Abstract
Temporal lobe epilepsy with (TLE-mts) and without (TLE-no) mesial temporal sclerosis display different patterns of cortical neuronal loss, suggesting that the distribution of white matter damage may also differ between the sub-groups. The purpose of this study was to examine patterns of white matter damage in TLE-mts and TLE-no and to determine if identified changes are related to neuronal loss at the presumed seizure focus. The 4 T diffusion tensor imaging (DTI) and T1-weighted data were acquired for 22 TLE-mts, 21 TLE-no and 31 healthy controls. Tract-based spatial statistics (TBSS) was used to compare fractional anisotropy (FA) maps and voxel-based morphometry (VBM) was used to identify grey matter (GM) volume atrophy. Correlation analysis was conducted between the FA maps and neuronal loss at the presumed seizure focus. In TLE-mts, reduced FA was identified in the genu, body and splenium of the corpus callosum, bilateral corona radiata, cingulum, external capsule, ipsilateral internal capsule and uncinate fasciculus. In TLE-no, FA decreases were identified in the genu, the body of the corpus callosum and ipsilateral anterior corona radiata. The FA positively correlated with ipsilateral hippocampal volume. Widespread extra-focal GM atrophy was associated with both sub-groups. Despite widespread and extensive GM atrophy displaying different anatomical patterns in both sub-groups, TLE-mts demonstrated more extensive FA abnormalities than TLE-no. The microstructural organization in the corpus callosum was related to hippocampal volume in both patients and healthy subjects demonstrating the association of these distal regions.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Patients suffering from temporal lobe epilepsy (TLE) can mainly be divided into two sub-groups: TLE with mesial temporal sclerosis as defined by MRI (TLE-mts) and those with normal appearing MRI on visual inspection (TLE-no). Structural MRI studies have shown different patterns of gray matter abnormalities (cortical thinning, volume loss) in the two sub-types: In TLE-mts the most prominent abnormalities are found in the ipsilateral mesial temporal lobe, but also in the parietal and frontal lobes and basal ganglia/thalamus. In TLE-no, abnormalities affect the ipsilateral inferior lateral temporal cortex, but also insular and frontal regions [1–4].
It may be hypothesized that neuronal loss at the seizure focus in TLE causes damage to white matter tracts connecting the focus to extra-focal brain regions which then causes the atrophy seen in the remote but anatomically connected brain regions. A recent study from our laboratory supports this hypothesis, demonstrating a positive relationship between atrophy at the seizure focus and distal cortical thinning in both TLE-mts and TLE-no [2]. This study demonstrated a significant correlation between the ipsilateral hippocampal volume loss and cortical thinning in the inferior medial, posterior temporal and occipital regions in TLE-mts and a significant correlation between neuronal loss of the inferior temporal gyrus and thinning in the lateral temporal lobe, opercular and frontal lobes in TLE-no. The loss of the connecting tracts may result in localized white matter damage whose distribution would depend on the localization of the focus and, hence, would show different patterns in TLE-mts and TLE-no.
One of the most commonly derived diffusion tensor imaging (DTI) measures is fractional anisotropy (FA), which may be interpreted as a marker for the microstructural organization of white matter. Previous studies have demonstrated reduced white matter FA in TLE-mts affecting the tracts associated with the mesial temporal lobe (e.g., uncinate fasciculus, UNC), the ipsilateral superior longitudinal fasciculus (SLF) and frontal lobe white matter extensions [5–10]. Fewer studies have investigated TLE-no diffusion abnormalities and how they differ from TLE-mts. Using region of interest tractography, Concha et al. [9] demonstrated that TLE-mts patients have a higher degree of and more extensive white matter abnormalities than TLE-no. Diffusion abnormalities were identified in the genu, tapetum of the corpus callosum and the external capsule in TLE-no. White matter tracts directly related to the mesial temporal structures were affected in TLE-mts but not in TLE-no. A second study, however, found no FA changes in TLE-no using a whole brain voxel-based approach (although there was identified a small area of increased mean diffusivity (MD) in the cingulum) [7]. In previous studies of other modalities (volumetric, cortical thickness), it has been demonstrated that the abnormalities associated with TLE-no affect inferior temporal neocortical areas; therefore, we may expected ipsilateral tracts connecting the temporal and frontal lobes, particularly the SLF and inferior longitudinal fasciculus (IFO), to be affected.
Despite the number of structural and DTI studies of TLE, only one previous study has examined the relationship of changes in brain gray matter (using structural MRI) and white matter (with DTI) in TLE. Bonilha et al. [6] identified a relationship between white matter damage in the parahippocampal gyrus in TLE-mts and grey matter (GM) atrophy in the putamen, pallidum, middle and inferior temporal areas, amygdala and cerebellum.
Therefore, the aims of this study were (1) To identify patterns of both GM atrophy (using structural MRI) and FA abnormalities in both TLE-mts and TLE-no, (2) To determine if neuronal loss in the presumed seizure focus (mesial temporal structures, i.e., hippocampus, parahippocampus in TLE-mts and inferior temporal lateral structures in TLE-no) co-relates to the degree of white matter damage in the rest of the brain.
Methods
Subjects
Twenty-two consecutive patients with TLE-mts (8 male, 14 female; median age = 48, IQR = 17 years), 21 patients with TLE-no (7 male, 14 female; median age = 42, IQR = 14 years) and 31 healthy controls (9 male, 22 female; median age = 38, IQR = 15 years) who agreed to undergo the 4 T research imaging protocol were included in this study. All patients were treatment resistant and were recruited during a pre-surgical evaluation from the University of California, San Francisco and the Pacific Epilepsy Program, California Pacific Medical Center. The committees of human research at the University of California, San Francisco (UCSF); California Pacific Medical Center, San Francisco; and VA Medical Center, San Francisco approved the study, written informed consent was obtained from each subject and have, therefore, been performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments. Laterality of seizure onset was determined from prolonged ictal and interictal scalp video-EEG telemetry (VET). Fourteen TLE-mts patients had a left temporal onset and eight TLE-mts patients had a right onset. In TLE-no, 11 patients were diagnosed with left temporal onset and ten patients with right temporal onset. Patients were categorized as TLE-mts or TLE-no based on evidence of hippocampal atrophy and signal changes on their 4 T MRI which were all reviewed by the a board-certified neuroradiologist (JB). Hippocampal volumetry was also used to confirm the presence (TLE-mts) or absence (TLE-no) of significant hippocampal volume loss (Summary in Table 1). So far, ten TLE-MTS and four TLE-no have had surgery and histopathological examination confirming the presence (TLE-MTS) and absence (TLE-no), respectively, of mesial temporal lobe sclerosis in these patients. There was a significant mean difference (p < 0.05) between the age that TLE-mts and TLE-no patients developed epilepsy (TLE-mts: median = 15, IQR = 16 years; TLE-no: median = 25, IQR = 17 years) and also the duration of years patients have had epilepsy (TLE-mts: median = 28, IQR = 19 years; TLE-no: median = 11, IQR = 17 years).
Data processing
MRI acquisition
All subjects underwent MR imaging with a Bruker MedSpec 4 T system (Bruker MedSpec, Madison, WI, USA) controlled by a Siemens Trio™ console and equipped with a USA instruments eight channel array coil (Aurora, OH, USA). Four echo-planar imaging (EPI) DTI images (TR/TE = 6 s/77 ms, voxel resolution 2 × 2 × 3 mm, 6 encoding gradients, b = 800 s/mm) were acquired and averaged. T1-weighted (T1w) and T2-weighted (T2w) structural MRI were also acquired. The volumetric T1w sequence was acquired with the following imaging parameter: 2,300/3/950 ms (TR/TE/TI); flip angle of 7°; 1 × 1 × 1 mm3 voxel resolution. The parameters of the T2-weighted turbo spin-echo sequence were TR/TE = 3,000/356 ms, variable flip angles and with the same resolution matrix and field of view of T1w.
MRI processing
The DICOM files of each DTI acquisition were converted into a single multivolume ANALYZE file. Next, they were corrected for eddy currents with FSL (http://www.fmrib.ox.ac.uk/fsl). The T1w and T2w structural images were used to correct the DTI images for geometric distortions resulting from inhomogeneities in the magnetic field [11]. Tensor estimation was performed using the TEEM software package (http://teem.sourceforge.net) and FA maps calculated. In order to combine left and right temporal onset patients in the analysis, the images of right TLE patients were right-to-left flipped so the ipsilateral hemisphere was on the left in all cases. Original and flipped control subject images were also included in the analysis to account for physiological hemispheric differences.
TBSS
Each FA image was non-linearly co-registered to the FSL FMRIB58_FA template in standard Montreal Neurological Institute (MNI) space using FSL’s non-linear registration tool, FNIRT. A mean FA image was generated by averaging all non-linearly registered images. The mean image was thinned to create a skeleton which represents the centers of all tracts the group has in common (for a more detailed description of this process see [12]). The skeleton was then thresholded at an FA of 0.2 to avoid analysis of non-white matter regions and/or areas of high inter-subject variability. To account for residual misalignments after the initial non-linear registration, each subject’s aligned FA data was projected onto the mean skeleton by assigning the maximum FA value in the tract-perpendicular direction to the corresponding skeletal point. For each subject, an individual FA skeleton was produced for analysis.
Free surfer
Based on previous structural MRI findings [1–3], the volume of the ipsilateral hippocampus in TLE-mts and the ipsilateral inferior temporal gyrus thickness in TLE-no were taken as surrogate markers for atrophy at the epileptogenic focus in each sub-group. Segmentation was carried out using FreeSurfer (version 3.05, http://surfer.nmr.mgh.harvard.edu). A full technical description of the reconstruction process of FreeSurfer can be found elsewhere [13–15]. FreeSurfer directly sub-divides the image data into a series of neuro-anatomically defined cortical and sub-cortical structures based on knowledge of MR intensity properties, atlas location, and location relative to each other [13, 16]. The FreeSurfer generated hippocampal labels were inspected and manually edited to improve their accuracy by an experienced rater and using R-view software (http://www.colin-studholme.net/software/software.html). Rater reliability was assessed using the intra-class correlation coefficient (ICC): 0.9.
Voxel-based morphometry
The T1 images were intensity inhomogeneity corrected and segmented into GM, WM, and CSF using the EMS toolbox [17] in SPM2 (http://www.fil.ion.ucl.ac.uk/spm), running in Matlab 6.1 (MathWorks, Natick, MA, USA). The EMS was used because its bias field correction algorithm performs better with the more pronounced bias field at 4 T than the bias field correction of the unified segmentation that is part of SPM8 [18]. DARTEL [19], as implemented in SPM8, was used to optimally warp the GM segmentation maps into a new reference space representing an average of all (left and right flipped) control subjects. The GM values were scaled by the Jacobian determinants of the deformations to account for local compression/expansion. The modulated GM maps were smoothed using an isotropic 8 mm FWHM Gaussian kernel.
Statistical analysis
Voxel-based TBSS analysis
The TBSS FA skeletons were first analyzed in groups comparing (1) Controls and TLE-mts, (2) Controls and TLE-no, (3) TLE-mts and TLE-no. Voxel-wise statistical analyses were performed across subjects at each voxel on the common skeleton. A general linear model was used to determine the effect of ‘group’ at each voxel. Threshold-free cluster enhancement (TFCE) permutation analysis with 10,000 random permutations was used to correct for multiple comparisons. The TFCE is similar to cluster based thresholding but has been shown to be more robust and removes the need for an arbitrarily selected threshold [20]. Regions with significant changes in white matter FA were later defined using the John Hopkins University (JHU) white matter atlases [21, 22]. To determine if reduction in FA was related to atrophy at the presumed epileptogenic focus, a general linear regression analysis was used to investigate the association of the volume/thickness of the presumed epileptogenic focus (volume of the ipsilateral hippocampus and the mean cortical thickness of the ipsilateral inferior temporal gyrus in both TLE-mts and controls; TLE-no and controls) with extra-focal changes in FA. Age and intracranial volume (ICV) were included as covariates in this analysis. It is hypothesized that the same regression coefficient is present in both control and patient groups, but in patient affected brain regions the inclusion of both sub-groups will aid to increase the range and the association. Again, a TFCE permutation analysis with 10,000 random permutations was used to correct for multiple comparisons.
Region of interest TBSS analysis
The JHU white matter probabilistic tractography atlas, supplied with FSL, was used for ROI analysis of the skeleton. Based on findings of other neuroimaging modalities in TLE, we expect white matter changes to be associated with the long association fibers connecting distal brain regions. The SLF and inferior frontal-occipital fasciculus (IFO) are investigated in both TLE-mts and TLE-no as well as the uncinate fasciculus (UNC) in TLE-mts and inferior longitudinal fasciculus (ILF) in TLE-no (Fig. 1). Tracts in the ipsilateral hemisphere only are examined so as to reduce the number of multiple tracts examined statistically, and; it is postulated that ipsilateral white matter damage may be greater than the contralateral side. Selected probabilistic tracts were thresholded at 20 %, masked to create an ROI and mean FA was calculated at each ROI on the skeleton for each individual subject. Mean FA differences were assessed between (1) Controls and TLE-mts, and (2) Controls and TLE-no for the above ROIs using independent sample t-tests. Partial correlations were used to assess the relationship between the volume/thickness of the presumed epileptogenic focus with ROIs that were found to be significant, correcting for age and ICV. Statistical analyses were carried out using SPSS (version 18).
Regions of interests (ROI) defined using the JHU white matter probabilistic tractography atlas. IFO inferior fronto-occipital fasciculus, ILF inferior longitudinal fasciculus, SLF superior longitudinal fasciculus, UNC uncinate fasciculus. The mean FA skeleton is represented in green, the ROI in blue
Voxel-based analysis
Independent two-sample t-tests were used to test for group differences at each GM voxel with intra-cranial volume and age added as a nuisance variable. A false discovery rate (FDR) of p ≤ 0.05 was applied to correct for multiple comparisons.
Results
TBSS voxel-based analysis
Compared with controls, TLE-mts patients showed clusters of reduced FA in the genu, body and splenium of the corpus callosum, bilateral corona radiata, cingulum, external capsule, ipsilateral anterior limb of the internal capsule, SLF and UNC. A cluster of reduced FA was identified in TLE-no which included the genu, body of the corpus callosum and ipsilateral anterior corona radiata (Fig. 2). There were no significant findings in the TLE-mts versus TLE-no analysis.
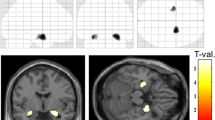
Clusters of voxels (p < 0.05, TFCE) with significantly reduced FA in a TLE-mts and b TLE-no compared with controls. The mean FA skeleton where analysis was carried out is represented in green. Significant FA differences after correction for multiple comparisons are presented in yellow/red. SLF superior longitudinal fasciculus, CC corpus callosum
Figure 3 shows regions with positive correlations between ipsilateral hippocampal volume loss and decreased extra-hippocampal FA in TLE-mts patients and controls corrected for age, ICV and multiple comparisons. The correlation indicates areas of FA reduction also found in the direct comparison between TLE-mts and controls. Clusters were identified in the genu, body of the corpus callosum and ipsilateral cingulum. The correlation between FA in the corpus callosum and hippocampus volume was also significant in TLE-no and controls. A post hoc interaction analysis was carried out to determine if the relationship between FA and hippocampal volume differed depending on group (patient or control), the result was not significant (p = 0.17) and a further exploratory analysis confirmed this finding was also significant in controls only. The correlation between ipsilateral inferior temporal gyrus thickness and FA was not significant after correction for multiple comparisons in any group.
Clusters of voxels (p < 0.05, TFCE) with significant positive correlations between ipsilateral hippocampal volume and FA in a TLE-mts and controls b TLE-no and controls. The mean FA skeleton where analysis was carried out is represented in green. Significant FA differences after correction for multiple comparisons are presented in yellow/red. Graphs show correlation between hippocampal volume and FA in the genu of the corpus callosum
Region-of-interest analysis
In TLE-mts versus the control subjects, group mean analysis of the preselected ROIs showed a group effect (p < 0.05) for the ipsilateral SLF, IFO and UNC, however, only the UNC survived correction for multiple comparisons. No significant mean differences were found in the TLE-no group (Table 2).
A positive correlation was identified between hippocampal volume and the UNC (r = 0.32, p = 0.004), in TLE-mts and controls. No other significant correlations were identified.
To further explore the heterogeneity of the TLE-no patient group, a post hoc individual analysis was subsequently carried out. For each ROI, z-scores were calculated for each subject based on control mean and standard deviations. To identify an ROI with an abnormal low FA in individual patients, a threshold of <−2 standard deviations are considered abnormal. On analysis, no TLE-no subject fell below two standard deviations for SLF, IFO or ILF ROIs.
Voxel-based morphometry
Figure 4 displays the results of GM atrophy in TLE-mts and TLE-no. In TLE-mts significant volume reduction was observed in the ipsilateral hippocampus, bilateral thalamus, insula, putamen, postcentral gyrus and cingulate gyrus. In TLE-no, volume loss was identified in bilateral insula, superior temporal gyrus, postcentral gyrus, cingulate gyrus and ipsilateral thalamus and inferior temporal gyrus. There was no significant GM volume loss in controls relative to either TLE sub-group and no significant findings in the TLE-mts versus TLE-no analysis.
Discussion
The main findings of this study were (1) Regions of decreased FA were found in both TLE subgroups. (2) Although no between-group FA differences were noted, TLE-mts demonstrated a higher degree of abnormality and a more extensive distribution than TLE-no when compared with controls despite the fact that widespread extra-focal GM atrophy was present in both subgroups. (3) FA was positively correlated with volume in the hippocampus in all groups with the highest correlation in TLE-mts and controls. Such a correlation between FA and ITG was absent in all groups.
TLE-mts
White matter regions of abnormally low FA in TLE-mts were identified in the genu and body and splenium of the corpus callosum, the cingulum, external and internal capsule, corona radiata bilaterally and the UNC and were accompanied by widespread, ipsilateral mesial temporal and bilateral parietal and thalamic GM atrophy. These findings are in good agreement with previous reports in TLE-mts (See [12] and [23] for recent reviews). In addition, there was a positive correlation between ipsilateral hippocampal volume and regions of FA reduction (corpus callosum and ipsilateral cingulum) that is suggestive of a disruption of the microstructural organization of the white matter pathways connecting the site of the seizure focus to these distal cortical regions. This conclusion is also supported by the findings of a recent study from Bonilha and co-workers [6], who were the first to demonstrate a significant relationship between white matter damage in the parahippocampal gyrus that encompasses some of the major fiber tracts from and to the hippocampus and regional GM atrophy involving the putamen, pallidum, middle and inferior temporal areas, amygdala and cerebellum. The relationship between the hippocampus volume and microstructural organization of the white matter in the corpus callosum is also confirmed in both healthy controls and TLE-no, where no abnormalities in hippocampal volume were observed, confirming the relationship between these distal brain regions. The higher correlation in the TLE-mts group would support the hypothesis that neuronal dysfunction/loss and white matter damage may be due to the excitotoxic effects of spreading epileptogenic activity in the hippocampus and in extrafocal brain regions that are directly or indirectly connected to it. However, we cannot speculate in this paper whether this relationship is a consequence of the disorder, or whether the atrophy of the hippocampus precedes the FA abnormalities. It is hoped that future longitudinal studies will begin to confirm these hypotheses.
TLE-no
In TLE-no the FA abnormalities were restricted to the anterior corpus callosum and the ipsilateral anterior corona radiata, i.e., they were considerably less extensive than in TLE-mts, and there was also no significant correlation between the region of the presumed epileptic focus (inferior temporal lobe, based on the findings of Mueller et al. [18]) and FA in the ipsilateral temporal lobe and beyond. The finding of a relatively circumscribed region of FA abnormalities in TLE-no is in agreement with a previous study by Concha et al. [9], who also identified low FA in the corpus callosum in both TLE sub-groups using tractography, but contrasts with Shon et al. [21], who employed a whole brain voxel-based method and found no FA decreases in TLE-no. Based on these findings and taking the different methodologies and study populations into account, these three studies seem to suggest that compared to TLE-mts focal and extrafocal white matter integrity is relatively preserved in TLE-no. The preserved white matter integrity is particularly interesting considering the fact that TLE-no has evidence for widespread bilateral inferior temporal, insular and medial prefrontal gray matter volume loss that, although differently distributed, matched the severity of GM loss observed in TLE-mts.
There are several potential explanations for this discrepancy, (1) TLE-no had a significantly shorter epilepsy duration compared to TLE-mts and, thus, it is possible that they have not yet had sufficient time/seizures to develop more severe white matter abnormalities. However, even though this explanation cannot be completely excluded, the fact that both TLE types had extensive gray matter volume losses makes it rather unlikely that the difference in duration of epilepsy alone explains the relatively preserved white matter integrity in TLE-no. (2) FA abnormalities in TLE-no are more heterogeneous in extent and severity than in TLE-mts and, therefore, more difficult to detect in group analyses. The findings of the single subject ROI analysis, however, in which none of the TLE-no patients demonstrated significant mean FA reductions in the SLF, IFO or ILF ROIs, refutes this reasoning. (3) The discrepancy between white matter and gray matter damage could indicate that white matter in TLE-no is relatively better protected from the excitotoxic effects of seizure spread than in TLE-MTS. In contrast to TLE-no whose seizures typically start in early adulthood or later life, TLE-mts already start having recurrent seizures in childhood or adolescence, i.e., at an age when the brain myelination is still ongoing and thus is likely to be negatively affected by seizures and seizure spread. The notion that white matter is better protected against the effects of seizures after the completion of the myelination is supported by animal models that demonstrated a protective effect of the myelin associated glycoprotein against the excitotoxic effects of kainate induced seizures [24]. (4) TLE-no have a different histopathological correlate for their epilepsy than TLE-mts. One possible correlate is microdysgenesis that can be quite widespread and is characterized by neuronal clustering within cortical layers, blurred gray white distinction, perivascular clustering of oligodendrocytes, isolated heterotopic gray matter neurons in white matter and white matter gilosis [25–27]. If it is assumed that the blurring of the gray/white boundary results in an underestimation of the gray matter rim by the segmentation algorithm and that the white matter gilosis obscures the effects of excitotoxic white matter damage, gray matter loss in combination with a relatively preserved white matter could indeed be the signature of microdysgenesis in quantitative MR imaging analyses.
The study has the following limitations: (1) Diagnosis of MTS was made based on 1.5 T MRI which was also in agreement with a high resolution 4 T hippocampal specific scan, where volumetric information of hippocampal sub-fields was available. Unfortunately, a number of patients had not undergone surgery to histologically confirm the presence or absence of MTS (10 out of 22), (2) Based on the findings of previous publications from our lab [2] and on electrophysiological findings [28] indicating a diffuse, medial–lateral temporal seizure onset zone in TLE with normal hippocampus, thickness of the inferior temporal lobe was selected as presumed seizure focus in TLE-no. However, only a subset of TLE-no had surgery, which in the case of postoperative seizure freedom is considered to be the ultimate confirmation of focus localization by EEG and; therefore, we cannot completely exclude that some of the TLE-no patients had mild hippocampal sclerosis not detected by MRI, (3) From this study we cannot conclude that TLE-mts and TLE-no are two distinctly different disorders given that we have not shown a significant difference between these two subgroups. It is highly probable that some cases represent grouping along a spectrum, particularly as we do not have histological information for all subjects and is a possible reason why we do not see a significant difference between TLE sub-groups, (4) To increase the power to detect group differences, right TLE were combined with left TLE for all analyses and; therefore, the images of right TLE patients were flipped so the ipsilateral hemisphere was on the left in all cases. Due to subtle asymmetries in the brain we cannot rule out that pooling subjects in this manner may lead to subtle FA changes going unnoticed, (5) In this study six gradient directions were measured, repeated four times and averaged. Although Tournier et al. [29] discuss that it is difficult to conclude that multiple repeated sets of a smaller number of orientations should not be used, Jones et al. [30] recommend >20 gradient directions for robust measures of anisotropy, with a lower number potentially leading to variability and false negative findings, (6) A limitation of DTI is the issue of crossing fibers where an image voxel can contain more than one dominant fiber orientation. The TBSS is currently being extended to account for crossing fibers [29] and should be considered for future TBSS studies, (7) Finally, a limitation of TBSS is that analysis of FA is restricted to the center of the main tracts and, therefore, FA information from the more peripheral regions is lost.
References
Scanlon C, Mueller SG, Tosun D, Cheong I, Garcia P, Barakos J, Weiner MW, Laxer KD (2011) Impact of Methodologic choice for automatic detection of different aspects of brain atrophy by using temporal lobe epilepsy as a model. Am J Neuroradiol. doi:10.3174/ajnr.A2578
Mueller SG, Laxer KD, Barakos J, Cheong I, Garcia P, Weiner MW (2009) Widespread neocortical abnormalities in temporal lobe epilepsy with and without mesial sclerosis. NeuroImage 46(2):353–359. doi:10.1016/j.neuroimage.2009.02.020
Mueller SG, Laxer KD, Schuff N, Weiner MW (2007) Voxel-based T2 relaxation rate measurements in temporal lobe epilepsy (TLE) with and without mesial temporal sclerosis. Epilepsia 48(2):220–228. doi:10.1111/j.1528-1167.2006.00916.x
Carne R, Cook M, MacGregor L, Kilpatrick C, Hicks R, O’brien T (2007) “Magnetic resonance imaging negative positron emission tomography positive” Temporal lobe epilepsy: FDG-PET pattern differs from mesial temporal lobe epilepsy. Mol Imag Biol 9(1):32–42
Gross DW (2011) Diffusion tensor imaging in temporal lobe epilepsy. Epilepsia 52(Suppl 4):32–34. doi:10.1111/j.1528-1167.2011.03149.x
Bonilha L, Edwards JC, Kinsman SL, Morgan PS, Fridriksson J, Rorden C, Rumboldt Z, Roberts DR, Eckert MA, Halford JJ (2010) Extrahippocampal gray matter loss and hippocampal deafferentation in patients with temporal lobe epilepsy. Epilepsia 51(4):519–528. doi:10.1111/j.1528-1167.2009.02506.x
Shon YM, Kim YI, Koo BB, Lee JM, Kim HJ, Kim WJ, Ahn KJ, Yang DW (2010) Group-specific regional white matter abnormality revealed in diffusion tensor imaging of medial temporal lobe epilepsy without hippocampal sclerosis. Epilepsia 51(4):529–535. doi:10.1111/j.1528-1167.2009.02327.x
Riley JD, Franklin DL, Choi V, Kim RC, Binder DK, Cramer SC, Lin JJ (2010) Altered white matter integrity in temporal lobe epilepsy: association with cognitive and clinical profiles. Epilepsia 51(4):536–545. doi:10.1111/j.1528-1167.2009.02508.x
Concha L, Beaulieu C, Collins DL, Gross DW (2009) White-matter diffusion abnormalities in temporal-lobe epilepsy with and without mesial temporal sclerosis. J Neurol Neurosurg Psychiatry 80(3):312–319. doi:10.1136/jnnp.2007.139287
Focke NK, Yogarajah M, Bonelli SB, Bartlett PA, Symms MR, Duncan JS (2008) Voxel-based diffusion tensor imaging in patients with mesial temporal lobe epilepsy and hippocampal sclerosis. NeuroImage 40(2):728–737. doi:10.1016/j.neuroimage.2007.12.031
Tao R, Fletcher P, Gerber S, Whitaker R (2009) A variational image-based approach to the correction of susceptibility artifacts in the alignment of diffusion weighted and structural MRI. Inf Process Med Imaging 21:664–675
Looi JCL, Lindberg O, Liberg B, Tatham V, Kumar R, Maller J, Millard E, Sachdev P, Högberg G, Pagani M (2008) Volumetrics of the caudate nucleus: reliability and validity of a new manual tracing protocol. Psychiatry Res: NeuroImage 163(3):279–288
Fischl B, Salat DH, Busa E, Albert M, Dieterich M, Haselgrove C, van der Kouwe A, Killiany R, Kennedy D, Klaveness S (2002) Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron 33(3):341–355
Dale AM, Fischl B, Sereno MI (1999) Cortical surface-based analysis I segmentation and surface reconstruction. NeuroImage 9(2):179–194
Fischl B, Sereno MI, Dale AM (1999) Cortical surface-based analysis II: inflation, flattening, and a surface-based coordinate system. NeuroImage 9(2):195–207
Desikan RS, Ségonne F, Fischl B, Quinn BT, Dickerson BC, Blacker D, Buckner RL, Dale AM, Maguire RP, Hyman BT (2006) An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. NeuroImage 31(3):968–980
Van Leemput K, Maes F, Vandermeulen D, Suetens P (1999) Automated model-based tissue classification of MR images of the brain. IEEE Transactions on Medical Imaging 18(10):897–908
Ashburner J, Friston KJ (2005) Unified segmentation. NeuroImage 26(3):839–851
Ashburner J (2007) A fast diffeomorphic image registration algorithm. NeuroImage 38(1):95–113. doi:10.1016/j.neuroimage.2007.07.007
Smith SM, Nichols TE (2009) Threshold-free cluster enhancement: addressing problems of smoothing, threshold dependence and localisation in cluster inference. NeuroImage 44(1):83–98
Hua K, Zhang J, Wakana S, Jiang H, Li X, Reich DS, Calabresi PA, Pekar JJ, van Zijl PCM, Mori S (2008) Tract probability maps in stereotaxic spaces: analyses of white matter anatomy and tract-specific quantification. NeuroImage 39(1):336–347. doi: 10.1016/j.neuroimage.2007.07.053
Wakana S, Caprihan A, Panzenboeck MM, Fallon JH, Perry M, Gollub RL, Hua K, Zhang J, Jiang H, Dubey P, Blitz A, van Zijl P, Mori S (2007) Reproducibility of quantitative tractography methods applied to cerebral white matter. NeuroImage 36(3):630–644. doi:10.1016/j.neuroimage.2007.02.049
Keller SS, Roberts N (2007) Voxel-based morphometry of temporal lobe epilepsy: an introduction and review of the literature. Epilepsia 49(5):741–757
Lopez PHH, Ahmad AS, Mehta NR, Toner M, Rowland EA, Zhang J, Doré S, Schnaar RL (2011) Myelin-associated glycoprotein protects neurons from excitotoxicity. J Neurochem 116(5):900–908
Thom M, Sisodiya S, Harkness W, Scaravilli F (2001) Microdysgenesis in temporal lobe epilepsy. Brain 124(11):2299–2309
Eriksson S, Malmgren K, Nordborg C (2005) Microdysgenesis in epilepsy. Acta Neurol Scand 111(5):279–290
Kasper BS, Stefan H, Paulus W (2003) Microdysgenesis in mesial temporal lobe epilepsy: a clinicopathological study. Ann Neurol 54(4):501–506
Vossler DG, Kraemer DL, Haltiner AM, Rostad SW, Kjos BO, Davis BJ, Morgan JD, Caylor LM (2004) Intracranial EEG in temporal lobe epilepsy: location of seizure onset relates to degree of hippocampal pathology. Epilepsia 45(5):497–503
Tournier JD, Mori S, Leemans A (2011) Diffusion tensor imaging and beyond. Magn Reson Med 65(6):1532–1556. doi:10.1002/mrm.22924
Jones DK (2004) The effect of gradient sampling schemes on measures derived from diffusion tensor MRI: a Monte Carlo study†. Magn Reson Med 51(4):807–815
Acknowledgments
We would like to thank Dr. Tom Fletcher, Assistant Professor at the Scientific Computing and Imaging Institute, University of Utah, for his help with the DTI processing and software. This work was supported by a NIH grant R01NS0311966 to K.D.L.
Conflicts of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Scanlon, C., Mueller, S.G., Cheong, I. et al. Grey and white matter abnormalities in temporal lobe epilepsy with and without mesial temporal sclerosis. J Neurol 260, 2320–2329 (2013). https://doi.org/10.1007/s00415-013-6974-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-013-6974-3