Abstract
The aim of the study was to characterize the clinical and radiological spectrum of posterior reversible encephalopathy syndrome (PRES) in a large cohort. The radiological report data bases of the authors′ university hospitals were searched for patients with PRES. Various imaging features at onset of symptoms and on follow-up as well as clinical and paraclinical data were tabulated in those patients fulfilling the criteria for PRES. Exploratory univariate analyses were performed. A total of 96 patients with PRES were included into the study. Wide differences in lesion location, diffusivity, distribution pattern, edema severity, hemorrhage, underlying diseases, symptoms, mean arterial pressure (MAP) and coagulation status were encountered. Hemorrhage occurred significantly more frequently in patients with altered coagulation state and was significantly associated with higher edema grades and with the presence of cytotoxic edema. There was a significant difference in MAP between toxic associations with higher MAP in infection, eclampsy and autoimmune disorders, while lower MAP was found in chemotherapy and immunsupression. In 82% of patients complete or near complete resolution of edema was noted during follow-up. Higher MAP levels were associated with incomplete edema resolution. In 43% of patients residual lesions were seen with a relatively even distribution between focal gliosis, infarction, posthemorrhagic residua, atrophy and laminar necrosis. PRES in this large hospital-based retrospective study comprises a wide radiological and clinical spectrum. Residual lesions were encountered more frequently than commonly expected. Our results point towards a differential contribution of high blood pressure to the course of PRES in different underlying etiologies.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Posterior reversible encephalopathy syndrome (PRES) was first described as a clinical and radiological disease entity by Hinchey and colleagues in 1996 [1]. The syndrome is defined by a potentially reversible predominantly vasogenic edema of the white matter with a predilection of parenchyma supplied by the posterior circulation. However, atypical imaging findings have been increasingly recognized in the recent past, including various distribution patterns, cytotoxic edema, infarction, hemorrhage and contrast enhancement [2–5]. PRES has been shown to manifest in the setting of a widening range of disorders and predisposing factors, all ultimately leading to endothelial dysfunction with failure of cerebral autoregulation and development of vasogenic edema such as severe hypertension, eclampsia, organ transplantation, exposure to various immunosuppressants and cytostatic drugs as well as systemic inflammatory conditions with impaired renal function [6–8]. The clinical syndrome can have variable symptoms and typically comprises holocephalic headache, focal neurological deficits such as visual disturbances, dysphasia and paresis, seizures and reduced consciousness. Data from larger European hospital-based studies on the clinical and radiological features of PRES patients are sparse. In order to better define the broadening radiological and clinical spectrum of this syndrome in a large group of patients, we conducted a retrospective study in all three university hospitals of Charité-Universitätsmedizin Berlin.
Methods
Patients
The radiological report data bases of the authors’ university hospitals with large neurology, cardiovascular, oncology, rheumatology, obstetrics, renal and pediatrics departments were searched for the following items cited on brain MR imaging reports from January 1999 to August 2010: PRES, posterior reversible encephalopathy, posterior reversible leucoencephalopathy, cyclosporine, FK-506, tacrolimus, preeclampsia, eclampsia, toxemia of pregnancy, hypertensive encephalopathy, hypertensive crisis, neurotoxicity, sclerodermia, systemic sclerosis, systemic lupus erythematosus (SLE) and Wegener’s granulomatosis. Brain MR imaging studies of identified candidates were scrutinized for signs compatible with PRES, i.e., variable degrees of vasogenic edema, variable degrees of reversibility and a clinical constellation compatible with PRES. If present, patients were included into the study. In equivocal cases patients were included by consensus agreement between the neuroradiologist (ES) and the neurologist (TL) after having additionally screened the clinical reports. The institutional ethics committee waived permission to collect all data necessary for this retrospective study.
Clinical evaluation
All clinical records available were screened with a focus on clinical settings known or suspected to be related to the development of PRES (see above listed search items). Demographic data, clinical presentation such neurological symptoms, co-morbidities, laboratory data as well as baseline and toxicity blood pressure were acquired. In cases with more than one clinical association, the clinically dominant was used for analysis and tabulation. Mean arterial pressure defined as two-thirds diastolic pressure + one-third systolic pressure at onset of PRES symptoms was calculated. Blood pressure was graded as normal (MAP ≤ 105 mmHg), slightly elevated (MAP 106–115 mmHg), and significantly elevated (MAP > 115 mmHg). Data on toxicity blood pressure could not be obtained in two patients. Coagulation state was categorized in (1) normal coagulation and (2) altered coagulation state including sub-therapeutic or therapeutic, oral or intravenous medication, e.g., heparin, marcumar, aspirin that may affect coagulation cascade, alter platelet function, or/and laboratory findings of elevated INR, PTT, PT and/or thrombocytopenia.
Imaging evaluation
MR imaging was performed at 1.5 or 3 T. Because of the retrospective nature of the study, protocols and parameters differed frequently. All studies comprised at least axial T1-weighted images and turbo spin-echo axial proton density and T2-weighted images. Axial fluid-attenuated inversion-recovery (FLAIR) images and diffusion-weighted imaging sequences were available in 86 and 85 patients, respectively. ADC maps had been calculated in 74 patients. Imaging features of PRES were evaluated by one experienced neuroradiologist (ES). In detail, the scans were assessed for the following items: location (left/right; frontal, parietal, occipital, temporal, striatum, pallidum, thalamus, hypothalamus, mesencephalon, pons, cerebellum, medulla oblongata, corpus callosum), extent (grade 1: limited cortex and white matter edema; grade 2: white matter and cortex edema with some deep white matter extension; grade 3: white matter and cortex edema with limited ventricle surface extension; grade 4: white matter and cortex edema, diffuse, widely confluent, extensive ventricle contact; grade 5: severe white matter and cortex edema, diffuse confluence, ventricle deformity, as previously done [2]), diffusivity of lesions (ADC increase, pseudonormalization, decrease), contrast enhancement (parenchymal, cortical, leptomeningeal), presence of hemorrhage (parenchymal hematoma, microbleeds, subarachnoid blood), topographic lesion pattern (holohemispheric watershed pattern, superior frontal sulcus pattern, dominant parieto-occipital pattern, partial or asymmetric expression, central pattern, as previously done [2]).
Follow-up imaging
If available, follow-up imaging was evaluated for restitution of edematous brain areas and development of residual structural lesions. Regression of edema was graded as complete, incomplete, constant or progressive edema. Residual structural lesions were divided into none, small gliosis in the hemispheric white matter as manifested by circumscript foci of T2 signal prolongation, infarction, post-hemorrhagic residua, atrophy and cortical laminar necrosis.
Statistical analysis
The t test, Wilcoxon or Kruskal–Wallis test were used to test differences in continuous variables, and the χ 2 test for those in proportions. All statistical tests were two-tailed, and statistical significance was set at p < 0.05. SPSS software (SPSS 18, Chicago) was used for all analyses.
Results
Patient demographics and clinical findings
Out of 154 patients that fulfilled the search criteria, 96 were identified as clinico-radiologically consistent with PRES. Sixty-five were female (67.7%), median age was 36 years (IQR 23–62). Toxicity and co-morbidities associated with PRES are shown in Table 1. Median MAP was 117 (IQR 103–130) with a maximum of 167 mmHg. The most common neurological symptoms were seizures occurring in 67 patients. Visual disturbances were found in 32 patients and were documented as blurred vision, homonymous hemianopsia or cortical blindness. Altered mental state was found in 45 patients, hemiparesis in 13 patients. Thirty-eight patients had altered coagulation as defined in the method section. Cerebral spinal fluid puncture was performed in 51, electroencephalography in 63 PRES patients. Seventy-four patients were seen by a neurologist at least once. In univariate analyses, hemorrhages were significantly associated with altered coagulation state (p = 0.025), but not with higher MAP or elevated CRP levels. A trend was found for altered creatinine levels (p = 0.065). There was a significant difference in MAP between toxic associations (p = 0.026). Higher mean MAP was found in infection (131 mmHg), eclampsy (119 mmHg) and autoimmune disorders (123 mmHg), while lower MAP was found in chemotherapy (110 mmHg) and immunosuppression (106 mmHg). Total recovery from neurological symptoms during hospital stay occurred in 71% of patients with average discharge in 21 days (IQR 11–59). Four patients died in hospital mostly due to clinical complication that accompanied PRES neurotoxicity.
Radiological spectrum
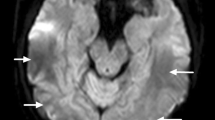
Extent of edematous lesions (Fig. 1)
The extent of edematous lesions was as follows: edema grade 1 was found in 15 patients, grade 2 in 40 patients, grade 3 in 25 patients, grade 4 in 11 patients and grade 5 in five patients. We did not observe significant laterality differences (deviation of more than two edema grades). Higher edema grades were significantly associated with occurrence of intracranial hemorrhages (p = 0.05). There was a trend for higher edema grades to be associated with higher systolic blood pressure values (p = 0.06). The extent of edematous lesions was not significantly correlated with the type of toxic association in our PRES patients.
Spectrum of vasogenic edema in PRES, ranging from discrete foci of cortico-juxtacortical edema (grade 1, arrows), limited edema with some deep white matter extension (grade 2, arrow), via circumscript ventricle contacting edema (grade 3), diffuse edema with extensive ventricle contact (grade 4) to extensive confluent and ventricle deforming edema (grade 5)
Lesion distribution
A detailed overview of lesion distribution is given in Table 2. In summary, edematous lesions were encountered within the entire brain with a predominance of the occipital and parietal lobes. However, frontal and temporal lobe involvement occurred in about 50% of cases. The basal ganglia were affected in about one-fourth of cases, the thalamus in about one-fifth of cases and infratentorial involvement was recognized in more than half of cases with the cerebellum and the pons being most frequently affected. Lesion distribution was not significantly correlated with the type of toxic association in our PRES patients.
Topographic imaging patterns (Fig. 2)
Several patterns of topographic lesion distribution have been recognized recently [2]. In our study the “classical” parieto-occipital pattern was encountered most frequently; however, nearly half of cases showed patterns other than the classical one with a relatively even distribution between superior frontal sulcus pattern, central pattern and holohemispheric watershed pattern as shown in Table 2. Lesion pattern was not associated with the type of toxic association.
Different topographic lesion distribution patterns in PRES. a Classic parieto-occipital pattern. b Central pattern with relative sparing of the lobes and predominance of lesions in the basal ganglia and brain stem. c Superior frontal sulcus pattern with predominance of edema along the anterior watershed. d Holohemispheric watershed pattern with edema along both the anterior and posterior watershed regions
Hemorrhage (Fig. 3)
Table 2 summarizes hemorrhagic sequelae in PRES. Hemorrhage occurred in 32% patients with minute hemorrhages, only visible on T2 gradient echo images being most frequent, followed by frank parenchymal hematoma and sulcal subarachnoid hemorrhage. Cortical laminar necrosis was found in 12 patients. Hemorrhage occurred significantly more frequently patients with altered coagulation state (p = 0.025) and was significantly associated with higher edema grades (p = 0.05) and with the presence of cytotoxic edema (p = 0.022) but not with higher MAP or elevated CRP levels. A non-significant trend was found for altered creatinine levels (p = 0.065).
Various types of hemorrhage in PRES. a Minute hemorrhages as shown by foci of increased susceptibility on T2-weighted sequences in areas of vasogenic edema. b Circumscript sulcal subarachnoid hemorrhage as displayed by CSF hypersignal on FLAIR sequences adjacent to areas of vasogenic edema along the superior frontal sulcus (arrow). c Parieto-occipital parenchymal hematoma surrounded by vasogenic edema
Cytotoxic edema
Foci or areas of cytotoxic edema, as determined by a reduction of the apparent diffusion coefficient, were observed in 28% of patients that had DWI including ADC performed. Vasogenic edema with correspondingly increased ADC values was observed in 63% of patients that had DWI including ADC performed, whereas in 9% of patients no ADC changes were visible in the edematous areas (pseudonormalization). Cytotoxic edema was significantly associated with intracranial hemorrhage (p = 0.022).
Follow-up
In 53 patients follow-up imaging was performed after at least a period of 5 days (maximum 12 months). Median follow-up imaging period was 26 days (IQR 9–52). Table 3 gives a summary on resolution of edema and development of residual structural lesions. In summary, in 82% of patients complete or near complete resolution of edema was noted during follow-up. Higher MAP levels were associated with incomplete resolution (p = 0.001). In 57% of patients no residual lesions were seen. In the case of residual structural lesions there was a relatively even distribution between focal gliosis, infarction, post-hemorrhagic residua, atrophy and laminar necrosis. In one patient changes in topographic distribution patterns occurred. During a follow-up of 35 days with multiple repeated MRI scans the patient who suffered from Goodpasture Syndrome showed moderate parieto-occipital lesions at the start of neurotoxic symptoms. He progressed to a superior frontal sulcus predominant pattern and further on to a holohemispheric pattern (Fig. 4). In two patients delayed intracranial hemorrhage after complete resolution of the edema was noted within a time span from 6 to 36 days after edema resolution and consisted of large parenchymal and intraventricular hematomas, respectively. In one patient massive parenchymal and intraventricular hemorrhage ensued on day six during the course of PRES. One patient suffered from a second episode of PRES which showed a central pattern whereas the first episode had shown a parieto-occipital pattern.
Change of distribution patterns in a patient with Goodpasture Syndrome. a MRI at onset of neurologic symptoms shows parieto-occipital edematous lesions. b On day 15 MRI depicts lesions along the superior frontal sulcus bilaterally with resolution of parieto-occipital edema. c On day 35, the patient progresses to confluent grade 5 holohemispheric PRES. d Follow-up MRI after 5 months shows atrophy and focal gliosis
Discussion
In our study we described the clinical and imaging features of 96 PRES patients, the largest European group of patients to the best of our knowledge. Since the first description of the posterior reversible encephalopathy syndrome (PRES) as a clinico-radiological disease entity by Hinchey and colleagues in 1996, PRES is diagnosed with increasing frequency [9]. In accordance with the recent literature our study shows that PRES is a heterogeneous syndrome with various clinical and imaging features [2, 4, 8]. In our study PRES occurred in a wide range of disorders and predisposing conditions ranging from hypertension, eclampsia to solid organ and bone-marrow transplantation, exposure to various immunosuppressants and cytostatic drugs as well as systemic (auto-)inflammatory conditions with impaired renal function such as SLE. There was a significant difference in MAP between toxic associations with higher MAP in infection, eclampsia and autoimmune disorders, while lower MAP was found in chemotherapy and immunosuppression. This finding may be explicable by the fact that endothelial dysfunction secondary to endotheliotoxic effects of immunosuppressant and chemotherapeutic drugs might play a pivotal pathophysiological role in the development of PRES in these patients. It seems in these cases that endothelial dysfunction alone might be sufficient to cause vasogenic cerebral edema secondary to blood–brain barrier disintegrity. Accordingly, several authors have pointed out the possible role of the endothelial damage in the pathogenesis of PRES [6, 8–11]. This also underlines the importance of withdrawing toxic drugs whenever possible, e.g., by readjusting immuno- or chemo-therapy regimens.
This study confirmed the results of prior studies regarding lesion distribution and topographic lesion patterns [2, 4, 8]. Although we found PRES to preferentially manifest in the parieto-occipital lobes, involvement of other regions, superficial, deep and infratentorial, was frequent. Especially, frontal lobe involvement occurred in more than half of cases and cerebellar involvement in nearly one-third of cases. Nearly one half of patients displayed imaging patterns other than the parieto-occipital one with an almost even distribution between superior frontal sulcus involvement, holohemispheric watershed pattern and central pattern. In accordance with previous observations, we found no significant association between topographic lesion pattern as well as lesion distribution and toxic associations [2].
The severity of the edematous parenchymal changes differed widely between cases, ranging from minimal cortico-subcortical foci to large confluent, ventricle contacting and space-occupying lesions. Interestingly, higher edema grades were significantly associated with occurrence of intracranial hemorrhages. One possible explanation may be that more severely affected patients as shown by edema grades are more likely to have hemorrhages. However, we did not measure overall disease severity or, in particular, PRES severity due to study design aspects. Furthermore, we found a trend for higher systolic blood pressures in higher edema grades, which is in agreement with the pathophysiological hypothesis of acute brain–barrier dysfunction. Interestingly, as MAP did not correlate with edema grades, there might be a particular impact of the maximum systolic blood pressure on the pathogenesis of edema evolution.
Hemorrhage was considered PRES associated only if the patients showed both clinical symptoms and toxic associations compatible with PRES as well as imaging findings compatible with PRES, i.e., areas of vasogenic edema in variable pattern and intensity extending well beyond an expected perihemorrhagic edema. Isolated hematomas manifesting in brain areas with perforating artery supply, such as basal ganglia, thalamus and brain stem, typical for hypertensive hematoma, were not considered in this study and care was taken to rule out neoplastic and vascular causes of hemorrhage such as tumors and venous sinus thrombosis. Hemorrhage associated with PRES occurred in about 32% of cases and was significantly associated with altered coagulation state as reported in the recent literature [3, 5]. In the present study, however, we could show a significant association of hemorrhage with higher edema grades as well as with the presence of cytotoxic edema which has not been reported in the literature to the best of our knowledge. Cytotoxic edema is known to occur in a subset of PRES patients and consists of intracellular swelling secondary to impaired active ion transport mechanisms of the cells [12, 13]. This can be due to energy impairment related to hypoperfusion in the setting of disturbed autoregulation of the cerebral vasculature or toxic substances, metabolites or drugs interfering with cell metabolism. Prolonged cytotoxic edema causes cell death, blood–brain barrier damage and renders the tissue prone to hemorrhages, a cascade well known from cerebral ischemia [14].
Borderline significance was found for the association of hemorrhage and altered creatinine levels. This finding might be explicable by a reduced clearance of toxic substances by the kidneys, possibly aggravating endothelial toxicity depending on the underlying disease. On the other hand, patients with reduced renal function are known to have renovascular hypertension, also possibly contributing to the development of a more severe form of PRES with subsequent hemorrhage.
This study also investigated the frequency of complications and residual structural lesions. Although PRES is generally regarded as a reversible phenomenon, permanent lesions such as hemorrhage or infarction are well recognized. In our study, in nearly one-third of cases hemorrhage occurred with minute hemorrhages and parenchymal hematomas being more frequent than sulcal subarachnoid bleedings. On follow-up three hemorrhages were seen, two of them after complete resolution of symptoms and edematous changes. This may signify that previous affection of parenchyma by PRES changes due to endothelial dysfunction or failed vascular autoregulation might render this area susceptible to future hemorrhage. At least foci of restricted diffusion either in the white matter or the cortex occurred in more than one-fifth of cases, which nearly equals frequency of either infarction or isolated cortical laminar necrosis on follow-up, signifying that these were permanent changes. However, large infarctions were noted only in three cases. Other sequelae of PRES were focal gliosis and atrophy. In 82% of patients complete or near complete resolution of edema was noted during follow-up. Higher MAP levels were associated with incomplete edema resolution in the investigated time span. Of note, the time span between initial and follow-up MRI investigations differed widely in individual cases and often after resolution of symptoms no MRI was performed although the last MRI had shown regressing or residual edema instead of complete resolution of edema. Yet, this finding may indicate that higher MAP levels might perpetuate and prolong the phase of blood–brain barrier disturbance or disturbed autoregulation underlying PRES. It underlines the importance of stringently adjusting blood pressure to normotension.
In a significant proportion (57%) of patients no residual lesions were seen, underlining the frequently reversible nature of PRES. Residual structural lesions were present in 43% of cases which is somewhat more frequent than previously reported and there was a relatively even distribution between focal gliosis, infarction, posthemorrhagic residua, atrophy and laminar necrosis [15]. This study also shows that PRES can manifest with more than one episode if predisposing factors are not eliminated. Furthermore, changes in topographic distribution patterns may occur during one episode.
In our study several limitations need to be considered, most of them inherent to retrospective studies in general. Since patient enrollment was initially based on a radiology report search, some cases may have been missed, e.g., if not reported on subtle findings or erroneous diagnoses. The study cohort is quite heterogeneous and risk factors were categorized according to the predominating clinical feature although in some cases multiple associations were present. Furthermore imaging protocols and timing differed between patients in the acute case as well as on follow-up. In retrospect, knowledge of clinical and paraclinical information was dependent on the documentation. Furthermore we noticed a growing awareness of both clinicians and radiologists regarding the syndrome with increased numbers of specific requests for PRES and subsequent diagnosis in patients with recognized risk factors which might have caused a bias in the study. In order to substantially enhance our understanding of PRES future prospective studies are needed. In the light of our study and the context of the literature we would consider the following criteria potentially helpful for future prospective studies:
(1) Acute development of clinical signs and symptoms typical for PRES, i.e., seizures, visual abnormalities, headaches, nausea or vomiting and other focal deficits. (2) Imaging signs typical of PRES, i.e., foci of vasogenic edema in variable distribution and severity with our without foci of restricted diffusion or hemorrhagic manifestations. So-called atypical distributions of edema or the above mentioned complications should not refrain one from diagnosing PRES if all criteria are fulfilled. The same holds true for the subsequent development of residual lesions. (3) Presence of toxic associations, often multiple, with possible endotheliotoxic effects and/or arterial hypertension to a variable degree from mild to severe.
To conclude, in this large European study posterior reversible encephalopathy syndrome comprised a wide clinical and radiological spectrum. Features that have in the recent past been regarded as atypical, such as frontal lobe, basal ganglia and brain stem involvement, hemorrhages and infarctions occur more frequently than commonly thought. Thus, the clinical and imaging differential diagnosis of PRES can be broad. Residual lesions were encountered more frequently than commonly expected. Significant clinicoradiological associations, not reported in the literature so far, were association of hemorrhage with edema severity as well as presence of cytotoxic edema and a trend for more severe edema with higher systolic blood pressure.
References
Hinchey J, Chaves C, Appignani B, Breen J, Pao L, Wang A, Pessin MS, Lamy C, Mas JL (1996) Caplan LR: a reversible posterior leukoencephalopathy syndrome. N Engl J Med 334:494–500
Bartynski WS, Boardman JF (2007) Distinct imaging patterns and lesion distribution in posterior reversible encephalopathy syndrome. Am J Neuroradiol 28:1320–1327
Hefzy HM, Bartynski WS, Boardman JF, Lacomis D (2009) Hemorrhage in posterior reversible encephalopathy syndrome: imaging and clinical features. Am J Neuroradiol 30:1371–1379
McKinney AM, Short J, Truwit CL, McKinney ZJ, Kozak OS, SantaCruz KS, Teksam M (2007) Posterior reversible encephalopathy syndrome: incidence of atypical regions of involvement and imaging findings. Am J Roentgenol 189:904–912
Sharma A, Whitesell RT, Moran KJ (2010) Imaging pattern of intracranial hemorrhage in the setting of posterior reversible encephalopathy syndrome. Neuroradiology 52:855–863
Bartynski WS (2008) Posterior reversible encephalopathy syndrome, part 2: controversies surrounding pathophysiology of vasogenic edema. Am J Neuroradiol 29:1043–1049
Bartynski WS (2008) Posterior reversible encephalopathy syndrome, part 1: fundamental imaging and clinical features. Am J Neuroradiol 29:1036–1042
Lee VH, Wijdicks EF, Manno EM, Rabinstein AA (2008) Clinical spectrum of reversible posterior leukoencephalopathy syndrome. Arch Neurol 65:205–210
Hinchey JA (2008) Reversible posterior leukoencephalopathy syndrome: what have we learned in the last 10 years? Arch Neurol 65:175–176
Benigni A, Morigi M, Perico N, Zoja C, Amuchastegui CS, Piccinelli A, Donadelli R, Remuzzi G (1992) The acute effect of fk506 and cyclosporine on endothelial cell function and renal vascular resistance. Transplantation 54:775–780
Liman T, Siebert E, Endres M (2010) headache and hypertension: myth and evidence. Nervenarzt 81:963–972
Covarrubias DJ, Luetmer PH, Campeau NG (2002) Posterior reversible encephalopathy syndrome: prognostic utility of quantitative diffusion-weighted mr images. Am J Neuroradiol 23:1038–1048
Schwartz RB, Mulkern RV, Gudbjartsson H, Jolesz F (1998) Diffusion-weighted mr imaging in hypertensive encephalopathy: clues to pathogenesis. Am J Neuroradiol 19:859–862
Sandoval KE, Witt KA (2008) Blood–brain barrier tight junction permeability and ischemic stroke. Neurobiol Dis 32:200–219
Roth C, Ferbert A (2010) Posterior reversible encephalopathy syndrome: long-term follow-up. J Neurol Neurosurg Psychiatry 81:773–777
Acknowledgments
The research leading to these results has also received funding from the Federal Ministry of Education and Research (BMBF) through the Grant Center for Stroke Research Berlin (01 EO 0801), from the German Science Foundation (DFG; Neurocure) and from the Volkswagen Foundation (Lichtenberg program to Matthias Endres).
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Liman, T.G., Bohner, G., Heuschmann, P.U. et al. The clinical and radiological spectrum of posterior reversible encephalopathy syndrome: the retrospective Berlin PRES study. J Neurol 259, 155–164 (2012). https://doi.org/10.1007/s00415-011-6152-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-011-6152-4