Abstract
Background
Medial temporal lobe epilepsy (mTLE) has been associated with widespread white mater (WM) alternations in addition to mesial temporal sclerosis (MTS). Herein, we aimed to investigate the correlation between disease duration and WM structural abnormalities in mTLE using diffusion MRI (DMRI) connectometry approach.
Method
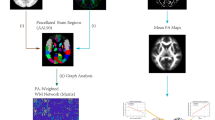
DMRI connectometry was conducted on 24 patients with mTLE. A multiple regression model was used to investigate white matter tracts with microstructural correlates to disease duration, controlling for age and sex. DMRI data were processed in the MNI space using q-space diffeomorphic reconstruction to obtain the spin distribution function (SDF). The SDF values were converted to quantitative anisotropy (QA) and used in further analyses.
Results
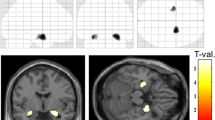
Connectometry analysis identified impaired white matter QA of the following fibers to be correlated with disease duration: bilateral retrosplenial cingulum, bilateral fornix, right inferior longitudinal fasciculus (ILF), and genu of corpus callosum (CC) (FDR = 0.009).
Conclusion
Our results were obtained from DMRI connectometry, which indicates the connectivity and the level of diffusion in nerve fibers rather just the direction of diffusion. Compared to previous studies investigating the correlation between duration of epilepsy and white matter integrity in mTLE patients, we detected broader and somewhat different associations in midline structures and component of limbic system. However, further studies with larger sample sizes are required to elucidate previous and current results.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Medial temporal lobe epilepsy (mTLE/TLE) is the most common form of focal epilepsy and is characterized by recurrent seizures that originate in the temporal lobe [1]. Apart from seizures, patients with mTLE experience deficits in a variety of neuropsychological domains such as attention, memory, personality, mood, and executive function [2]. Although antiepileptic drugs are effective first choice options in management of mTLE, up to 90% of pediatric patients and most adult patients with mTLE eventually become medically intractable [3]. A majority of these patients benefit from surgical resection. Importantly, presence of mesial temporal sclerosis (MTS) predicts better surgical outcomes and lower risk of recurrence after surgery [4,5,6,7]. On the other hand, a longer disease duration and an earlier age of mTLE onset are associated with increased seizure frequency [8], increased frequency of MTS [9], and multilobar involvement, which is in turn predictive of surgery failure [10]. Disease duration and earlier onset also predict lower quality of life and associate with cognitive impairment [7, 11].
The “temporo-polar blurring” in MRI, indicative of MTS, emerges relatively late in the disease process and predicts poor cognitive performance in mTLE patients [12]. Also, white matter abnormalities, detected through conventional MRI, poorly correlate with the duration of symptoms and the success of surgical intervention [13]. Conventional MRI is unable to locate ultrastructural changes in temporal pole white matter or other epileptogenic foci. On the other hand, diffusion tensor imaging (DTI) studies have revealed disturbed microstructure in white matter tracts connected to the affected temporal lobe in patients with mTLE [14]. Importantly, diffusion metrics of white matter microstructure in temporal [15,16,17] and extra-temporal white matter [18] have shown association with the duration of the disease.
Diffusion MRI (DMRI) connectometry is a novel analytical approach that uses the concept of local connectome to identify white matter trajectories in the brain. Conventional DTI calculates the velocity of water diffusion, i.e., how fast the water tends to diffuse along different directions of each voxel, while DMRI connectometry uses the diffusion density, i.e., how many water molecules diffuse in each direction, to characterize the white matter fibers [19]. Conventional DTI calculates the average of the water diffusion metrics of all voxels in a white matter tract using predefined atlas-based trajectories. DMRI connectometry uses a model-free method to track white matter trajectories and characterize their diffusometric features, not the average diffusion metrics. Based on this difference, conventional DTI loses spatial resolution in areas of kissing or crossing white matter fibers, commonly encountered in the white matter tracts originating or crossing the medial temporal lobe.
Mean diffusivity (MD) and fractional anisotropy (FA) are two most popular diffusion metrics used by conventional DTI [20]. In DMRI connectometry, significant connectomes, i.e., local patterns of water diffusivity, are built by tracking the density of water diffusion along any given fiber orientation, a parameter named spin distribution function (SDF) [21]. Quantitative anisotropy (QA) is a secondary parameter calculated from SDF and used in regression analyses [22]. QA is a reflection of local connectome fingerprint of individual voxels, and therefore less susceptible to partial volume effects [23, 24]. These features confer DMRI connectometry with more accurate and reproducible results when identifying fibers with microstructural correlates to a variable of interest or identifying between group differences in connectomics [25,26,27].
We previously used DMRI connectometry to identify white matter tracts with altered connectomics between patients with left and right mTLE [28]. To our knowledge, no study has yet used DMRI connectometry to identify alterations in white matter tracts relative to disease characteristics. Using this approach, we investigated white matter tracts with altered local connectome in mTLE patients. Next, we used DMRI connectometry in a multiple regression model to investigate whether disease duration could predict white matter changes.
Materials and methods
Participants
We used images of 24 patients with unilateral mTLE. All patients underwent epileptic surgery and achieved an Engel class I outcome after resection of their mesial temporal lobe. Patients consisted of two groups of 12 right-sided mTLE (male/female: 7:5, age: 43.3 ± 11.4 years) and 12 left-sided mTLE patients (male/female: 6:6, age: 40.1 ± 13.7 years) (Table 1). The laterality of seizure focus was determined based on non-invasive electroencephalogram and confirmed through histopathological examination of surgical specimen. None of the patients had a history of previous brain surgery, brain tumors, or brain trauma. All patients underwent pre-surgical DMRI. The study population and enrollment details are described elsewhere [29].
Images of a group of 12 age- and sex-matched healthy adults were obtained from the freely available “information extraction from images” database (http://biomedic.doc.ic.ac.uk/brain-development/). The subjects included six males and six females and were 42.2 ± 11.5 years old.
Data acquisition
All patients underwent pre-surgical imaging using a 3-Tesla scanner to collect 25-angle diffusion MRI with field of view = 224 × 224 mm, voxel size: 1.96 × 1.96 × 2.6 mm3, repetition time = 10,000 ms, echo time = 76 ms, at b = 1000 s/mm2, implementing the spoiled gradient echo protocol, and one b0 image. In addition, a 3D T1-weighted structural scan (SPGR) with voxel size = 0.39 × 0.39 × 2.0 mm3, repetition time = 10,400 ms, echo time = 300 ms, and flip angle = 15° was obtained.
Diffusion MRI processing
The DMRI data were corrected for subject motion, eddy current distortions, and susceptibility artifacts due to the magnetic field inhomogeneity, using the Explore DTI toolbox [30]. We further performed a visual quality control in T1-weighted images to rule out significant brain abnormalities, including white matter lesions, silent brain infarction, brain tumors, hydrocephalus, or any other anatomical disturbing. None of the patients in either groups were excluded after quality check.
Q-space diffeomorphic reconstruction
DMRI data were reconstructed in the MNI space in order to obtain the SDF. SDF was calculated for each voxel, using q-space diffeomorphic reconstruction (QSDR) method [31]. QSDR uses a model-free algorithm to quantify the density of water diffusion at different orientations of each voxel. In other words, QSDR first builds a matrix of orientation distribution function of the diffusing spins. QSDR then determines the peak distribution value for each voxel orientation, i.e., SDF, which is then converted to a new value, quantitative anisotropy, QA. QA gives a unique identity to each voxel based on the probability of the voxel being connected to its adjacent voxels. This probability is simply called “connectivity” and the high dimensional vector of all SDF/QAs magnitudes and directions is called “local connectome” []. QA in DMRI connectometry is analogous to FA in conventional DTI, both referring to structural integrity and white matter fiber health. Herein and after, QA and connectivity are used as alternates of local connectome, when referring to the statistical analyses performed afterwards.
DMRI connectometry
In the next step, DMRI connectometry was used to construct white matter tracts with significant changes in local connectome in mTLE patients compared to controls [20]. The analysis was done using the software DSI Studio (http://dsi-studio.labsolver.org), which is publicly available. DSI Studio adopts a multiple regression model with age and sex as covariates to investigate white matter fibers where connectivity is significantly correlated with disease duration. DSI Studio allows for tuning of three parameters of the fiber tracking algorithm: (1) T threshold, (2) length threshold, and (3) seeding density. The algorithm then reports a p value estimate for the identified null distribution of fiber tracts. The raw estimates are then corrected for type 1 error inflation due to multiple testing via false discovery rate (FDR) estimation.
To identify the significant local connectomes using DMRI connectometry, we conducted a deterministic fiber tracking algorithm along the core pathway of the fiber bundle to identify the null distribution of fiber tracts. The connectometry approach was then followed by a permutation testing to assign a corrected p value for the association of white matter connectivity with the study variable, here disease duration. In order to estimate the FDR, 2000 random permutations were applied to the group label, to estimate and control the type I error inflation. Adopting the highest permutation count of 2000 ensured the most accurate estimation of FDR. Importantly, FDR is different from other correction methods, e.g., Bonferroni correction, as it controls the number of false discoveries only in tests that result in significant fibers, but not all tests. This offers a higher statistical power than other conventional methods of p value correction.
Adding to the validity of the results, the T-score threshold was assigned to the highest level of 3, to select local connectomes, ensuring highest specificity in fiber tracking. Finally, the length threshold of 20 voxels distance was considered to choose tracks; the track density was 20 per voxel.
Review of literature
In a final step, we performed a comprehensive search in the literature using the key words: (“diffusion MRI” OR “diffusion magnetic resonance imaging” OR “diffusion tensor imaging” OR DTI) AND (“Temporal lobe epilepsy” OR TLE) using the search engines PubMed, Scopus, and Embase. Two of the co-authors independently reviewed the resulting 362 articles for their relevance to the question regarding the association between white matter diffusion parameters and disease duration in mTLE. Articles were initially screened based on title and abstract and then full text. Any disagreements between the authors were dismissed by a third party reviewing the articles.
Results
Our study population consisted of 24 patients with mTLE, 12 with right-sided seizure focus, and 12 with left-sided seizure, confirmed through post temporal resection surgery pathology. Patients with right and left mTLE were comparable in terms of age, disease duration, and presence of MTS so that there was no inhomogeneity regarding the laterality of the connectometry results.
In the first step, we investigated white matter tracts with altered connectivity in mTLE patients relative to controls. Higher connectivity in DMRI connectometry refers to higher QA of white matter fibers. Higher QA reflects, and can be regarded as a signature for higher microstructural integrity and fiber health, while lower connectivity is indicative of microstructural disorganization and demise [32, 33]. DMRI connectometry yielded the following white matter tracts with decreased connectivity in mTLE patients: left arcuate fasciculus (AF) and left posterior limb of internal capsule (PLIC) (FDR = 0.002). DMRI connectometry also revealed several fibers with increased connectivity compared to healthy controls: bilateral corticospinal tracts (CST), bilateral retrosplenial cingulum, genu of corpus callosum (CC), bilateral fornix, forceps minor, and right inferior longitudinal fasciculus (ILF) (FDR = 0.008).
Next, the multiple regression model helped narrow down the result to white matter regions where connectivity was negatively correlated with disease duration after adjustment for subject’s age and sex. We identified connectivity in bilateral retrosplenial cingulum, bilateral fornix, right ILF, and genu of CC to negatively correlate with disease duration (FDR = 0.009) (Fig. 1).
In the final step, we reviewed the literature addressing the effect of disease duration on white matter microstructure in mTLE using diffusion tensor imaging, as described in the final paragraph of the methods section. Five articles were finally included after reviewing a total of 362 relevant publications. Compared to the previous results using DTI, our finding with DMRI connectometry identified distinct areas within cortical and subcortical white matter where microstructural disorganization directly correlated with increased duration of mTLE. These areas were more widespread and corroborated to both intratemporal and extratemporal white matter tracts, consistent with the network disruption hypothesis in mTLE [34]. Table 2 summarizes the results of the five previous articles along with those of the current study.
Discussion
Using DMRI tractography, we identified fibers with increased connectivity in patients with mTLE. Among these, connectivity in bilateral retrosplenial cingulum and genu of corpus callosum as well as right ILF showed inverse correlation with disease duration. A decrease in connectivity/QA of white matter fibers usually results from microstructural disruption of the fibers, indicating reduced axonal density and myelin sheath disruption [35]. We also identified decreased white matter connectivity in the left AF and left PLIC fibers, yet with no correlation with disease duration.
Diffusion-based imaging has been previously used to study white matter alterations in patients with mTLE [15,16,17,18, 36]. Disrupted white matter microarchitecture in terms of increased MD and reduced FA is generally observed in patients with mTLE, predominantly in temporal lobe white matter adjacent to the epileptogenic focus, including the ipsilateral uncinate and arcuate fasciculi (UF and AF), and ILF [14]. The contralateral fibers are also significantly, but less prominently, affected [14], providing bases for the hypothesis that white matter changes in mTLE follow a network-based pattern, with microstructural disintegrity observed in fiber tracts belonging to a specific network regardless of their relative anatomical distance [37]. This is associated with compensatory changes in fibers outside the network, despite their relative adjacency to the medial temporal lobe, as we found in CC and forceps minor.
Similar to the findings of our study, a review of literature revealed several studies that identified a correlation between a disruption in conventional diffusion metrics, i.e., decreased FA and increased MD, with disease severity and duration of seizures in patients with mTLE [15,16,17,18, 36]. Reduced FA in the UF [36] and increased MD and linear and spherical anisotropy in the left AF and left UF [17] were shown to be correlated with longer duration of disease in mTLE patients. Longer disease duration was also shown to be correlated with reduced FA in parahippocampal gyrus and thalamus [18] and in the ILF ipsilateral to the epileptogenic focus [15]. We provided similar evidence on white matter dysconnectivity in left AF and PLIC, as well as compensatory changes in the genu of CC, CST, fornix, and right ILF, which was not reported by previous DTI studies. Our discovery of reduced connectivity in bilateral retrosplenial cingulum, fornix, and genu of CC, associated with longer disease duration, was also never reported in the DTI literature.
According to the previous studies, ILF, AF, and UF are among the most common fiber tracts, with direct interconnection to the temporal lobe, which have shown lower FA in mTLE patients [17, 38]. These changes are shown to be more widespread in the ipsilateral side [15, 36] and correlate with longer duration of epilepsy and earlier age of seizure onset [36, 39]. Spherical diffusion indices, which have higher specificity in areas with kissing or crossing white matter fibers, are also shown to be increased in bilateral UF and CST as well as left ILF and AF in mTLE patients [17, 40]. It appears that this increase in mean and spherical diffusivity indices is indicative of disrupted white matter microstructure and is more prominent in the dominant hemisphere, i.e., in the left side fibers, which is responsible for language semantics and memory processing. This is confirmed by our finding of reduced connectivity in left AF and left PLIC, which is independent of the laterality of the epileptogenic focus, considering the fact that our group encompassed an equal number of patients with right and left TLE. Fornix is another temporal lobe white matter fiber, which has shown structural abnormalities, especially in the ipsilateral side to the MTS in mTLE patients [38, 41]. Concordant with the higher number of patients with MTS in our group, we identified altered fornix connectivity in mTLE patients to inversely correlate with disease duration.
Besides the described changes in white matter fibers projecting in or out of the temporal lobe, extensive microstructural disruptions are reported in white matter tracts that “pass through” or are “adjacent” to the temporal lobe [14]. Similarly, previous studies report that the correlation between disease duration and white matter integrity extends beyond the temporal lobe and involves the parahippocampal gyri, thalamus, and corpus callosum [39]. As an example, the retrosplenial part of cingulum, which we identified to have reduced connectivity associated with longer disease duration, is known to encompass fibers that travel directly from the temporal pole to the retrosplenial cortex [42]. We also identified reduced connectivity in the cingulum bundle, which is an extra-temporal fiber with reduced FA in both ipsilateral and contralateral hemispheres according to previous studies [16, 35]. Crucially, it has been shown that cingulum bundle microstructural changes are more prominent in mTLE associated with MTS and correlate with the degree of hippocampal atrophy in these patients [16, 43]. Similarly, it has been shown that reduced axonal density in the cingulum fiber in mTLE patients is correlated with the degree of atrophy in the hippocampal gyrus, as well as fornix and UF, which are intra-temporal fibers [44], supporting the network-based pattern of white matter degeneration in mTLE.
A summary of our literature review on the associations between disease duration in mTLE patients and DTI diffusion indices is presented in Table 2. Herein, we used DMRI connectometry to embody white matter connectivity, as a proxy of the density of water diffusion, yielding more sensitive outcomes than those found through conventional DTI. Our results revealed wider associations compared to previous studies using DTI, including both intra-temporal, such as UF and AF, and extra-temporal white matter fibers from the default mode network.
One major limitation of our study is our small sample size. Therefore, repeating these analyses in a larger group of mTLE patients might revalidate the correlation between the mentioned tracts and disease duration. Furthermore, performing multimodal imaging techniques, e.g., volumetric methods with DTI or DMRI, and longitudinal assessment in a single study population, would be of great value.
Conclusion
Results of the current study were obtained through the novel DMRI connectometry approach, which confers a higher sensitivity and more reproducible results when investigating white matter microstructural abnormalities in areas with highly crowded fibers. Compared to previous studies investigating the correlation between duration of epilepsy and white matter integrity using DTI, we detected broader associations in temporal white matter, fibers from language circuitry, midline structures, and components of the default mode network.
References
Engel J Jr (1996) Introduction to temporal lobe epilepsy. Epilepsy Res 26(1):141–150
Mameniskiene R, Rimsiene J, Puronaite R (2016) Cognitive changes in people with temporal lobe epilepsy over a 13-year period. Epilepsy Behav: E&B 63:89–97
Kwan P, Sperling MR (2009) Refractory seizures: try additional antiepileptic drugs (after two have failed) or go directly to early surgery evaluation? Epilepsia 50(Suppl 8):57–62
Lau T, Miller T, Klein T, Benbadis SR, Vale FL (2014) Temporal lobe surgery in medically refractory epilepsy: a comparison between populations based on MRI findings. Seizure 23(1):20–24
Jardim AP, Neves RS, Caboclo LO, Lancellotti CL, Marinho MM, Centeno RS, Cavalheiro EA, Scorza CA, Yacubian EM (2012) Temporal lobe epilepsy with mesial temporal sclerosis: hippocampal neuronal loss as a predictor of surgical outcome. Arq Neuropsiquiatr 70(5):319–324
Di Gennaro G, D'Aniello A, De Risi M, Grillea G, Quarato PP, Mascia A, Grammaldo LG, Casciato S, Morace R, Esposito V, Picardi A (2015) Temporal pole abnormalities in temporal lobe epilepsy with hippocampal sclerosis: clinical significance and seizure outcome after surgery. Seizure 32:84–91
Pauli C, Thais ME, Claudino LS, Bicalho MA, Bastos AC, Guarnieri R, Nunes JC, Lin K, Linhares MN, Walz R (2012) Predictors of quality of life in patients with refractory mesial temporal lobe epilepsy. Epilepsy Behav: E&B 25(2):208–213
Coan AC, Campos BM, Yasuda CL, Kubota BY, Bergo FP, Guerreiro CA, Cendes F (2014) Frequent seizures are associated with a network of gray matter atrophy in temporal lobe epilepsy with or without hippocampal sclerosis. PLoS One 9(1):e85843
Helmstaedter C, May TW, von Lehe M, Pfaefflin M, Ebner A, Pannek HW, Elger CE, Stefan H, Schramm J (2014) Temporal lobe surgery in Germany from 1988 to 2008: diverse trends in etiological subgroups. Eur J Neurol 21(6):827–834
Barba C, Rheims S, Minotti L, Guenot M, Hoffmann D, Chabardes S, Isnard J, Kahane P, Ryvlin P (2016) Temporal plus epilepsy is a major determinant of temporal lobe surgery failures. Brain J Neurol 139(Pt 2):444–451
Amlerova J, Cavanna AE, Bradac O, Javurkova A, Raudenska J, Marusic P (2014) Emotion recognition and social cognition in temporal lobe epilepsy and the effect of epilepsy surgery. Epilepsy Behav : E&B 36:86–89
Garbelli R, Milesi G, Medici V, Villani F, Didato G, Deleo F, D'Incerti L, Morbin M, Mazzoleni G, Giovagnoli AR, Parente A, Zucca I, Mastropietro A, Spreafico R (2012) Blurring in patients with temporal lobe epilepsy: clinical, high-field imaging and ultrastructural study. Brain J Neurol 135(Pt 8):2337–2349
Kuba R, Tyrlikova I, Pazourkova M, Hermanova M, Horakova I, Brazdil M, Rektor I (2012) Grey-white matter abnormalities in temporal lobe epilepsy associated with hippocampal sclerosis: inter-observer analysis, histopathological findings, and correlation with clinical variables. Epilepsy Res 102(1–2):78–85
Otte WM, van Eijsden P, Sander JW, Duncan JS, Dijkhuizen RM, Braun KP (2012) A meta-analysis of white matter changes in temporal lobe epilepsy as studied with diffusion tensor imaging. Epilepsia 53(4):659–667
Kreilkamp BA, Weber B, Richardson MP, Keller SS (2017) Automated tractography in patients with temporal lobe epilepsy using TRActs constrained by UnderLying anatomy (TRACULA). NeuroImage Clin 14:67–76
Chiang S, Levin HS, Wilde E, Haneef Z (2016) White matter structural connectivity changes correlate with epilepsy duration in temporal lobe epilepsy. Epilepsy Res 120:37–46
Govindan RM, Makki MI, Sundaram SK, Juhasz C, Chugani HT (2008) Diffusion tensor analysis of temporal and extra-temporal lobe tracts in temporal lobe epilepsy. Epilepsy Res 80(1):30–41
Keller SS, Schoene-Bake JC, Gerdes JS, Weber B, Deppe M (2012) Concomitant fractional anisotropy and volumetric abnormalities in temporal lobe epilepsy: cross-sectional evidence for progressive neurologic injury. PLoS One 7(10):e46791
Ansari M, Adib Moradi S, Ghazi Sherbaf F, Hedayatnia A, Aarabi MH (2019) Comparison of structural connectivity in Parkinson's disease with depressive symptoms versus non-depressed: a diffusion MRI connectometry study. International psychogeriatrics 31(1):5–12. https://doi.org/10.1017/S1041610218000170
Yeh FC, Badre D, Verstynen T (2016) Connectometry: a statistical approach harnessing the analytical potential of the local connectome. NeuroImage 125:162–171
Yeh FC, Wedeen VJ, Tseng WY (2011) Estimation of fiber orientation and spin density distribution by diffusion deconvolution. NeuroImage 55(3):1054–1062
Yeh F-C, Tang P-F, Tseng W-YI (2013) Diffusion MRI connectometry automatically reveals affected fiber pathways in individuals with chronic stroke. Neuro Image: Clinical 2:912–921
Sobhani S, Rahmani F, Aarabi MH, Sadr AV (2017) Exploring white matter microstructure and olfaction dysfunction in early parkinson disease: diffusion MRI reveals new insight. Brain Imaging and Behavior. https://doi.org/10.1007/s11682-017-9781-0
Ghazi Sherbaf F, Rahmani F, Jooyandeh SM, Aarabi MH (2018) Microstructural changes in patients with Parkinson disease and REM sleep behavior disorder: depressive symptoms versus non-depressed. Acta neurologica Belgica 118 (3):415-421. https://doi.org/10.1007/s13760-018-0896-x
Abhinav K, Yeh FC, Pathak S, Suski V, Lacomis D, Friedlander RM, Fernandez-Miranda JC (2014) Advanced diffusion MRI fiber tracking in neurosurgical and neurodegenerative disorders and neuroanatomical studies: a review. Biochim Biophys Acta 1842(11):2286–2297
Yeh FC, Verstynen TD, Wang Y, Fernandez-Miranda JC, Tseng WY (2013) Deterministic diffusion fiber tracking improved by quantitative anisotropy. PLoS One 8(11):e80713
Yeh FC, Vettel JM, Singh A, Poczos B, Grafton ST, Erickson KI, Tseng WI, Verstynen TD (2016) Quantifying differences and similarities in whole-brain White matter architecture using local connectome fingerprints. PLoS Comput Biol 12(11):e1005203
Sanjari Moghaddam H, Rahmani F, Aarabi MH, Nazem-Zadeh M-R, Davoodi-Bojd E, Soltanian-Zadeh H (2019) White matter microstructural differences between right and left mesial temporal lobe epilepsy. Acta neurologica Belgica. https://doi.org/10.1007/s13760-019-01074-x
Nazem-Zadeh MR, Elisevich K, Air EL, Schwalb JM, Divine G, Kaur M, Wasade VS, Mahmoudi F, Shokri S, Bagher-Ebadian H, Soltanian-Zadeh H (2016) DTI-based response-driven modeling of mTLE laterality. Neuro Image Clin 11:694–706
Leemans A, Jeurissen B, Sijbers J, Jones D (2009). ExploreDTI: a graphical toolbox for processing, analyzing, and visualizing diffusion MR data. In: 17th Annual Meeting of Intl Soc Mag Reson Med p 3537
Yeh FC, Tseng WY (2011) NTU-90: a high angular resolution brain atlas constructed by q-space diffeomorphic reconstruction. NeuroImage 58(1):91–99
Zhang T, Kong J, Jing K, Chen H, Jiang X, Li L, Guo L, Lu J, Hu X, Liu T (2015) Multi-scale and multimodal fusion of tract-tracing, myelin stain and DTI-derived fibers in macaque brains. Med Image Comp Comp-Assisted Interv : MICCAI International Conference on Medical Image Computing and Computer-Assisted Intervention 9350:246–254
Deleo F, Thom M, Concha L, Bernasconi A, Bernhardt BC, Bernasconi N (2018) Histological and MRI markers of white matter damage in focal epilepsy. Epilepsy Res 140:29–38
Voets NL, Beckmann CF, Cole DM, Hong S, Bernasconi A, Bernasconi N (2012) Structural substrates for resting network disruption in temporal lobe epilepsy. Brain J Neurol 135(Pt 8):2350–2357
Rodríguez-Cruces R, Concha L (2015) White matter in temporal lobe epilepsy: clinico-pathological correlates of water diffusion abnormalities. Quant Imaging Med Surg 5(2):264–278
Lin JJ, Riley JD, Juranek J, Cramer SC (2008) Vulnerability of the frontal-temporal connections in temporal lobe epilepsy. Epilepsy Res 82(2):162–170
Liu M, Bernhardt BC, Hong SJ, Caldairou B, Bernasconi A, Bernasconi N (2016) The superficial white matter in temporal lobe epilepsy: a key link between structural and functional network disruptions. Brain J Neurol 139(Pt 9):2431–2440
Ahmadi ME, Hagler DJ Jr, McDonald CR, Tecoma ES, Iragui VJ, Dale AM, Halgren E (2009) Side matters: diffusion tensor imaging tractography in left and right temporal lobe epilepsy. AJNR Am J Neuroradiol 30(9):1740–1747
Keller SS, Ahrens T, Mohammadi S, Gerdes JS, Moddel G, Kellinghaus C, Kugel H, Weber B, Ringelstein EB, Deppe M (2013) Voxel-based statistical analysis of fractional anisotropy and mean diffusivity in patients with unilateral temporal lobe epilepsy of unknown cause. Journal of Neuroimaging : official journal of the American Society of Neuroimaging 23(3):352–359
Dell'Acqua F, Simmons A, Williams SC, Catani M (2013) Can spherical deconvolution provide more information than fiber orientations? Hindrance modulated orientational anisotropy, a true-tract specific index to characterize white matter diffusion. Hum Brain Mapp 34(10):2464–2483
Concha L, Beaulieu C, Collins DL, Gross DW (2009) White-matter diffusion abnormalities in temporal-lobe epilepsy with and without mesial temporal sclerosis. J Neurol Neurosurg Psychiatry 80(3):312–319
Jones D, Christiansen K, Chapman R, Aggleton J (2013) Distinct subdivisions of the cingulum bundle revealed by diffusion MRI fibre tracking: implications for neuropsychological investigations. Neuropsychologia 51(1):67–78
Scanlon C, Mueller SG, Cheong I, Hartig M, Weiner MW, Laxer KD (2013) Grey and white matter abnormalities in temporal lobe epilepsy with and without mesial temporal sclerosis. J Neurol 260(9):2320–2329
Urbach H, Egger K, Rutkowski K, Nakagawa JM, Schmeiser B, Reisert M, Brandt A, Steinhoff BJ, Schulze-Bonhage A, Hammen T (2017) Bilateral cingulum fiber reductions in temporal lobe epilepsy with unilateral hippocampal sclerosis. Eur J Radiol 94:53–57
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
Ethics approval was provided by Henry Ford Health System Institutional Review Board, Detroit, Michigan, USA.
Informed consent
De-identified retrospective data was used in this research.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Glossary (adopted from: F.C Yeh; NeuroImage: Clinical 2 (2013): 912–921)
- Diffusion MRI
-
A magnetic resonance imaging method that generates images related to the density, direction, or velocity of water diffusion in the tissue.
- Local connectomics
-
A model to quantify local anatomical integration of brain voxels as nodes of a network using graph theory methods. The model generates a connectivity matrix.
- Diffusion MRI connectometry
-
A model free method to analyze diffusion MRI connectomics using permutation test to find the association of white matter pathways with a study factor. Connectometry essentially uses the “tracking the difference” paradigm.
- Local connectome fingerprinting
-
A reconstruction method that calculates the density of diffusing water along the major fiber bundles from the diffusion MRI data. Local connectome finger print is found to be highly unique for each individual.
- Quantitative anisotropy
-
The quantity/density of the water diffusion in each direction of a given voxel/node of the connectivity matrix.
Rights and permissions
About this article
Cite this article
Ashraf-Ganjouei, A., Rahmani, F., Aarabi, M. et al. White matter correlates of disease duration in patients with temporal lobe epilepsy: updated review of literature. Neurol Sci 40, 1209–1216 (2019). https://doi.org/10.1007/s10072-019-03818-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10072-019-03818-2