Abstract
Purpose
Although epilepsy in the elderly has attracted attention recently, there are few systematic studies of neuroimaging in such patients. In this study, we used structural MRI and diffusion tensor imaging (DTI) to investigate the morphological and microstructural features of the brain in late-onset temporal lobe epilepsy (TLE).
Methods
We recruited patients with TLE and an age of onset > 50 years (late-TLE group) and age- and sex-matched healthy volunteers (control group). 3-Tesla MRI scans, including 3D T1-weighted images and 15-direction DTI, showed normal findings on visual assessment in both groups. We used Statistical Parametric Mapping 12 (SPM12) for gray and white matter structural normalization and comparison and used Tract-Based Spatial Statistics (TBSS) for fractional anisotropy and mean diffusivity comparisons of DTI. In both methods, p < 0.05 (family-wise error) was considered statistically significant.
Results
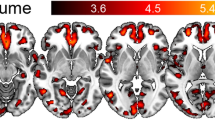
In total, 30 patients with late-onset TLE (mean ± SD age, 66.8 ± 8.4; mean ± SD age of onset, 63.0 ± 7.6 years) and 40 healthy controls (mean ± SD age, 66.6 ± 8.5 years) were enrolled. The late-onset TLE group showed significant gray matter volume increases in the bilateral amygdala and anterior hippocampus and significantly reduced mean diffusivity in the left temporofrontal lobe, internal capsule, and brainstem. No significant changes were evident in white matter volume or fractional anisotropy.
Conclusions
Our findings may reflect some characteristics or mechanisms of cryptogenic TLE in the elderly, such as inflammatory processes.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Epilepsy is a common neurological disease, with significant prevalence and incidence in the elderly [1]. Epilepsy in the elderly population is clinically important because it is usually treatable with appropriate anti-epileptic drugs, although the semiology of subtle seizures can often make accurate diagnosis difficult [2]. According to an epidemiological survey, the incidence of epilepsy shows a bimodal peak distribution in both childhood and late life, with the second peak beginning at around 50 years of age [3].
Despite the available epidemiological and therapeutic data in the elderly population with epilepsy, there is a lack of systematic neuroimaging studies in this population. Stroke, neoplasm, and trauma are often etiologic factors in late-onset epilepsy, although around 30–50% of patients have no evidence of a lesion, so-called cryptogenic late-onset epilepsy [4, 5]. Recently, the involvement of autoimmune and inflammatory processes in late-onset seizures has attracted attention [6, 7]. In the current study, we expected that statistical neuroimaging methods could provide considerable insights or evidence into the potential etiology and mechanisms of cryptogenic late-onset epilepsy. Voxel-based morphometry (VBM) and diffusion tensor imaging (DTI) have been conventionally and widely used in many neuropsychiatric diseases; the former estimates the morphological features of the brain [8] and the latter determines microstructural parameters such as fractional anisotropy (FA) and mean diffusivity (MD) mainly in the white matter tract [9].
Over 90% of patients with late-onset epilepsy have focal epilepsy [4], of which temporal lobe epilepsy (TLE) is the most common type in adults [10]. Because different focal epilepsies yield different VBM/DTI findings [11], a focus-specific investigation would be beneficial. In this study, using VBM and DTI analyses, we investigated the morphological and microstructural features of cryptogenic late-onset TLE.
Methods
Subjects
We recruited patients with TLE and an age of onset > 50 years who were diagnosed at our institution between December 2013 and March 2016. The diagnosis of TLE was based on the presence of focal seizures consistent with mesial temporal lobe onset and a focal epileptiform discharge predominantly in the temporal area on conventional scalp electroencephalogram (EEG). All patients underwent conventional MRI for visual evaluation of epileptogenic lesions by experienced neuroradiologists, and we excluded patients with a significant medical history of neurosurgery, acute encephalitis (or highly suspected chronic encephalitis), meningitis, severe head trauma, or ischemic encephalopathy and those with obvious lesions such as tumors, contusions, or infarcts on MRI.
In total, 30 patients were enrolled; all had normal brain MRI based on visual clinical interpretation. We also recruited 40 age- and sex-matched volunteers as healthy controls with no history of neurological or psychiatric diseases and no use of medication affecting the central nervous system. We found no possible structural anomalies or abnormalities in the controls on MRI. Visual assessment of MRI for both groups was performed by two independent neuroradiologists with 15 and 30 years of experience.
All participants provided written informed consent. The study was approved by the Institutional Review Board.
MRI acquisitions
The MRI scans for all participants were performed on a 3.0-T MR system with a 32-channel coil (Philips Medical Systems, Best, Netherlands). The parameters of the sequences were as follows: for three-dimensional (3D) sagittal T1-weighted images, repetition time (TR)/echo time (TE), 7.12 ms/3.4 ms; flip angle, 10°; number of excitations (NEX), 1; effective slice thickness, 0.6 mm with no gap; slices, 300; matrix, 260 × 320; and field of view (FOV), 26 × 24 cm; and for diffusion-weighted images, TR/TE, 6700 ms/58 ms; flip angle, 90°; NEX, 2; effective slice thickness, 3.0 mm with no gap; slices, 60; matrix, 80 × 78; and FOV, 24 × 24 cm. Diffusion was measured along 15 non-collinear directions using diffusion-weighted factor b in each direction of 1000 s/mm2, and one image was acquired with no diffusion gradient.
We also performed a routine MRI examination with the following protocols: for transverse turbo spin echo T2-weighted imaging, TR/TE, 4704/80 ms; flip angle, 90°; NEX, 2; thickness, 3.0 mm with a 1.5-mm gap; slices, 34; matrix, 368 × 215; and FOV, 23 × 18 cm; and for coronal fluid-attenuated inversion recovery (FLAIR) imaging, TR/TE, 10000/120 ms; inversion time, 2450 ms; flip angle, 120°; NEX, 2; thickness, 3.0 mm with 1.5-mm gap; slices, 34; matrix, 272 × 144; FOV, 23 × 18 cm. The axial and coronal T1-weighted images were obtained by reconstruction of the 3D T1-weighted images and used for visual assessment.
This study adopted a cross-sectional design and the MRI scan for each participant was performed only once.
Voxel-based morphometry
To evaluate morphological differences in gray and white matter volumes, the 3D T1 images were segmented and spatially normalized with the Statistical Parametric Mapping 12 software program (SPM12; http://www.fil.ion.ucl.ac.uk/spm/) running in MATLAB2014a and the DARTEL (Diffeomorphic Anatomical Registration through Exponentiated Lie) method [12]. The spatial normalization process of each subject was performed using a standard template. Each gray and white matter image was then smoothed by an 8-mm full-width at half-maximum (FWHM) Gaussian kernel.
Tract-based spatial statistics
For DTI analyses, we used Tract-Based Spatial Statistics (TBSS) software [9]. After eddy current correction and brain extraction with FMRIB Software Library (FSL) version 5.0.8 [9, 13], the FSL software yielded voxel-wise maps of FA and MD for each subject. The skeletonized FA and MD were analyzed to evaluate the difference between the two groups with age and sex as covariates, using the Threshold-Free Cluster Enhancement option in FSL Randomize with 10,000 permutations.
Statistics
To confirm morphological differences between the late-onset TLE and control groups, we subjected normalized gray and white matter images to two-sample t test analysis in SPM12 software with age, sex, and intracranial volume (calculated by SPM12) as nuisance covariates. A height threshold of p < 0.05 (family-wise error [FWE] correction) was deemed significant. Likewise, p < 0.05 with FWE correction was considered significant in the TBSS comparison.
Results
Demographics
Table 1 provides general information on the demographic characteristics of both groups. The mean duration between seizure onset and the MRI scan was 3.8 years. No patients showed conflicting seizure semiology of lateralization with the abnormal EEG side. Among the 30 patients, epileptiform discharges were prominent in the anterior temporal area in 17 patients and in the middle temporal area in the remaining 13 patients. At the time of the MRI, four patients were already seizure free, and finally, none of the patients needed presurgical evaluation because they had a favorable drug response. None of the patients had a history of febrile seizure.
Voxel-based morphometry
VBM comparisons showed significant gray matter increases in the bilateral amygdala and hippocampal head in the late-onset TLE group (p < 0.01, FWE; Fig. 1), with no white matter volume changes. The coordinates and detailed p values are shown in Table 2.
Tract-based spatial statistics
TBSS showed significant MD reductions in the late-onset TLE group (p < 0.05, FWE), particularly in the left temporal white matter (Fig. 2). The decreased MD areas comprised the left orbitofrontal lobe, temporal lobe, internal capsule, and brainstem. There were no significant FA changes.
TBSS differences in MD images between late-onset TLE patients and controls. Green represents the mean MD skeleton of all participants; red and yellow denote regions with decreased MD in late-onset TLE patients (p < 0.05, family-wise error). A significantly reduced MD was found in the left temporofrontal lobe and brainstem
Discussion
In this study, we investigated VBM and TBSS findings in late-onset TLE patients and found significant gray matter increases in the bilateral mesial temporal lobes and MD reductions in several areas. There are many previous studies of VBM [14,15,16,17,18,19] and DTI [20] in TLE, but without a specific focus on late-onset cases. The present study provides additional insight by focusing on elderly patients with late onset and cryptogenic etiologies.
Conventionally, the brain morphological features of TLE have been regarded as volume loss in medial temporal structures [14], the extrahippocampal temporal lobe [16], related subcortical structures [15], and related extratemporal cortices [18], and these volume changes can correlate with clinical parameters [14, 19]. The widespread atrophy is more consistent in TLE with hippocampal sclerosis [17, 21], which is the most common etiology of TLE. As for cryptogenic TLE, the morphological findings are relatively heterogeneous [18, 21]. Our findings of VBM are different from those of most in the literature, probably because we focused on late-onset cases. A previous study suggested that this volume loss would be due to an early neurological insult in childhood or result from recurrent seizures [14]. Both causes might not fit with our late-onset cases with shorter disease duration. Thus, we may have to consider differing mechanisms of late-onset TLE in elderly people.
One previous neuroimaging study of late-onset epilepsy focused on the association with occult cerebrovascular disease [22]. The 16 patients with late-onset epilepsy (age of onset > 50 years) who were assessed using T1-weighted, FLAIR, and arterial spin labeling MRI showed lower cortical volume, higher white matter hyperintensity, prolonged arterial arrival time, and unchanged cerebral blood flow. In particular, findings of reduced gray matter differ from ours of increased regional gray matter. We attribute this difference to differences in patient selection. This previous study recruited patients with various types of epilepsies, whereas we recruited only TLE patients. Because different types of epilepsies can yield different morphological and microstructural findings [11], we performed a focus-specific investigation.
We found a significant bilateral increase in gray matter in the amygdala and anterior hippocampus. Recently, there have been an increasing number of reports on TLE with amygdala enlargement, with several studies finding an association with a later age of onset [23,24,25,26]. However, these investigations were not limited to elderly patients. As a systematic neuroimaging survey of late-onset TLE, our results may provide additional support for these studies. The mesial temporal gray matter increases, perhaps caused by swelling, might reflect some susceptibility to limbic abnormal electrical activity or an inflammatory process in elderly patients. Additionally, although some studies adopted a left-right difference or asymmetry index for the definition of amygdala enlargement [23, 24], this procedure could overlook bilateral enlargements, such as in our patients.
The pathological significance of amygdala enlargement is still controversial however. It can occur in TLE with hippocampal sclerosis [27, 28], and a recent paper revealed unspecific amygdala enlargement in extratemporal focal epilepsies [29]. Thus, we should bear in mind the potentially low specificity of amygdala enlargement when interpreting the present results.
Regarding the white matter volume, we found no significant differences between the two groups, which seems consistent with previous studies reporting no white matter volume changes in visually MRI-negative TLE [17] or TLE with amygdala enlargement [30].
In this study, late-onset TLE also showed significant MD reductions in the left orbitofrontal lobe, temporal lobe, internal capsule, and brainstem, without any FA changes. The left dominant reductions may reflect our selection of patients (more left TLE than right or bilateral TLE). However, most DTI studies on TLE report abnormal MD increases [20]. Recently, amygdala enlargement was partly linked to inflammatory or autoimmune processes [6, 31], which may have caused a reduction in diffusion in our cohort. On the other hand, a previous DTI study showed a specific FA reduction in the ipsilateral anterior cingulum in TLE with amygdala enlargement [30]. This differing finding could be because the previous study was not limited to elderly patients, used a left-right flipping procedure to evaluate focus/contralateral sides, and/or excluded bilateral TLE. Left-right flipping is sometimes used to analyze the left and right TLE together [11, 30, 32] by making one hemisphere the ipsilateral side.
This study has several limitations. First, it lacks detailed clinical information, including cerebrospinal antibodies, which may be important to exclude limbic encephalitis with amygdala swelling. We excluded clinically highly suspected encephalitis, although chronic encephalitis is sometimes difficult to diagnose accurately. Additionally, there are antibody-negative cases with TLE and amygdala enlargement [6, 31]. We also did not perform a left-right flipping procedure for focus/contralateral evaluation. Because late-onset epilepsy has a favorable response to anti-epileptic drugs [4, 5], the patients did not need comprehensive presurgical examinations such as video-EEG monitoring or nuclear imaging. Considering the limited value of focus lateralization by interictal discharges on scalp EEG [33], we decided not to use the flipping procedure to avoid misleading results. The limited value of focus localization is a clear limitation of this study but, given the good prognosis of late-onset epilepsy, we consider the study design to be reasonable at this stage. Finally, the cross-sectional design may yield only descriptive findings, and a future longitudinal study is needed for more accurate understanding of late-onset TLE. Single-point measurements have limitations, especially in dynamic pathological conditions such as epilepsy or inflammation, given the recent literature reporting significant amygdala volume changes over time in limbic encephalitis [34]. For a more solid foundation, further longitudinal studies should be performed to assess changes over time.
Conclusions
As possible features of the brain in late-onset TLE, significant gray matter increases were found in the bilateral mesial temporal structures and MD reduction was observed in the left temporofrontal lobes and brainstem in the late-onset TLE group, findings that were statistically significant in our small cohort of patients in this cross-sectional study. These findings may reflect some characteristics or mechanisms of TLE in the elderly such as inflammatory processes. For stronger and more reliable data, further longitudinal studies should be performed to assess the changes in the values.
References
Brodie MJ, Kwan P (2005) Epilepsy in elderly people. BMJ 331(7528):1317–1322. https://doi.org/10.1136/bmj.331.7528.1317
Stefan H (2011) Epilepsy in the elderly: facts and challenges. Acta Neurol Scand 124(4):223–237. https://doi.org/10.1111/j.1600-0404.2010.01464.x
Sillanpaa M, Kalviainen R, Klaukka T, Helenius H, Shinnar S (2006) Temporal changes in the incidence of epilepsy in Finland: nationwide study. Epilepsy Res 71(2–3):206–215. https://doi.org/10.1016/j.eplepsyres.2006.06.017
Stefan H, May TW, Pfafflin M, Brandt C, Furatsch N, Schmitz B, Wandschneider B, Kretz R, Runge U, Geithner J, Karakizlis C, Rosenow F, Kerling F (2014) Epilepsy in the elderly: comparing clinical characteristics with younger patients. Acta Neurol Scand 129(5):283–293. https://doi.org/10.1111/ane.12218
Tanaka A, Akamatsu N, Shouzaki T, Toyota T, Yamano M, Nakagawa M, Tsuji S (2013) Clinical characteristics and treatment responses in new-onset epilepsy in the elderly. Seizure 22(9):772–775. https://doi.org/10.1016/j.seizure.2013.06.005
Malter MP, Widman G, Galldiks N, Stoecker W, Helmstaedter C, Elger CE, Wagner J (2016) Suspected new-onset autoimmune temporal lobe epilepsy with amygdala enlargement. Epilepsia 57(9):1485–1494. https://doi.org/10.1111/epi.13471
von Podewils F, Suesse M, Geithner J, Gaida B, Wang ZI, Lange J, Dressel A, Grothe M, Kessler C, Langner S, Runge U, Bien CG (2017) Prevalence and outcome of late-onset seizures due to autoimmune etiology: a prospective observational population-based cohort study. Epilepsia 58(9):1542–1550. https://doi.org/10.1111/epi.13834
Ashburner J, Friston KJ (2000) Voxel-based morphometry--the methods. NeuroImage 11(6 Pt 1):805–821. https://doi.org/10.1006/nimg.2000.0582
Smith SM, Jenkinson M, Johansen-Berg H, Rueckert D, Nichols TE, Mackay CE, Watkins KE, Ciccarelli O, Cader MZ, Matthews PM, Behrens TE (2006) Tract-based spatial statistics: voxelwise analysis of multi-subject diffusion data. NeuroImage 31(4):1487–1505. https://doi.org/10.1016/j.neuroimage.2006.02.024
Engel J Jr (1996) Introduction to temporal lobe epilepsy. Epilepsy Res 26(1):141–150
Campos BM, Coan AC, Beltramini GC, Liu M, Yassuda CL, Ghizoni E, Beaulieu C, Gross DW, Cendes F (2015) White matter abnormalities associate with type and localization of focal epileptogenic lesions. Epilepsia 56(1):125–132. https://doi.org/10.1111/epi.12871
Ashburner J (2007) A fast diffeomorphic image registration algorithm. NeuroImage 38(1):95–113. https://doi.org/10.1016/j.neuroimage.2007.07.007
Smith SM (2002) Fast robust automated brain extraction. Hum Brain Mapp 17(3):143–155. https://doi.org/10.1002/hbm.10062
Keller SS, Wieshmann UC, Mackay CE, Denby CE, Webb J, Roberts N (2002) Voxel based morphometry of grey matter abnormalities in patients with medically intractable temporal lobe epilepsy: effects of side of seizure onset and epilepsy duration. J Neurol Neurosurg Psychiatry 73(6):648–655
Bonilha L, Rorden C, Castellano G, Pereira F, Rio PA, Cendes F, Li LM (2004) Voxel-based morphometry reveals gray matter network atrophy in refractory medial temporal lobe epilepsy. Arch Neurol 61(9):1379–1384. https://doi.org/10.1001/archneur.61.9.1379
McMillan AB, Hermann BP, Johnson SC, Hansen RR, Seidenberg M, Meyerand ME (2004) Voxel-based morphometry of unilateral temporal lobe epilepsy reveals abnormalities in cerebral white matter. NeuroImage 23(1):167–174. https://doi.org/10.1016/j.neuroimage.2004.05.002
Keller SS, Roberts N (2008) Voxel-based morphometry of temporal lobe epilepsy: an introduction and review of the literature. Epilepsia 49(5):741–757. https://doi.org/10.1111/j.1528-1167.2007.01485.x
Riederer F, Lanzenberger R, Kaya M, Prayer D, Serles W, Baumgartner C (2008) Network atrophy in temporal lobe epilepsy: a voxel-based morphometry study. Neurology 71(6):419–425. https://doi.org/10.1212/01.wnl.0000324264.96100.e0
Yasuda CL, Betting LE, Cendes F (2010) Voxel-based morphometry and epilepsy. Expert Rev Neurother 10(6):975–984. https://doi.org/10.1586/ern.10.63
Otte WM, van Eijsden P, Sander JW, Duncan JS, Dijkhuizen RM, Braun KP (2012) A meta-analysis of white matter changes in temporal lobe epilepsy as studied with diffusion tensor imaging. Epilepsia 53(4):659–667. https://doi.org/10.1111/j.1528-1167.2012.03426.x
Mueller SG, Laxer KD, Cashdollar N, Buckley S, Paul C, Weiner MW (2006) Voxel-based optimized morphometry (VBM) of gray and white matter in temporal lobe epilepsy (TLE) with and without mesial temporal sclerosis. Epilepsia 47(5):900–907. https://doi.org/10.1111/j.1528-1167.2006.00512.x
Hanby MF, Al-Bachari S, Makin F, Vidyasagar R, Parkes LM, Emsley HC (2015) Structural and physiological MRI correlates of occult cerebrovascular disease in late-onset epilepsy. Neuroimage Clin 9:128–133. https://doi.org/10.1016/j.nicl.2015.07.016
Bower SP, Vogrin SJ, Morris K, Cox I, Murphy M, Kilpatrick CJ, Cook MJ (2003) Amygdala volumetry in "imaging-negative" temporal lobe epilepsy. J Neurol Neurosurg Psychiatry 74(9):1245–1249. https://doi.org/10.1136/jnnp.74.9.1245
Mitsueda-Ono T, Ikeda A, Inouchi M, Takaya S, Matsumoto R, Hanakawa T, Sawamoto N, Mikuni N, Fukuyama H, Takahashi R (2011) Amygdalar enlargement in patients with temporal lobe epilepsy. J Neurol Neurosurg Psychiatry 82(6):652–657. https://doi.org/10.1136/jnnp.2010.206342
Sone D, Ito K, Taniguchi G, Murata Y, Nakata Y, Watanabe Y, Okazaki M, Sato N, Matsuda H, Watanabe M (2015) Evaluation of amygdala pathology using (11)C-methionine positron emission tomography/computed tomography in patients with temporal lobe epilepsy and amygdala enlargement. Epilepsy Res 112:114–121. https://doi.org/10.1016/j.eplepsyres.2015.02.018
Beh SM, Cook MJ, D'Souza WJ (2016) Isolated amygdala enlargement in temporal lobe epilepsy: a systematic review. Epilepsy Behav 60:33–41. https://doi.org/10.1016/j.yebeh.2016.04.015
Coan AC, Morita ME, Campos BM, Bergo FP, Kubota BY, Cendes F (2013) Amygdala enlargement occurs in patients with mesial temporal lobe epilepsy and hippocampal sclerosis with early epilepsy onset. Epilepsy Behav 29(2):390–394. https://doi.org/10.1016/j.yebeh.2013.08.022
Sone D, Ikemura M, Saito Y, Taniguchi G, Kunii N (2017) Marked accumulation of oligodendroglia-like cells in temporal lobe epilepsy with amygdala enlargement and hippocampal sclerosis. Neuropathology. https://doi.org/10.1111/neup.12410
Reyes A, Thesen T, Kuzniecky R, Devinsky O, McDonald CR, Jackson GD, Vaughan DN, Blackmon K (2017) Amygdala enlargement: temporal lobe epilepsy subtype or nonspecific finding? Epilepsy Res 132:34–40. https://doi.org/10.1016/j.eplepsyres.2017.02.019
Sone D, Ota M, Maikusa N, Kimura Y, Sumida K, Yokoyama K, Imabayashi E, Watanabe M, Watanabe Y, Okazaki M, Sato N, Matsuda H (2016) White matter abnormalities in patients with temporal lobe epilepsy and amygdala enlargement: comparison with hippocampal sclerosis and healthy subjects. Epilepsy Res 127:221–228. https://doi.org/10.1016/j.eplepsyres.2016.09.011
Lv RJ, Sun ZR, Cui T, Guan HZ, Ren HT, Shao XQ (2014) Temporal lobe epilepsy with amygdala enlargement: a subtype of temporal lobe epilepsy. BMC Neurol 14:194. https://doi.org/10.1186/s12883-014-0194-z
Sone D, Matsuda H, Ota M, Maikusa N, Kimura Y, Sumida K, Yokoyama K, Imabayashi E, Watanabe M, Watanabe Y, Okazaki M, Sato N (2016) Graph theoretical analysis of structural neuroimaging in temporal lobe epilepsy with and without psychosis. PLoS One 11(7):e0158728. https://doi.org/10.1371/journal.pone.0158728
Rathore C, Radhakrishnan K (2015) Concept of epilepsy surgery and presurgical evaluation. Epileptic Disord 17(1):19–31; quiz 31. https://doi.org/10.1684/epd.2014.0720
Wagner J, Weber B, Elger CE (2015) Early and chronic gray matter volume changes in limbic encephalitis revealed by voxel-based morphometry. Epilepsia 56(5):754–761. https://doi.org/10.1111/epi.12968
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This study was funded in part by grants from the Japan Epilepsy Research Foundation (JERF TENKAN 17009) and the Japan Society for the Promotion of Science (KAKENHI Grant Number JP17H07385) (both to DS).
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Rights and permissions
About this article
Cite this article
Sone, D., Sato, N., Kimura, Y. et al. Brain morphological and microstructural features in cryptogenic late-onset temporal lobe epilepsy: a structural and diffusion MRI study. Neuroradiology 60, 635–641 (2018). https://doi.org/10.1007/s00234-018-2019-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00234-018-2019-z