Abstract
Introduction
Impaired mitochondrial function is a key factor attributing to the lung ischemia reperfusion injury (LIRI). Methylene blue (MB) has been reported to attenuate brain and renal ischemia–reperfusion injury. We hypothesized that MB also could have a protective effect against LIRI by preventing mitochondrial oxidative damage.
Methods
Isolated rat lungs were assigned to the following four groups (n = 6): a sham group: perfusion for 105 min without ischemia; I/R group: shutoff of perfusion and ventilation for 45 min followed by reperfusion for 60 min; and I/R + MB group and I/R + glutathione (GSH) group: 2 mg/kg MB or 4 μM glutathione were intraperitoneally administered for 2 h, and followed by 45 min of ischemia and 60 min of reperfusion.
Results
MB lessened pulmonary dysfunction and severe histological injury induced by ischemia–reperfusion injury. MB reduced the production of reactive oxygen species and malondialdehyde and enhanced the activity of superoxide dismutase. MB also suppressed the opening of the mitochondrial permeability transition pore and partly preserved mitochondrial membrane potential. Moreover, MB inhibited the release of cytochrome c from the mitochondria into the cytosol and decreased apoptosis. Additionally, MB downregulated the mRNA expression levels of pro-inflammatory cytokines (TNF-α, IL-1β, IL-6, and IL-18).
Conclusion
MB protects the isolated rat lungs against ischemia–reperfusion injury by attenuating mitochondrial damage.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Lung ischemia–reperfusion injury (LIRI) occurs in many clinical situations such as lung transplantation, pulmonary embolism, cardiopulmonary resuscitation, and pulmonary thrombosis [1, 2]. However, currently there are no effective therapies available for LIRI in clinical practice [3, 4].
Impaired mitochondrial function is strongly and directly involved in pathogenesis of LIRI [5]. As soon as reperfusion, mitochondrial respiratory chain dysfunction leads to the augmentation of reactive oxygen species (ROS). ROS directly attack the mitochondrial membrane, trigger lipid peroxidation, and activate the opening of the mitochondrial permeability transition pore (MPTP), finally resulted in mitochondrial damage [6, 7]. In the injured mitochondria, cytochrome c is released into the cytosol through MPTP, activating caspase-3 to induce apoptosis [8]. On the basis of the aforementioned findings, we assume that an effective pharmacological compound, which can decrease the production of free radicals and enhance mitochondrial function, may be a promising strategy to prevent LIRI.
Methylene blue (MB) is traditionally used to treat methemoglobinemia, lithangiuria, and nitrite poisoning. Recent investigations suggest that MB has multiple biological functions [9]. Among these biological functions, the anti-oxidative properties of MB have been extensively investigated. MB easily crosses the cellular membrane, accumulates in the mitochondrial matrix, and restores mitochondrial functions such as enhancing cytochrome c oxidase activity, oxygen consumption, and ATP production while reducing ROS generation [10, 11]. Notably, several studies have shown that MB could attenuate ischemia–reperfusion injury in major organs (heart [12], brain [13], kidney [14], and gut [15]). Weinbroum designed a dose–response study to investigate the protective effects of MB on pancreas ischemia/reperfusion-induced lung injury [16]. Additionally, Abreu et al. [17] reported that MB attenuated pulmonary injury in lung transplantation. However, the precise role of MB on LIRI remains to be determined.
We hypothesized that MB could exert a protective effect in LIRI. Ex vivo lung perfusion has emerged as an essential tool for evaluating therapeutic interventions to reverse acute lung injury or end-stage lung diseases. In this study, we used ex vivo lung perfusion to determine the protective effects of MB on rat LIRI and found that MB protected the isolated rat lungs from LIRI by attenuating mitochondrial oxidative damage.
Materials and Methods
Drugs and Reagents
MB was obtained from Jumpcan Pharmaceutical Group (Taizhou, China). GSH (NO.70-18-8) and rhodamine 123 (NO. 62669-70-9) were purchased from Sigma-Aldrich (St. Louis, MO). The content of LDH in the perfusate (NO.A020-2, reactive oxygen species(ROS, NO.E004), malondialdehyde (MDA, NO.A003-1), adenosine triphosphate (ATP, NO.A095-1), and the activity of superoxide dismutase (SOD, NO.A001-3) assay kits were all obtained from Nanjing Jiancheng Bioengineering Institute (China). Mitochondria isolation kit NO. C1260) from the lung tissue was obtained from Beijing Applygen Technology (China). TUNEL assay was conducted with a commercial kit (NO. CW2574S, Kangwei biotechnology co. LTD, Beijing, China). Anti-cytochrome c antibody and anti-Cox-IV from Cell Signaling Technology (USA); anti-β-actin antibody from Abcam (Cambridge, MA, USA). All other reagents were of analytical grade and commercially available.
Animals and Study Groups
Male Sprague–Dawley rats weighing 280 ± 40 g were obtained from SLAC Laboratory Animal Co. Ltd. (Shanghai, China). All animal experiments were conducted in accordance with protocols approved by the Animal Care and Use Committee of Jiangnan University.
Rats were divided randomly into four groups (n = 6 for each group). In the sham group, the lungs were continuously perfused for 105 min. In I/R groups, the lungs were exposed to 45 min of ischemia followed by 60 min reperfusion. In I/R + MB group, rats were intraperitoneally injected with 2 mg/kg of methylene blue 2 h before surgery and then ischemia 45 min plus 60 min reperfusion [18]. In I/R + GSH group, the lungs were subjected to ischemia 45 min and perfused with the perfusate containing 4 μM of GSH for 60 min.
Isolated Rat Lung Perfusion Model
An isolated rat lung perfusion system (interleukin [IL-2 type; Hugo Sachs Elektronik Harvard Apparatus, March Hugstetten, Germany) was used for ex vivo lung perfusion (Fig. 1). Briefly, Rats anesthetized with an intraperitoneal injection of sodium pentobarbital (80 mg/kg) were intubated after tracheotomy and ventilated during the operation. After systemic heparinization (500 IU), a cannula was inserted into the pulmonary artery and another cannula into the left atrium through a median sternotomy. Two cannulas were connected to the perfusion circuit driven by two roller pumps, and lung perfusion was started with a low flow rate (1 mL/min). Finally, the lungs were removed and suspended in a sealed chamber. All of isolated lungs were allowed to equilibrate and stabilize for 20 min before ischemia. The baseline time is defined as the end of the stabilization period. Tidal volume (V T), airway dynamic compliance (Cdyn), airway resistance (R aw), and pulmonary vein oxygen partial pressure (PaO2) were measured during the perfusion.
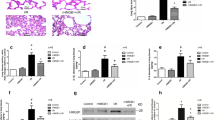
a Expose the trachea and put the trachea cannula into the trachea. The trachea cannula is connected to the ventilation circuit (80 breaths/min; 10 mL/kg tidal volume). b The chest cavity is opened to expose the lung and the heart. c Transect the cardiac apex. Two cannulas are inserted into the main pulmonary artery from the right ventricle and the left atrium from the left ventricle, respectively. A modified Krebs–Henseleit buffer (2 mL/min) is injected into the pulmonary artery. d Free the heart–lung block by dissecting the surrounding tissues. e Carefully remove the hear-lung block from the chest cavity and place it in the perfusion circuit. f Remove the ventilation circuit, and the lung is ventilated by negative pressure (end-inspiratory chamber pressure − 8 cmH2O; end-expiratory chamber pressure – 2 cmH2O). The trachea cannula is connected with the pressure sensor of the perfusion system to record the ventilation data
Pulmonary Edema
At the end of experiment, the right middle lung lobe was weighed and then dried at 60 °C overnight to calculate the wet-to-dry weight ratio [19].
Histological analysis
Lung specimens were fixed in paraformaldehyde, and embedded in paraffin. Paraffin-embedded lung samples were excised into 5 μm-thick sections and stained with hematoxylin and eosin.
Oxidative Stress Assays
The lung tissues and perfusate were collected at the end of reperfusion. The content of LDH in the perfusate was determined according to the manufacturer’s recommended protocol. The content of reactive oxygen species, malondialdehyde (MDA), adenosine triphosphate (ATP), and the activity of superoxide dismutase (SOD) in lung tissues were determined using commercially available kits (Jiancheng, Nanjing, China).
Mitochondrial Swelling Assay
Mitochondria from the lung tissue were isolated using a discontinuous percoll gradient method according to the manufacturer’s instructions (Applygen, Beijing, China). Mitochondrial swelling due to MPTP opening was assessed as follows [20, 21]: the freshly prepared mitochondria (0.5 mg protein/mL) were added to the assay buffer (125 mmol/L sucrose, 50 mmol/L KCl, 2 mmol/L KH2PO4, and 10 mmol/L HEPES). Swelling was initiated by the addition of CaCl2 (200 μmol/L). The decrease in OD at A520, which indicated the extent of opening of MPTP, was measured at 37 °C for 10 min.
Mitochondrial Membrane Potential Assay
Changes in mitochondrial membrane potential (MMP) were measured in the presence of 0.2 mM rhodamine 123 (Sigma-Aldrich, St. Louis, MO) as described by Emaus et al. [22]. The isolated mitochondria (0.5 mg protein/mL) were incubated in the assay buffer (225 mmol/L mannitol, 70 mmol/L sucrose, 5 mmol/L HEPES, pH 7.2). The discharge of Rh123 was induced by CaCl2 (40 μmol/L). The fluorescence intensity was measured using a fluorospectrophotometer (Shanghai instrument analysis instrument, China) at 503 nm excitation and 525 nm emission wavelengths.
Western Blotting
For western blotting analysis, equal amount of proteins (40 μg) was loaded onto SDS–polyacrylamide gel for separation and electrotransferred onto nitrocellulose membranes. The membranes were incubated with anti-cytochrome c antibody, anti-Cox-IV (Cell Signaling Technology), or anti-β-actin antibody (Abcam, Cambridge, MA). After incubation with HRP-conjugated secondary antibodies, specific protein bands on the blots were visualized with a chemiluminescence detection system.
Terminal-deoxynucleotidyl Transferase-Mediated Nick End Labeling (TUNEL) Assay
TUNEL assay was conducted with a commercial kit (Kangwei biotechnology co. LTD, Beijing, China). TUNEL-positive cells were counted below five non-continuous high-power fields (× 400) in each group. The percentage of TUNEL-positive cells was calculated.
Quantitative Real-time PCR (qRT-PCR)
The total RNA was isolated using Trizol (Invitrogen) and reverse transcribed into cDNA using a PrimeScript RT reagent Kit (TaKaRa). The cDNA was subjected to the Light Cycler® 480 (Roche) using SYBR Fast qPCR Mix (TaKaRa). Gene expression was normalized to GAPDH. All primers used in this study are listed in Table 1.
Statistical Analysis
Data were analyzed with SPSS 13.0 software and expressed as mean ± standard deviation (SD). The nature of hypothesis testing was two tailed. For comparing the parameters (tidal volume, lung compliance, and PaO2) between groups during 90 min of observation, a two-way analysis of variance (ANOVA) for repeated measurements was used followed by the post hoc least significant difference test or Dunnett’s t test, treat I/R as a control, and compared all other groups against it. The difference was considered significant if P < 0.05 was observed.
Results
MB Improved LIRI-Induced Changes in Pulmonary Parameters
The IL-2-type lung isolation system can monitor the pulmonary physiology and mechanics in real time. We first evaluated the potential protective effects of MB on LIRI-induced pulmonary dysfunction. At baseline, the parameters had no significant differences among the groups. In this ex vivo ischemia–reperfusion rat model, lungs in the I/R group showed a significant decrease in V T, C dyn, and PaO2 and increase in R aw after 30 and 60 min of reperfusion compared to the sham group. In contrast, the lungs in the I/R with MB or GSH treated groups showed a significant increase in V T, C dyn, and PaO2 and lower R aw (Table 2).
MB Attenuated LIRI-induced Pulmonary Injury
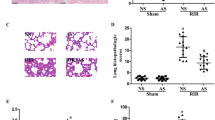
To further explore the beneficial effects of MB on IR-induced pulmonary injury, we investigated the pulmonary morphology, wet/dry ratio, and the activity of LDH in the perfusate. As shown in Fig. 2b, there were severe perivascular edema and intra-alveolar hemorrhage in the I/R group, whereas MB and GSH decreased histological damages (Fig. 2c, d). Additionally, the pulmonary wet/dry ratio significantly decreased in I/R + MB group and I/R + GSH group compared to that in the I/R group, indicating that the lungs treated with MB or GSH could reduce LIRI-induced pulmonary edema (Fig. 2e). Furthermore, MB and GSH significantly diminished the IR injury induced release of LDH in lung tissues (Fig. 2f).
Effects of MB on the pulmonary morphology, wet/dry ratio, and the content of LDH in the perfusates of the different groups. a–d Histological observation of pulmonary injury. Typical images were chosen from each experimental group (original magnification ×200); a sham group; b I/R group; c I/R + MB group; and d I/R + GSH group. e wet/dry ratio. f The content of LDH in the perfusate. All values are expressed as mean ± SD. The experiment was repeated three times (n = 6 for each group). *P < 0.05 versus the sham group, # P < 0.05 versus I/R group
MB Protected Against LIRI-Induced Oxidative Stress
Considering that excess ROS has key destructive effects on LIRI, we then examined whether MB could inhibit LIRI-induced oxidative stress. As shown in Fig. 3a, c, ROS production and MDA concentration significantly increased in I/R group when compared with that in the sham group. Interestingly, MB or GSH treatment markedly reduced the production of ROS and MDA content. Moreover, MB or GSH ameliorated the decrease in SOD activity induced by LIRI (Fig. 3b). These results indicated that MB and GSH alleviated LIRI-induced oxidative stress.
MB Attenuated LIRI-induced Mitochondrial Dysfunction
To define the role of MB in improving mitochondrial functions, we isolated the mitochondria and evaluated their function. The decrease of absorbance at 540 nm indicated that mitochondrial swelling induced by I/R was inhibited by treatment of MB or GSH (Fig. 4a). Similarly, MMP in the I/R group was significantly lower than that in the sham group, whereas there was a significant increase in MMP in the I/R + MB group and the I/R + GSH group (Fig. 4b). To further define the role of MB in improving mitochondrial functions, we examined ATP concentrations in the pulmonary tissues. Consistent with the aforementioned data, the results showed that ATP concentration was markedly reduced in I/R group when compared with that in the sham group. Importantly, MB or GHS treatment apparently increased ATP production (Fig. 4c).
MB Blocked Cytochrome c Release From the Mitochondria into the Cytosol and Alleviated Apoptosis
It is well accepted that apoptosis plays a causal role in LIRI. Therefore, we evaluated whether the protective properties of MB against LIRI involved inhibition of apoptosis. To analyze LIRI-induced apoptotic cells, pulmonary sections were subjected to TUNEL staining, and the apoptosis indices were calculated. As shown in Fig. 5a–d, most of the pulmonary epithelial cells were positive for TUNEL staining in the I/R group. However, the numbers of TUNEL-positive cells were significantly decreased in the I/R + MB group and the I/R + GSH group (Fig. 5e). The results of western blotting showed that the release of cytochrome c from the mitochondria into the cytosol was increased in the I/R group, whereas MB or GSH treatment inhibited this release (Fig. 5f). These data showed that MB could inhibit LIRI-induced apoptosis.
Effects of MB on apoptosis in lung tissues. (a–d) Histological images of TUNEL-stained cells. e The apoptosis index. All values are expressed as mean ± SD (n = 6 for each group). *P < 0.05 versus sham group, # P < 0.05 versus I/R group. f The content of cytochrome c in the mitochondria and cytosol was assessed by western blotting. β-actin and Cox-IV were used as a protein loading control. The experiment was repeated three times; representative results of three independent experiments were shown
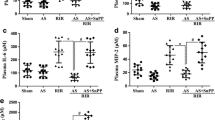
MB Downregulated the mRNA Expression Levels of Pro-inflammatory Cytokines Induced by LIRI
qRT-PCR results indicated that the mRNA expression levels of TNF-α, IL-1β, IL-6, and IL-18 were significantly elevated in the I/R group compared to those in the sham group. MB treatment downregulated the mRNA expression levels of TNF-α, IL-1β, IL-6, and IL-18 (Fig. 6).
Effects of MB on the gene expression levels of pro-inflammatory cytokines including TNF-α (a), IL-1β (b), IL-6 (c), and IL-18 (d) in lung tissues. All values are expressed as mean ± SD. The experiment was repeated three times (n = 6 for each group). *P < 0.05 versus sham group, # P < 0.05 versus I/R group, + P < 0.05 versus I/R + GSH group
Discussion
In the present study, we demonstrated for the first time that MB could protect the isolated rat lungs from LIRI, as confirmed by pulmonary function parameters, pulmonary morphology, wet/dry ratio, and LDH activity. Considering that the mitochondrion is an important target of MB, to identify the potential mechanism by which MB inhibited LIRI, we focused on the effect of MB on mitochondrial functions. We found that MB treatment significantly attenuated LIRI-induced decrease in SOD activity and ATP generation and increase in ROS production and MDA concentration. We also found that MB preserved MMP and inhibited mitochondrial swelling. Concomitantly, lungs from MB-treated rats showed less cytochrome c release into the cytosol and decreased cell apoptosis rate. In addition, treatment with MB significantly down regulated the expression levels of the pro-inflammatory cytokines. Taken together, these findings strongly support the protective effects of MB on the isolated rat lungs affected by LIRI through its anti-oxidative, anti-apoptotic, and anti-inflammatory effects.
Isolated lung perfusion is a powerful experimental tool to research the treatment of acute lung injury (ALI). Many kinds of ALI animal models can be easily and rapidly developed using this approach [23]. Moreover, these models can be used to assess the histological characterization and inflammatory cytokine content in pulmonary tissues [24]. Most importantly, the model of isolated lung perfusion is a convenient tool to monitor the physiological indices such as V T, C dyn, and PaO2 and R aw in real time. VT is the volume of gas inspired or expired during each respiratory cycle; C dyn is an index of functional stiffness of the lung and reflects changes to small airways; R aw is defined as the pressure difference required for a unit flow change. In this study, we successfully established the isolated LIRI model on the IL-2-type perfused lung isolation system, as evidenced by abnormal lung function, pulmonary morphology, increased wet/dry ratio, and LDH content in the perfusion. Interestingly, MB could attenuate these abnormal conditions.
In this study, GSH was selected to serve as a positive control drug. Although the protective effect of GSH on LIRI was comparable to that of MB, the very short half-life of GSH [25] and its insufficiency to rapidly cross the lipid-rich cell membrane [26] hinder its application to treat oxidative stress-associated lung diseases. MB has some unique advantages over GSH to treat LIRI. First, MB is water soluble and can rapidly cross the cell membrane [27, 28]. Second, MB has positive features such as universal availability, cost-effectiveness, and safety. Finally, MB as an FDA-approved agent has been used clinically for over 130 years. We found that MB treatment markedly improved lung function, lessened pulmonary damage, enhanced mitochondrial function, and inhibited apoptosis. These results implied that MB may be a potential agent to treat LIRI.
Mitochondrial damage is a central event in the development of IR injury [1, 5]. Recent investigations found that MB enhanced mitochondrial respiration at low concentrations (0.5–2 mM) by shuttling electrons to oxygen in the electron transport chain [9, 15, 28]. Furthermore, MB treatment could attenuate IR injury such as stroke [12] and other brain injuries [13], and those to the kidney [14] and the gut [15]. In this study, we further found that MB treatment reduced the production of ROS and the content of MDA and enhanced the activity of SOD. We also found that MB preserved MMP and blocked the opening of MPTP. These results demonstrated the beneficial effect of MB on mitochondrial function.
Excess apoptosis always contributes to IR injury [29]. The generation of ROS combined with calcium overload lead to the opening of MPTP [30]. The opening of MPTP and the rupture of the outer membrane cause cytochrome c release from mitochondria to cytosol. We found that MB blocked cytochrome c release into the cytosol and alleviated apoptosis. This result showed the anti-apoptotic effect of MB on LIRI.
It is well known that LIRI triggers an intense inflammatory response accompanied with increased levels of pro-inflammatory cytokines [31]. Pro-inflammatory cytokines cause long-term damage in the infracted tissue [32]. Therefore, suppression of inflammatory response might be a promising therapy for controlling organ injury [33, 34]. Consistent with Fenn’s finding that MB attenuated inflammation in traumatic brain [35], we observed down-regulation of the mRNA levels of pro-inflammatory cytokines after MB treatment.
Our study has some limitations. First, although the ex vivo lung perfusion has many advantages in lung research, we did not use the in vivo IR injury model to test the protective role of MB on LIRI. Second, we only investigated the protective effect of MB before LIRI. Future studies should investigate the beneficial effect of MB after LIRI.
In conclusion, the present results indicate that MB protects the isolated rat lungs from IR injury and that the potential mechanisms of this protective effect may be associated with the restoration of mitochondrial function and the suppression of inflammatory response and apoptosis. Therefore, our findings suggest that application of MB may have a curative effect on LIRI.
References
Ferrari RS, Andrade CF (2015) Oxidative stress and lung ischemia-reperfusion injury. Oxidative med cell longev 2015:590987. https://doi.org/10.1155/2015/590987
Ovechkin AV, Lominadze D, Sedoris KC, Robinson TW, Tyagi SC, Roberts AM (2007) Lung ischemia-reperfusion injury: implications of oxidative stress and platelet-arteriolar wall interactions. Arch Physiol Biochem 113(1):1–12. https://doi.org/10.1080/13813450601118976
Eltzschig HK, Eckle T (2011) Ischemia and reperfusion–from mechanism to translation. Nat Med 17(11):1391–1401. https://doi.org/10.1038/nm.2507
Eltzschig HK, Bratton DL, Colgan SP (2014) Targeting hypoxia signalling for the treatment of ischaemic and inflammatory diseases. Nat Rev Drug Discov 13(11):852–869. https://doi.org/10.1038/nrd4422
Silachev DN, Plotnikov EY, Pevzner IB, Zorova LD, Babenko VA, Zorov SD, Popkov VA, Jankauskas SS, Zinchenko VP, Sukhikh GT, Zorov DB (2014) The mitochondrion as a key regulator of ischaemic tolerance and injury. Heart Lung Circ 23(10):897–904. https://doi.org/10.1016/j.hlc.2014.05.022
Nederlof R, Eerbeek O, Hollmann MW, Southworth R, Zuurbier CJ (2014) Targeting hexokinase II to mitochondria to modulate energy metabolism and reduce ischaemia-reperfusion injury in heart. Br J Pharmacol 171(8):2067–2079. https://doi.org/10.1111/bph.12363
Murphy E, Steenbergen C (2008) Mechanisms underlying acute protection from cardiac ischemia-reperfusion injury. Physiol Rev 88(2):581–609. https://doi.org/10.1152/physrev.00024.2007
Souidi N, Stolk M, Seifert M (2013) Ischemia-reperfusion injury: beneficial effects of mesenchymal stromal cells. Curr Opin Organ Transplant 18(1):34–43. https://doi.org/10.1097/MOT.0b013e32835c2a05
Yang SH, Li W, Sumien N, Forster M, Simpkins JW, Liu R (2015) Alternative mitochondrial electron transfer for the treatment of neurodegenerative diseases and cancers: methylene blue connects the dots. Prog Neurobiol. https://doi.org/10.1016/j.pneurobio.2015.10.005
Kuliaviene I, Baniene R, Virketyte S, Kincius M, Jansen E, Gulbinas A, Kupcinskas L, Trumbeckaite S, Borutaite V (2016) Methylene blue attenuates mitochondrial dysfunction of rat kidney during experimental acute pancreatitis. J dig dis 17(3):186–192. https://doi.org/10.1111/1751-2980.12328
Lin X, Wu BB, Xing QY, Wang SD, Li J (2015) Methylene blue, a potential therapeutic target for mitochondria dysfunction. Int Immunopharmacol 28(2):1106–1107. https://doi.org/10.1016/j.intimp.2015.08.024
Ahmed ME, Tucker D, Dong Y, Lu Y, Zhao N, Wang R, Zhang Q (2016) Methylene blue promotes cortical neurogenesis and ameliorates behavioral deficit after photothrombotic stroke in rats. Neuroscience 336:39–48. https://doi.org/10.1016/j.neuroscience.2016.08.036
Wiklund L, Sharma A, Sharma HS (2016) Neuroprotection by Methylene blue in cerebral global ischemic injury induced blood-brain barrier disruption and brain pathology. CNS Neurol Disord Drug Target 15(9):1181–1187
Sarac F, Kilincaslan H, Kilic E, Koldas M, Terzi EH, Aydogdu I (2015) Methylene blue attenuates renal ischemia-reperfusion injury in rats. J Pediatr Surg 50(6):1067–1071. https://doi.org/10.1016/j.jpedsurg.2014.06.018
Collange O, Charles AL, Bouitbir J, Chenard MP, Zoll J, Diemunsch P, Thaveau F, Chakfe N, Piquard F, Geny B (2013) Methylene blue protects liver oxidative capacity after gut ischaemia-reperfusion in the rat. Eur J Vasc Endovasc Surg 45(2):168–175. https://doi.org/10.1016/j.ejvs.2012.11.011
Weinbroum AA (2009) Methylene blue attenuates pancreas ischemia-reperfusion (IR)-induced lung injury: a dose response study in a rat model. J Gastrointest Surg 13(9):1683–1691. https://doi.org/10.1007/s11605-009-0945-0
Abreu Mda M, Pazetti R, Almeida FM, Correia AT, Parra ER, Silva LP, Vieira RP, Pego-Fernandes PM, Jatene FB (2014) Methylene blue attenuates ischemia- reperfusion injury in lung transplantation. J Surg Res 192(2):635–641. https://doi.org/10.1016/j.jss.2014.07.043
Chen JL, Dai L, Zhang P, Chen W, Cai GS, Qi XW, Hu MZ, Du B, Pang QF (2015) Methylene blue attenuates acute liver injury induced by paraquat in rats. Int Immunopharmacol 28(1):808–812. https://doi.org/10.1016/j.intimp.2015.04.044
Setzer F, Oschatz K, Hueter L, Schmidt B, Schwarzkopf K, Schreiber T (2013) Susceptibility to ventilator induced lung injury is increased in senescent rats. Crit Care 17(3):R99. https://doi.org/10.1186/cc12744
Kristian T, Gertsch J, Bates TE, Siesjo BK (2000) Characteristics of the calcium-triggered mitochondrial permeability transition in nonsynaptic brain mitochondria: effect of cyclosporin A and ubiquinone O. J Neurochem 74(5):1999–2009
Galindo MF, Jordan J, Gonzalez-Garcia C, Cena V (2003) Reactive oxygen species induce swelling and cytochrome c release but not transmembrane depolarization in isolated rat brain mitochondria. Br J Pharmacol 139(4):797–804. https://doi.org/10.1038/sj.bjp.0705309
Emaus RK, Grunwald R, Lemasters JJ (1986) Rhodamine 123 as a probe of transmembrane potential in isolated rat-liver mitochondria: spectral and metabolic properties. Biochim Biophys Acta 850(3):436–448
Roman M, Gjorgjimajkoska O, Neil D, Nair S, Colah S, Parmar J, Tsui S (2015) Comparison between cellular and acellular perfusates for ex vivo lung perfusion in a porcine model. J Heart Lung Transplant 34(7):978–987. https://doi.org/10.1016/j.healun.2015.03.023
Nelson K, Bobba C, Ghadiali S, Hayes D Jr, Black SM, Whitson BA (2014) Animal models of ex vivo lung perfusion as a platform for transplantation research. World J Exp Med 4(2):7–15. https://doi.org/10.5493/wjem.v4.i2.7
Wendel A, Jaeschke H, Gloger M (1982) Drug-induced lipid peroxidation in mice–II. Protection against paracetamol-induced liver necrosis by intravenous liposomally entrapped glutathione. Biochem Pharmacol 31(22):3601–3605
Schauer RJ, Gerbes AL, Vonier D, Meissner H, Michl P, Leiderer R, Schildberg FW, Messmer K, Bilzer M (2004) Glutathione protects the rat liver against reperfusion injury after prolonged warm ischemia. Ann Surg 239(2):220–231. https://doi.org/10.1097/01.sla.0000110321.64275.95
Atamna H, Mackey J, Dhahbi JM (2012) Mitochondrial pharmacology: electron transport chain bypass as strategies to treat mitochondrial dysfunction. BioFactors 38(2):158–166. https://doi.org/10.1002/biof.197
Gabrielli D, Belisle E, Severino D, Kowaltowski AJ, Baptista MS (2004) Binding, aggregation and photochemical properties of methylene blue in mitochondrial suspensions. Photochem Photobiol 79(3):227–232
Sommer SP, Sommer S, Sinha B, Wiedemann J, Otto C, Aleksic I, Schimmer C, Leyh RG (2011) Ischemia-reperfusion injury-induced pulmonary mitochondrial damage. J Heart Lung Transplant 30(7):811–818. https://doi.org/10.1016/j.healun.2011.02.001
Brinkkoetter PT, Song H, Losel R, Schnetzke U, Gottmann U, Feng Y, Hanusch C, Beck GC, Schnuelle P, Wehling M, van der Woude FJ, Yard BA (2008) Hypothermic injury: the mitochondrial calcium, ATP and ROS love-hate triangle out of balance. Cell Physiol Biochem 22(1–4):195–204. https://doi.org/10.1159/000149797
Guo Z, Yu S, Chen X, Ye R, Zhu W, Liu X (2016) NLRP3 Is Involved in Ischemia/Reperfusion Injury. CNS Neurol Disord: Drug Targets 15(6):699–712
Mao LL, Hao DL, Mao XW, Xu YF, Huang TT, Wu BN, Wang LH (2015) Neuroprotective effects of bisperoxovanadium on cerebral ischemia by inflammation inhibition. Neurosci Lett 602:120–125. https://doi.org/10.1016/j.neulet.2015.06.040
Shen X, Du J, Zhao Y, Guan W (2014) Phosphatase Wip1 as a new therapeutic target for intestinal ischemia-reperfusion injury. Expert Rev Clin Immunol 10(12):1591–1595. https://doi.org/10.1586/1744666X.2014.975211
Fang T, Zhou D, Lu L, Tong X, Wu J, Yi L (2016) LXW7 ameliorates focal cerebral ischemia injury and attenuates inflammatory responses in activated microglia in rats. Braz J Med Biol Res 49(9):e5287. https://doi.org/10.1590/1414-431X20165287
Fenn AM, Skendelas JP, Moussa DN, Muccigrosso MM, Popovich PG, Lifshitz J, Eiferman DS, Godbout JP (2015) Methylene blue attenuates traumatic brain injury-associated neuroinflammation and acute depressive-like behavior in mice. J Neurotrauma 32(2):127–138. https://doi.org/10.1089/neu.2014.3514
Funding
This work was supported by the following Grants: National Natural Science Foundation of China (81270126); Fundamental Research Funds for the Central Universities (JUSRP51412B); The Science Funds for Health and Family Planning Commission of Sichuan Province (150234); Jiangsu Province Natural Science Foundation (BK20161141); and Graduate Student Research Innovation Project (KYZZ16_0312, KYLX15_1196).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical Approval
This research involved rats. The study was approved by the Animal Care and Use Committee of Jiangnan University.
Rights and permissions
About this article
Cite this article
Tian, Wf., Zeng, S., Sheng, Q. et al. Methylene Blue Protects the Isolated Rat Lungs from Ischemia–Reperfusion Injury by Attenuating Mitochondrial Oxidative Damage. Lung 196, 73–82 (2018). https://doi.org/10.1007/s00408-017-0072-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00408-017-0072-8