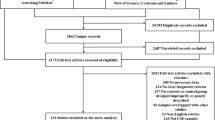
We obtained a total 2279 records from the PubMed database and 3472 records from the Web of Science Embase and Cochrane databases. We screened them by titles and abstracts. Initial screening provided us with 129 articles for full-text scrutiny but 94 were excluded, because they did not meet the inclusion criteria. Finally, we included 28 studies, which contained 1223 MSA patients, 1600 PD patients and 1868 HCs. A flowchart summarizing the study selection process is presented in Fig. 1 [8,9,10,11,12,13, 17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38]. The article quality analysis result is shown in Figures S1 and S2. The results indicate that the overall quality of the included articles is high, and our conclusions have a high degree of credibility.
Fixed-effect meta-analysis showed that five biomarkers were significantly different in the CSF of MSA patients compared with HCs: α-syn (Hedges g − 183.37; 95% CI − 213.35 to − 153.40; P < 0.00001), NFL (Hedges g 3.13; 95% CI 0.87–5.38; P = 0.007), p-tau (Hedges g − 7.90; 95% CI − 11.67 to − 4.14; P < 0.00001), t-tau (Hedges g − 6.89; 95% CI − 9.85 to − 3.93; P < 0.00001) and Aβ42 (Hedges g − 71.66; 95% CI − 106.21 to − 37.10; P < 0.00001). Fixed-effect meta-analysis showed that blood levels of three biomarkers in MSA patients also differed compared with HCs: HCY (Hedges g 3.51; 95% CI 2.63–4.40; P < 0.00001), UA (Hedges g − 45.96; 95% CI − 63.66 to − 28.97; P < 0.00001) and CoQ10 (Hedges g − 251.89; 95% CI − 361.78 to − 142.01; P < 0.0001). These eight biomarkers all showed low levels of heterogeneity in the two comparisons, indicating that they could be used to distinguish MSA patients from HCs. Random-effect meta-analysis showed that compared with HCs, MSA patients had higher levels of α-syn in plasma (Hedges g 3.13; 95% CI 0.87–5.38; P = 0.007) and lower levels of FLT3 in CSF (Hedges g − 20.23; 95% CI − 23.59 to − 16.86; P = 0.04). However, the levels of FLT3 in the CSF and α-syn in the plasma showed high levels of heterogeneity in the comparison of MSA and HCs, indicating that further studies related to their roles are needed. Furthermore, levels of YKL-40 and CRP did not show significant differences between MSA patients and HCs (Table 1; Figs. 2, 3). Several potential moderators could account for the heterogeneity in the meta-analysis, which includes theoretically relevant categorical variables (sampling source, assay type, and medication status) and continuous variables (age, sex, disease duration, and disease severity). The low levels of heterogeneity in our results indicate that these variables had little effect on our results.
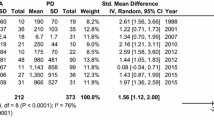
Three biomarkers mentioned above also significantly differed in the CSF of MSA patients compared with PD patients in fixed-effect meta-analysis: α-syn (Hedges g − 81.29; 95% CI − 105.00 to − 57.58; P < 0.00001), NFL (Hedges g 805.23; 95% CI 672.37–938.10; P < 0.00001) and t-tau (Hedges g 79.11; 95% CI 50.52–107.70; P < 0.00001)(Table 1; Fig. 4). Additionally, those biomarkers showed low levels of heterogeneity, thus increasing their diagnostic value.
We also did gender subgroup analysis for UA in the comparison of MSA and HCs in serum (Figure S3), but no significant differences were found. Meta-regression analyses revealed that age may have no significant moderating effects on most outcomes of the meta-analysis (Figures S4–S9); however, a significant association was found between the SD in α-syn levels and mean control age (Figure S4) [regression coefficient (SE) 44.3820 (− 0.3429); 95% CI − 0.0.063621 to 0.16101; P = 0.03906], indicating that age may have a negative impact on α-syn level. Sensitivity analysis suggested that a single study might influence the significant differences for p-tau in CSF in the MSA and PD comparison, for UA in serum in the MSA and PD comparison and for FLT3 in CSF in the MSA and PD comparison.
The Egger’s test results showed no apparent publication bias for α-syn, p-tau, HCY, UA and Aβ42, but there was bias for NFL and t-tau in the comparison of MSA and HCs (Table 1). In the comparison of MSA and PD, the α-syn, p-tau and t-tau in CSF showed no obvious publication bias. However, there was publication bias for NFL in the comparison of MSA and PD in CSF.
Discussion
To our knowledge, this is the first meta-analysis investigating alterations of different biomarkers in MSA patients compared with PD patients and HCs. We included 28 studies with 1223 MSA patients, 1600 PD patients and 1868 HC subjects estimating 11 biomarkers. We found that compared with PD patients and HCs, the reduction of α-syn and the elevation of NFL in the CSF were potential biomarkers for MSA diagnosis. Additionally, the reduction of t-tau in CSF could distinguish MSA and HCs, and elevation of t-tau in CSF could differentiate MSA and PD. Along with α-syn, NFL and t-tau, another seven biomarkers are available to distinguish MSA patients from HCs: p-tau, Aβ42 and FLT3 in CSF; and α-syn, UA, HCY and COQ10 in plasma. Levels of α-syn, NFL and t-tau in CSF might differentiate MSA from PD patients. It would be difficult to differentiate MSA from PD patients using UA, HCY, p-tau, Aβ42 and FLT3 in CSF. The COQ10 is a potential biomarker but more studies are needed to confirm this. It is unlikely that CRP and YKL-40 are suitable for MSA diagnosis.
In our study, α-syn decreased in the CSF of both MSA and PD patients, with greater reduction in MSA compared with PD. A previous study demonstrated that low α-syn levels in the CSF might reflect the decrease of “free” α-syn circulating in the CSF due to α-syn aggregation or mis-metabolism [39]. Thus, the lower level of α-syn in MSA compared to PD suggests that the neurodegeneration in MSA is wider and faster than in PD [11]. In addition, the majority of α-syn is present in red blood cells and α-syn is abundant during different steps of erythropoiesis [40, 41]. In our study, α-syn was elevated in the plasma of MSA patients compared with HCs, possibly due to the residue of α-syn in erythrocytes. Interestingly, another meta-analysis also found that α-syn was increased in the plasma of PD patients, consistent with expression of α-syn in MSA patients [42]. However, more research is needed to confirm the blood levels of α-syn in MSA and PD patients.
In our meta-analysis, NFL in CSF for MSA patients was higher than for PD patients and HCs, which could serve as a diagnostic marker for MSA. The NFL is important in forming the neuronal cytoskeleton and is released into the CSF during axonal damage [43]. Interestingly, the levels of NFL in blood correlated with the CSF levels for human synucleinopathies [44]. Other studies also found that serum NFL was useful to discriminate MSA from PD, and that the expression level of NFL was correlated with clinical parameters in MSA [45, 46]. The NFL could also be used as a promising biomarker for degenerative ataxias, such as differentiating of MSA-C from sporadic adult-onset ataxia [47]. However, it was difficult to distinguish MSA from other atypical Parkinsonism disorders (APDs), including progressive supranuclear palsy, and it suggested that the combination of biomarkers might be helpful to solve these problems [46]. Tau phosphorylation also affected axonal transportation and impaired intraneuronal signaling with subsequent cell death [48]. In CSF of MSA patients, t-tau decreased compared to HCs and increased compared to PD patients. Interestingly, t-tau in the CSF might correlate with cognitive decline in Alzheimer’s disease and possibly in PD [49, 50]. Additionally, previous studies found that the tau level was positively correlated with the severity of ataxia in MSA-C [51] and might be positively correlated with the motor changes in PD [52]. However, a study failed to find any relationship of t-tau with disease severity and the subtype of MSA [27]. The relationship between t-tau and cognition decline and disease phase in MSA needs to be confirmed through further experiments. Additionally, it was difficult to distinguish MSA from PD using p-tau. Another study failed to find any differences between MSA and APD [53]. As a result, combining α-syn with other biomarkers, such as t-tau and NFL, may be useful for MSA diagnosis but further confirmation is needed.
Amyloid precursor protein can be cleaved by β secretase to a peptide of 42 amino acids, Aβ42, which can induce neurotoxicity and help the diagnosis of Alzheimer’s disease [54]. Several studies also found changes in MSA. However, our analysis showed no difference in CSF levels of Aβ42 between MSA and PD [11, 31]. The FLT3 ligand can be found as a transmembrane or soluble protein and has a major role in activating the immune system [26]. Several studies have demonstrated its alteration in MSA [38]; however, we failed to find any diagnostic value for comparing MSA with PD patients and HCs due to its high heterogeneity.
Although lumbar puncture is inconvenient to monitor disease progression, we still need blood-based biomarker detection for at-risk individuals. In our results, UA decreased and HCY increased in MSA patients compared to HCs. The UA is an inverse risk factor and may protect neurons from apoptosis as a natural anti-oxidative stress substance for PD and MSA [55]. These findings demonstrated that lower UA levels might correlate with severity of MSA and PD because of their anti-oxidative protective effect, and might be a modifier in MSA [56]. Another study demonstrated that gender might influence UA metabolism [19]; however, we failed to find any difference in our meta-analysis. The small size of the previous studies might explain this inconsistency. The HCY, another blood biomarker related to neuroinflammation, could activate NMDA receptors and so, results in neurotoxicity [57]. The NMDA receptors are closely associated with cognitive function, which might explain the relationship between HCY and cognitive status for MSA patients in another study [22]. However, HCY and UA might not be useful to distinguish MSA from PD according to our meta-analysis and further studies are needed.
Compared with HCs, CoQ10 increased in MSA patients but there was a relatively clear publication bias. The functionally impaired variant of CoQ2, the gene encoding CoQ10, was associated with an increased risk of MSA [4]. The enzyme CoQ10 is involved in the mitochondrial respiratory chain, takes part in the pathogenesis of MSA and is a potential biomarker [13]. However, its diagnostic utility remains to be elucidated using large-scale comparative research.
The duration, severity, and subtype of the diseases may play roles in the expression of the biomarkers. Some studies have shown a negative correlation between disease severity of MSA and levels of α-syn in plasma [34]. A positive correlation between disease severity and NFL was found in the serum of atypical Parkinsonism disorder [46]. However, other studies failed to find any correlation of disease duration and severity and subtypes of MSA with levels of α-syn [8], t-tau [27], NFL, or other biomarkers [24]. Due to lack of sufficient data, we were unable to analyze this further. More detailed investigations on the effects of duration, severity, and subtypes of disease on the biomarkers are warranted.
In conclusion, the elevation of NFL and the reduction of α-syn in the CSF might be helpful to discriminate MSA from PD, APD and HCs. Additionally, t-tau decreased compared to HCs and increased compared to PD, which might aid in MSA diagnosis. The joint measurement of α-syn, NFL and t-tau might increase accuracy of diagnosis and more studies are needed to determine their roles in blood. The reduction of p-tau and Aβ42 in CSF, the elevation of UA, and reduction of HCY and COQ10 in plasma can only distinguish MSA patients from HCs. More studies are needed to determine other biomarkers of MSA.
References
Gilman S et al (2008) Second consensus statement on the diagnosis of multiple system atrophy. Neurology 71(9):670–676
Fanciulli A, Wenning GK (2015) Multiple-system atrophy. N Engl J Med 372(3):249–263
Zhang L et al (2018) Brain morphological alteration and cognitive dysfunction in multiple system atrophy. Quant Imaging Med Surg 8(10):1030–1038
Multiple-System Atrophy Research Collaboration (2013) Mutations in COQ2 in familial and sporadic multiple-system atrophy. N Engl J Med 369(3):233–244
Stefanova N et al (2009) Multiple system atrophy: an update. Lancet Neurol 8(12):1172–1178
Nuber S et al (2018) Abrogating native alpha-synuclein tetramers in mice causes a l-DOPA-responsive motor syndrome closely resembling Parkinson's disease. Neuron 100(1):75–90 (e4)
Wong YC, Krainc D (2017) Alpha-synuclein toxicity in neurodegeneration: mechanism and therapeutic strategies. Nat Med 23(2):1–13
Mondello S et al (2014) CSF alpha-synuclein and UCH-L1 levels in Parkinson's disease and atypical parkinsonian disorders. Parkinsonism Relat Disord 20(4):382–387
Wang Y et al (2012) Phosphorylated alpha-synuclein in Parkinson's disease. Sci Transl Med 4(121):121ra20
Magdalinou NK et al (2015) A panel of nine cerebrospinal fluid biomarkers may identify patients with atypical parkinsonian syndromes. J Neurol Neurosurg Psychiatry 86(11):1240–1247
Shi M et al (2011) Cerebrospinal fluid biomarkers for Parkinson disease diagnosis and progression. Ann Neurol 69(3):570–580
Chen D et al (2015) Contra-directional expression of serum homocysteine and uric acid as important biomarkers of multiple system atrophy severity: a cross-sectional study. Front Cell Neurosci 9:247
Mitsui J et al (2016) Plasma coenzyme Q10 levels in patients with multiple system atrophy. JAMA Neurol 73(8):977–980
Moher D et al (2009) Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. J Clin Epidemiol 62(10):1006–1012
Xu M et al (2017) Oxidative damage induced by arsenic in mice or rats: a systematic review and meta-analysis. Biol Trace Elem Res 176(1):154–175
Crippa A, Orsini N (2016) Dose–response meta-analysis of differences in means. BMC Med Res Methodol 16:91
Aerts MB et al (2012) CSF alpha-synuclein does not differentiate between parkinsonian disorders. Neurobiol Aging 33(2):430 (e1–3)
Bech S et al (2012) Amyloid-related biomarkers and axonal damage proteins in parkinsonian syndromes. Parkinsonism Relat Disord 18(1):69–72
Cao B et al (2013) Uric acid is associated with the prevalence but not disease progression of multiple system atrophy in Chinese population. J Neurol 260(10):2511–2515
Constantinescu R et al (2010) Consecutive analyses of cerebrospinal fluid axonal and glial markers in Parkinson's disease and atypical Parkinsonian disorders. Parkinsonism Relat Disord 16(2):142–145
Du J et al (2018) Clinical correlates of decreased plasma coenzyme Q10 levels in patients with multiple system atrophy. Parkinsonism Relat Disord 57:58–62
Guo Y et al (2017) Serum Klotho, vitamin D, and homocysteine in combination predict the outcomes of Chinese patients with multiple system atrophy. CNS Neurosci Ther 23(8):657–666
Hall S et al (2018) Cerebrospinal fluid concentrations of inflammatory markers in Parkinson's disease and atypical parkinsonian disorders. Sci Rep 8(1):13276
Hall S et al (2012) Accuracy of a panel of 5 cerebrospinal fluid biomarkers in the differential diagnosis of patients with dementia and/or parkinsonian disorders. Arch Neurol 69(11):1445–1452
Hansson O et al (2017) Blood-based NfL: a biomarker for differential diagnosis of parkinsonian disorder. Neurology 88(10):930–937
Herbert MK et al (2015) CSF neurofilament light chain but not FLT3 ligand discriminates Parkinsonian disorders. Front Neurol 6:91
Herbert MK et al (2014) CSF levels of DJ-1 and tau distinguish MSA patients from PD patients and controls. Parkinsonism Relat Disord 20(1):112–115
Holmberg B et al (2003) Cerebrospinal fluid Abeta42 is reduced in multiple system atrophy but normal in Parkinson's disease and progressive supranuclear palsy. Mov Disord 18(2):186–190
Holmberg B et al (2001) CSF-neurofilament and levodopa tests combined with discriminant analysis may contribute to the differential diagnosis of Parkinsonian syndromes. Parkinsonism Relat Disord 8(1):23–31
Lee PH et al (2006) The plasma alpha-synuclein levels in patients with Parkinson's disease and multiple system atrophy. J Neural Transm (Vienna) 113(10):1435–1439
Mollenhauer B et al (2011) Alpha-synuclein and tau concentrations in cerebrospinal fluid of patients presenting with parkinsonism: a cohort study. Lancet Neurol 10(3):230–240
Sakuta H et al (2017) Serum uric acid levels in Parkinson's disease and related disorders. Brain Behav 7(1):e00598
Starhof C et al (2018) Cerebrospinal fluid pro-inflammatory cytokines differentiate parkinsonian syndromes. J Neuroinflamm 15(1):305
Sun ZF et al (2014) Increase of the plasma alpha-synuclein levels in patients with multiple system atrophy. Mov Disord 29(3):375–379
Tullberg M et al (1998) CSF neurofilament and glial fibrillary acidic protein in normal pressure hydrocephalus. Neurology 50(4):1122–1127
Zhou L et al (2016) Oxidative stress and environmental exposures are associated with multiple system atrophy in chinese patients. Can J Neurol Sci 43(5):703–709
Olsson B et al (2013) The glial marker YKL-40 is decreased in synucleinopathies. Mov Disord 28(13):1882–1885
Silajdzic E et al (2014) Flt3 ligand does not differentiate between Parkinsonian disorders. Mov Disord 29(10):1319–1322
Eller M, Williams DR (2009) Biological fluid biomarkers in neurodegenerative parkinsonism. Nat Rev Neurol 5(10):561–570
Barbour R et al (2008) Red blood cells are the major source of alpha-synuclein in blood. Neurodegener Dis 5(2):55–59
Scherzer CR et al (2008) GATA transcription factors directly regulate the Parkinson's disease-linked gene alpha-synuclein. Proc Natl Acad Sci USA 105(31):10907–10912
Bougea A et al (2019) Plasma alpha-synuclein levels in patients with Parkinson's disease: a systematic review and meta-analysis. Neurol Sci 40(5):929–938
Khalil M et al (2018) Neurofilaments as biomarkers in neurological disorders. Nat Rev Neurol 14(10):577–589
Bacioglu M et al (2016) Neurofilament light chain in blood and CSF as marker of disease progression in mouse models and in neurodegenerative diseases. Neuron 91(2):494–496
Ge F et al (2018) Cerebrospinal fluid NFL in the differential diagnosis of parkinsonian disorders: a meta-analysis. Neurosci Lett 685:35–41
Marques TM et al (2019) Serum NFL discriminates Parkinson disease from atypical parkinsonisms. Neurology 92(13):e1479–e1486
Wilke C et al (2018) Serum neurofilament light is increased in multiple system atrophy of cerebellar type and in repeat-expansion spinocerebellar ataxias: a pilot study. J Neurol 265(7):1618–1624
Rodriguez-Martin T et al (2013) Tau phosphorylation affects its axonal transport and degradation. Neurobiol Aging 34(9):2146–2157
Schrag A et al (2017) Clinical variables and biomarkers in prediction of cognitive impairment in patients with newly diagnosed Parkinson's disease: a cohort study. Lancet Neurol 16(1):66–75
Ritchie C et al (2017) CSF tau and the CSF tau/ABeta ratio for the diagnosis of Alzheimer's disease dementia and other dementias in people with mild cognitive impairment (MCI). Cochrane Database Syst Rev 3:CD010803
Brouillette AM, Oz G, Gomez CM (2015) Cerebrospinal fluid biomarkers in spinocerebellar ataxia: a pilot study. Dis Markers 2015:413098
Zhang J et al (2013) Longitudinal assessment of tau and amyloid beta in cerebrospinal fluid of Parkinson disease. Acta Neuropathol 126(5):671–682
Sussmuth SD et al (2010) Differential pattern of brain-specific CSF proteins tau and amyloid-beta in Parkinsonian syndromes. Mov Disord 25(9):1284–1288
Qiang X et al (2017) DL-3-n-butylphthalide-Edaravone hybrids as novel dual inhibitors of amyloid-beta aggregation and monoamine oxidases with high antioxidant potency for Alzheimer's therapy. Bioorg Med Chem Lett 27(4):718–722
Schwarzschild MA et al (2014) Inosine to increase serum and cerebrospinal fluid urate in Parkinson disease: a randomized clinical trial. JAMA Neurol 71(2):141–150
Lee JE et al (2011) Uric acid as a potential disease modifier in patients with multiple system atrophy. Mov Disord 26(8):1533–1536
Boldyrev A et al (2013) Why is homocysteine toxic for the nervous and immune systems? Curr Aging Sci 6(1):29–36
Funding
This study was funded by the “Liaoning BaiQianWan Talents Program” and was also sponsored by Shenyang Science and technology plan project (19-112-4-045) and 345 Talent Project of Shengjing Hospital of China Medical University.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare that they have no conflict of interest.
Ethical standards
This article does not contain any studies with human participants or animals performed by any of the authors.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Cong, S., Xiang, C., Wang, H. et al. Diagnostic utility of fluid biomarkers in multiple system atrophy: a systematic review and meta-analysis. J Neurol 268, 2703–2712 (2021). https://doi.org/10.1007/s00415-020-09781-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-020-09781-9