ABSTRACT
Artesunate (AS), a semi-synthetic derivative of Artemisia, has been shown to exert a wide range of pharmacological effects, such as anti-inflammatory and antioxidant functions. However, the protective functions of AS on renal ischemia reperfusion injury (RIR)-stimulated lung inflammation remain unclear. In this research, acute lung injury (ALI) was stimulated by renal ischemia reperfusion injury (RIR). AS (15 mg/kg) was intraperitoneal administrated to rat 1 h before RIR stimulation. Serum and pulmonary NO, MDA, IL-6, MIP-2, and PGE2 levels, arterial blood gas and biochemistry, lung wet/dry weight ratio and MPO activity, total cell number and protein concentration in BALF, tissue histology, and NF-κB expression were determined. The results indicated that serum and pulmonary NO, MDA, IL-6, MIP-2, and PGE2 levels, lung wet/dry weight ratio and MPO activity, total cell number, and protein concentration in BALF enhanced after RIR stimulation. These alterations were mitigated by AS. AS attenuated lung wet/dry weight ratio and MPO activity, total cell number, and protein concentration in BALF. AS attenuated RIR-stimulated pulmonary NF-κB phosphorylation. In addition, these previously mentioned actions of AS were antagonized by suppressing HO-1 pathway. However, RIR-stimulated arterial blood gas and biochemistry and lung histopathology were also attenuated by AS. In summary, AS inhibited RIR-stimulated lung inflammation by activating HO-1 pathway.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
Renal ischemia reperfusion injury (RIR) is a common clinical syndrome of kidney inflammation responses with a high rate of complications and death [1]. RIR can result in remote organ injury such as the liver, heart, and lung [2,3,4]. Lung inflammation are the most common complication that caused by multiple ischemia reperfusion injury (IR) [5,6,7]. This experimental model was extensive used to investigate the underling mechanisms of lung inflammation and exploit new lung-protective drugs [1]. Previous reports have demonstrated that RIR-stimulated inflammatory molecules releases in rat model of lung inflammation [8,9,10]. These inflammatory molecules resulted in lung tissue edema and Pathological damage [11].
Heme oxygenase-1 (HO-1) is a kind of anti-inflammation protein that plays an important role in the development of lung inflammation [12,13,14]. Nuclear factor-kappa B (NF-κB) signaling plays a critical role in the expression of lung inflammatory mediators mRNA and protein [15, 16]. Researches indicated that NF-κB was activated via stimuli, which also enhance the expression of HO-1 gene [17]. Upregulates in HO-1 expression in macrophages results in suppression of NF-κB-induced innate immune responses [18]. Recent studies indicated that enhanced-HO-1 activity ameliorates IR-stimulated lung inflammation [5, 7], and inhibition of NF-κB activation had protective effects against IR-stimulated lung inflammation [19, 20].
Artesunate (AS), a semi-synthetic derivative of Artemisia, has been shown to possess anti-inflammatory and antioxidant functions [21,22,23,24,25]. Previous researches indicated that AS protects lipopolysaccharide (LPS) or cecal ligation and puncture (CLP)-stimulated lung inflammation though inhibition of NF-κB activation [21, 25] and attenuates paraquat- and cigarette smoke-induced lung injury by inhibiting the inflammatory response [26, 27]. AS was found to inhibit LPS-induced pro-inflammatory responses in microglia [24]. AS also attenuated hepatic fibrosis stimulated by various inflammation factors via inhibiting NF-κB activation [23]. Moreover, AS has been shown to improve severe acute pancreatitis though inhibition of pro-inflammatory cytokines expression and NF-κB activation [22]. However, the roles of AS on RIR-stimulated lung inflammation remain unclear. We hypothesized that AS would attenuate the RIR-stimulated lung inflammation via activating HO-1 pathway and suppressing NF-κB pathway.
MATERIALS AND METHODS
Ethics Declaration
Experimental procedures were performed in the accordance with the US NIH Guide for the Care and Use of Laboratory Animals and authorized by the Animal Care and Use Ethics Committee of Shanghai First People’s Hospital (Approval ID: 20151210-3).
Materials
AS and SnPP (A specific inhibitor for HO-1) were obtained from Sigma (St. Louis, MO, USA). Malonyldialdehyde (MDA) and myeloperoxidase (MPO) assay kits were purchased from R&D Systems (Minneapolis, MN). NO, IL-6, MIP-2, and PGE2 ELISA kits were provided by Santa Cruz Biotechnology Inc. (Santa Cruz, CA, USA). Antibodies against NF-κB, Iκ-B-α, and β-actin were provided by the Nanjing Jiancheng Biology Engineering Institute (Nanjing, China).
Animals
Adult male Sprague-Dawley rats (200 to 250 g) were provided by the Center of Experimental Animals of Shanghai First People’s Hospital (Shanghai, China). All animals were fed with a standard diet and sterile water under specific pathogen-free conditions.
Experimental Scheme
Rat acute lung injury model was stimulated by RIR. To assess the protection functions of AS on RIR-stimulated lung inflammation, 60 rats were randomly divided into five groups: the sham group, the sham plus AS group, RIR group, RIR plus AS group, and RIR plus AS plus SnPP (A specific inhibitor for HO-1, 30 mg/kg), group. AS (15 mg/kg) was given to the rat 1 h before RIR stimulation. The dose of AS chosen was determined by referring to previous studies [21, 28] and our pre-experiments. The serum and lung samples were gathered 24 h after RIR stimulation for subsequent assay.
Measurement of NO, IL-6, MIP-2, and PGE2
NO, IL-6, MIP-2, and PGE2 production in serum and lung samples were determined by applying commercial ELISA kits provided by Santa Cruz Biotechnology Inc. (Santa Cruz, CA, USA) in accordance with the manufacturer’s manuals.
MDA and MPO Activities Determination
Serum and pulmonary MDA levels were measured by using MDA assay kit in accordance with the manufacturer’s manuals (R&D Systems, Minneapolis, MN). MPO activity in lung samples was measured by applying relevant assay kits provided by R&D Systems (Minneapolis, MN) in accordance with the manuals.
Measurement of Lung Inflammation
Broncho alveolar lavage fluid (BALF) and lung tissues were gathered at 24 h after RIR stimulation. The total cell number in the BALF was calculated with a hemcytometer, and the protein level in the BALF was evaluated by the BCA method. The lung samples collected from the left upper lobe were dried out, and then it was weighed to obtain wet weight and then placed in the oven at 80 °C to get dry weight. Wet/dry weight ratio was calculated as a maker of lung edema.
Histological Evaluation
Lung samples were fixed in 10% buffered formalin and then embedded with paraffin. After fixation and stained with hematoxylin-eosin (H&E), characterizing lung injury was observed by microscopic evaluation.
Western Blot
Lung samples were homogenized and centrifuged, and then nuclear and cytoplasmic proteins was collected from the lung samples in accordance with the method instructed by the protein extract kit (Thermo, USA). Protein levels were measured by applying the Bradford protein assay (Bio-Rad, United Kingdom). Protein extracts were heated, denatured, and loaded on 12% sodium dodecyl sulfate polyacrylamide gel and transferred onto polyvinylidene difluoride membranes. The membranes were treated overnight at 4 °C with the primary antibodies. After washing three times, the membranes were treated with HRP-conjugated secondary antibodies at 37 °C for 1 h. Ultimately, the membranes were measured by applying the ECL western blot determination system in accordance with the manufacturer’s manual.
Statistical Analysis
All experimental data were expressed as mean ± SD. The biochemical data were determined using one-way analysis of variance (ANOVA) and Student’s t test applying the GraphPad 5.0 software. A P < 0.05 is considered as statistically significant.
RESULT
AS Suppresses RIR-Stimulated Serum NO, MDA, IL-6, MIP-2, and PGE2 Production
Serum NO, MDA, IL-6, MIP-2, and PGE2 production were used to assess the degree of RIR-induced systemic inflammatory responses. In comparison with the sham group, serum NO, MDA, IL-6, MIP-2, and PGE2 production augmented evidently in RIR group (Fig. 1, P < 0.05). Nevertheless, administration of AS suppress RIR-stimulated serum NO, MDA, IL-6, MIP-2, and PGE2 production (Fig. 1, P < 0.05). Meanwhile, the inhibition functions of AS on RIR-stimulated serum NO, MDA, IL-6, MIP-2, and PGE2 production were reversed by SnPP (A specific inhibitor for HO-1; Fig. 1, P < 0.05).
Effects of artesunate (AS) on serum inflammatory molecule levels after renal ischemia reperfusion injury (RIR) in rats. AS markedly reduces the serum levels of NO (a), MDA (b), IL-6 (c), MIP-2 (d), and PGE2 (e) in the rat model of RIR. Data were shown as mean ± SD. *P < 0.05 vs. sham group, # P < 0.05 vs. RIR group, $ P < 0.05 vs. RIR + AS group.
AS Suppresses RIR-Stimulated Pulmonary NO, MDA, IL-6, MIP-2, and PGE2 Production
Pulmonary NO, MDA, IL-6, MIP-2, and PGE2 production were used to assess the degree of RIR-induced pulmonary inflammatory responses. In comparison with the sham group, pulmonary NO, MDA, IL-6, MIP-2, and PGE2 production augmented evidently in RIR group (Fig. 2, P < 0.05). Nevertheless, administration of AS suppress RIR-stimulated pulmonary NO, MDA, IL-6, MIP-2, and PGE2 production (Fig. 2, P < 0.05). Meanwhile, the inhibition functions of AS on RIR-stimulated pulmonary NO, MDA, IL-6, MIP-2, and PGE2 production were reversed by SnPP (A specific inhibitor for HO-1; Fig. 2, P < 0.05).
Effects of artesunate (AS) on pulmonary inflammatory molecule levels after renal ischemia reperfusion injury (RIR) in rats. AS markedly reduces the lung levels of NO (a), MDA (b), IL-6 (c), MIP-2 (d), and PGE2 (e) in the rat model of RIR. Data were shown as mean ± SD. *P < 0.05 vs. Sham group, # P < 0.05 vs. RIR group, $ P < 0.05 vs. RIR + AS group.
AS Suppresses RIR-Stimulated the Indicators of Pulmonary Inflammation.
The indicators of pulmonary inflammation, such as total cell number and protein in BALF, pulmonary wet/dry weight ratio, and MPO activity, were used to assess the degree of RIR-induced pulmonary inflammation. In comparison with the sham group, the indicators of pulmonary inflammation production augmented evidently in RIR group (Fig. 3, P < 0.05). Nevertheless, administration of AS suppress RIR-stimulated the indicators of pulmonary inflammation production (Fig. 3, P < 0.05). Meanwhile, the inhibition functions of AS on RIR-stimulated the indicators of pulmonary inflammation production were reversed by SnPP (A specific inhibitor for HO-1; Fig. 3, P < 0.05).
Effects of artesunate (AS) on pulmonary inflammatory response levels after renal ischemia reperfusion injury (RIR) in rats. AS markedly reduces the BALF levels of total cell number (a) and total protein concentration (b) and the lung levels of wet/dry weight ratio (c) and MPO activity (d) in the rat model of RIR. Data were shown as mean ± SD. *P < 0.05 vs. Sham group, # P < 0.05 vs. RIR group, $ P < 0.05 vs. RIR + AS group.
Effects of AS on RIR-Stimulated Lung Histopathology
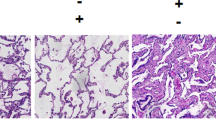
Histological evaluation of lung samples was used to determine the protection functions of AS on RIR-stimulated lung inflammation. As indicated in Fig. 4, lung samples of the RIR group indicated intra-alveolar hemorrhage, alveolar septa edema, and neutrophil exudation. Administration of AS evidently mitigated these pathological alteration stimulated by RIR. However, the inhibition functions of AS on RIR-stimulated lung histopathology were reversed by SnPP (A specific inhibitor for HO-1; Fig. 4, P < 0.05).
Artesunate (AS) ameliorate RIR-induced histologic changes in the lung. a Sham group. b AS group. c RIR group. d RIR + AS group. e RIR + AS + SnPP group (HE × 200). f The lung injury scores. Data were shown as mean ± SD. *P < 0.05 vs. Sham group, # P < 0.05 vs. RIR group, $ P < 0.05 vs. RIR + AS group.
AS Suppresses RIR-Stimulated Pulmonary NF-κB Translocation
Pulmonary NF-κB p65 and p-IκB-α were used to evaluate the extent of RIR-induced pulmonary NF-κB translocation. In comparison with the sham group, pulmonary NF-κB p65 in the nuclear and p-IκB-α in the cytosolic augmented evidently in RIR group (Fig. 5, P < 0.05). Nevertheless, administration of AS suppress RIR-stimulated pulmonary NF-κB translocation (Fig. 5, P < 0.05). Meanwhile, the inhibition functions of AS on RIR-stimulated pulmonary NF-κB translocation were reversed by SnPP (A specific inhibitor for HO-1; Fig. 5, P < 0.05).
Effects of artesunate (AS) on pulmonary NF-κB activation after renal ischemia reperfusion injury (RIR) in rats. NF-κB (p65) in the nucleus (a), NF-κB (p65) in the cytosol (b), and p-IκB-α (c) in the rat model of RIR was detected by western blot. Data were shown as mean ± SD. *P < 0.05 vs. Sham group, # P < 0.05 vs. RIR group, $ P < 0.05 vs. RIR + AS group.
DISCUSSION
AS, a derivative of artemisinin, has been shown to exert antioxidant and anti-inflammatory functions [28]. Nevertheless, the previous studies did not exam weather AS inhibits the lung inflammation induced by RIR, and effects the HO-1 level and NF-κB activity in RIR-induced ALI. RIR-stimulated ALI model in rat was commonly used to investigate original lung protection strategies [29]. In this research, we explored the protective functions of AS on RIR-stimulated ALI in rat. The results have demonstrated that AS attenuated serum and pulmonary inflammatory molecules production, the markers of the degree of lung inflammation. Moreover, histopathological injury evaluation directly demonstrated that AS mitigated RIR-stimulated ALI. AS represents an important therapeutic reagents for mitigating ALI.
Previous reports indicated that lipid peroxidation might participate in RIR-stimulated ALI [30]. MDA, an end product of oxidative injury, enhanced evidently in rat subjected to RIR [31]. In this report, we demonstrated that AS inhibited RIR-stimulated MDA production. Meanwhile, the levels of total cell number and protein in BALF, pulmonary wet/dry weight ratio, and MPO activity suppressed by RIR were increased by adminisration of AS. NF-κB is a transcription factor that adjusts inflammatory molecule gene production, which has been shown to exert anti-inflammatory effects [32]. Reports indicated that inhibition of NF-κB had protection functions against RIR-stimulated lung inflammation [8]. Consequently, we deduced that AS represented anti-inflammatory functions by inhibiting NF-κB signaling pathway. In order to validate this hypothesis, the roles of AS on NF-κB activation were determined. Our results indicated that AS attenuated the activation and translocation of NF-κB. These data indicated that AS attenuated RIR-stimulated lung inflammation by inhibiting NF-κB signaling pathway.
Growing evidences showed that inflammatory molecules, such as NO, IL-6, MIP-2, and PGE2, had vital functions in the initiating and maintenance of lung inflammation [33]. In this research, elevated NO, IL-6, MIP-2, and PGE2 production were determined in RIR-induced ALI model. In addition, AS attenuated RIR-stimulated NO, IL-6, MIP-2, and PGE2 levels. NF-κB signaling pathway is in charge of processing and regulation of the HO-1 activity [34]. To clarify whether the lung protective action of AS is via the activation of HO-1 activity. The actions of AS on RIR-stimulated HO-1 activity were determined in this paper. Our results indicated that HO-1 activity enhanced evidently following RIR administration. AS enhanced RIR-stimulated HO-1 activity. Lately, it has been shown that HO-1 adjusted NF-κB phosphorylation in RIR-stimulated lung inflammation. Reports indicated that HO-1 activity was indispensable to the NF-κB phosphorylation [35]. To further elucidate the potential mechanism, the functions of AS on RIR-stimulated HO-1 activity were determined. The results demonstrated that AS mitigated HO-1 activity was stimulated by RIR. And the upregulation of AS on the HO-1 stimulated by RIR may result from the decreased expression of NF-κB phosphorylation.
An important limitation of the current study is the pre-treatment with AS. It would be more clinically relevant if administered after RIR. In addition, the in vivo nature of the findings was an important limitation of the current study. We will add cell mechanism studies in future experiments. In this paper, we are not trying to multiple doses of AS in vivo. Therefore, the results showed the inhibition effects of AS on RIR-induced ALI were not dose-dependent. Investigation on the multiple doses of AS in vivo is warranted in the future studies. The tolerance dose of AS may be different between humans and animals; thereby, the clinical use of AS for its anti-inflammatory property still needs further careful evaluation.
In summary, our studies indicated that AS serves as a novel approach to protect RIR-stimulated lung inflammation. AS confers protection against RIR-stimulated lung inflammation by up-regulating the HO-1 signal pathway and suppressing NF-κB phosphorylation.AS may be an important therapeutic strategy for mitigating RIR-induced ALI.
References
Li, W.F., K. Yang, P. Zhu, H.Q. Zhao, Y.H. Song, K.C. Liu, and W.F. Huang. 2017. Genistein ameliorates ischemia/reperfusion-induced renal injury in a SIRT1-dependent manner. Nutrients 9 (4): 403.
Seifi, B., M. Kadkhodaee, A. Najafi, and A. Mahmoudi. 2014. Protection of liver as a remote organ after renal ischemia-reperfusion injury by renal ischemic postconditioning. Int J Nephrol 2014: 120391.
Youssef, M.I., A.A. Mahmoud, and R.H. Abdelghany. 2015. A new combination of sitagliptin and furosemide protects against remote myocardial injury induced by renal ischemia/reperfusion in rats. Biochemical Pharmacology 96: 20–29.
Gu, J., J. Chen, P. Xia, G. Tao, H. Zhao, and D. Ma. 2011. Dexmedetomidine attenuates remote lung injury induced by renal ischemia-reperfusion in mice. Acta Anaesthesiologica Scandinavica 55: 1272–1278.
Meng, Q.T., C. Cao, Y. Wu, H.M. Liu, W. Li, Q. Sun, R. Chen, Y.G. Xiao, L.H. Tang, Y. Jiang, Y. Leng, S.Q. Lei, C.C. Lee, D.M. Barry, X. Chen, and Z.Y. Xia. 2016. Ischemic post-conditioning attenuates acute lung injury induced by intestinal ischemia-reperfusion in mice: Role of Nrf2. Laboratory Investigation 96: 1087–1104.
Ota, S., T. Yazawa, K. Tojo, Y. Baba, M. Uchiyama, T. Goto, and K. Kurahashi. 2016. Adrenaline aggravates lung injury caused by liver ischemia-reperfusion and high-tidal-volume ventilation in rats. J Intensive Care 4: 8.
Wu, S.Y., S.E. Tang, F.C. Ko, G.C. Wu, K.L. Huang, and S.J. Chu. 2015. Valproic acid attenuates acute lung injury induced by ischemia-reperfusion in rats. Anesthesiology 122: 1327–1337.
Mitaka, C., M.K. Si, M. Tulafu, Q. Yu, T. Uchida, S. Abe, M. Kitagawa, S. Ikeda, Y. Eishi, and M. Tomita. 2014. Effects of atrial natriuretic peptide on inter-organ crosstalk among the kidney, lung, and heart in a rat model of renal ischemia-reperfusion injury. Intensive Care Med Exp 2: 28.
Domenech, P., T. Perez, A. Saldarini, P. Uad, and C.G. Musso. 2017. Kidney-lung pathophysiological crosstalk: its characteristics and importance. International Urology and Nephrology 11: 1–5.
Tulafu, M., C. Mitaka, M.K. Hnin Si, S. Abe, M. Kitagawa, S. Ikeda, Y. Eishi, S. Kurata, and M. Tomita. 2014. Atrial natriuretic peptide attenuates kidney-lung crosstalk in kidney injury. The Journal of Surgical Research 186: 217–225.
Kao, M.C., C.H. Yang, W.C. Chou, J.R. Sheu, and C.J. Huang. 2015. Cepharanthine mitigates lung injury in lower limb ischemia-reperfusion. The Journal of Surgical Research 199: 647–656.
Gao, W., Y. Guo, and H. Yang. 2017. Platycodin D protects against cigarette smoke-induced lung inflammation in mice. International Immunopharmacology 47: 53–58.
Hsieh, Y.H., J.S. Deng, H.P. Pan, J.C. Liao, S.S. Huang, and G.J. Huang. 2017. Sclareol ameliorate lipopolysaccharide-induced acute lung injury through inhibition of MAPK and induction of HO-1 signaling. International Immunopharmacology 44: 16–25.
Li, J., D. Tong, J. Liu, F. Chen, and Y. Shen. 2016. Oroxylin A attenuates cigarette smoke-induced lung inflammation by activating Nrf2. International Immunopharmacology 40: 524–529.
Shen, B., C. Zhao, C. Chen, Z. Li, Y. Li, Y. Tian, and H. Feng. 2017. Picroside II protects rat lung and A549 cell against LPS-induced inflammation by the NF-kappaB pathway. Inflammation 1: 1–0.
Yao, H., Y. Sun, S. Song, Y. Qi, X. Tao, L. Xu, L. Yin, X. Han, Y. Xu, H. Li, H. Sun, and J. Peng. 2017. Protective effects of Dioscin against lipopolysaccharide-induced acute lung injury through inhibition of oxidative stress and inflammation. Frontiers in Pharmacology 8: 120.
Paine, A., B. Eiz-Vesper, R. Blasczyk, and S. Immenschuh. 2010. Signaling to heme oxygenase-1 and its anti-inflammatory therapeutic potential. Biochemical Pharmacology 80: 1895–1903.
Mitterstiller, A.M., D. Haschka, S. Dichtl, M. Nairz, E. Demetz, H. Talasz, M.P. Soares, E. Einwallner, H. Esterbauer, F.C. Fang, S. Geley, and G. Weiss. 2016. Heme oxygenase 1 controls early innate immune response of macrophages to Salmonella Typhimurium infection. Cellular Microbiology 18: 1374–1389.
Matsuda, A., W.L. Yang, A. Jacob, M. Aziz, S. Matsuo, T. Matsutani, E. Uchida, and P. Wang. 2014. FK866, a visfatin inhibitor, protects against acute lung injury after intestinal ischemia-reperfusion in mice via NF-kappaB pathway. Annals of Surgery 259: 1007–1017.
Mao, Y.F., X.F. Zheng, J.M. Cai, X.M. You, X.M. Deng, J.H. Zhang, L. Jiang, and X.J. Sun. 2009. Hydrogen-rich saline reduces lung injury induced by intestinal ischemia/reperfusion in rats. Biochemical and Biophysical Research Communications 381: 602–605.
Cao, T.H., S.G. Jin, D.S. Fei, K. Kang, L. Jiang, Z.Y. Lian, S.H. Pan, M.R. Zhao, and M.Y. Zhao. 2016. Artesunate protects against sepsis-induced lung injury via Heme Oxygenase-1 modulation. Inflammation 39: 651–662.
Cen, Y., C. Liu, X. Li, Z. Yan, M. Kuang, Y. Su, X. Pan, R. Qin, X. Liu, J. Zheng, and H. Zhou. 2016. Artesunate ameliorates severe acute pancreatitis (SAP) in rats by inhibiting expression of pro-inflammatory cytokines and Toll-like receptor 4. International Immunopharmacology 38: 252–260.
Lai, L., Y. Chen, X. Tian, X. Li, X. Zhang, J. Lei, Y. Bi, B. Fang, and X. Song. 2015. Artesunate alleviates hepatic fibrosis induced by multiple pathogenic factors and inflammation through the inhibition of LPS/TLR4/NF-kappaB signaling pathway in rats. European Journal of Pharmacology 765: 234–241.
Wang, D., J. Shi, S. Lv, W. Xu, J. Li, W. Ge, C. Xiao, D. Geng, and Y. Liu. 2015. Artesunate attenuates lipopolysaccharide-stimulated proinflammatory responses by suppressing TLR4, MyD88 expression, and NF-kappaB activation in microglial cells. Inflammation 38: 1925–1932.
Zhao, D., J. Zhang, G. Xu, and Q. Wang. 2017. Artesunate protects LPS-induced acute lung injury by inhibiting TLR4 expression and inducing Nrf2 activation. Inflammation 40 (3): 798–805.
Jiang, L., Y. Zhang, Y. Sun, L. Hu, and D. Gao. 2015. Artesunate attenuates lung injury in paraquat-intoxicated rats via downregulation of inflammatory cytokines. Clinical Laboratory 61: 1601–1607.
Ng, D.S., W. Liao, W.S. Tan, T.K. Chan, X.Y. Loh, and W.S. Wong. 2014. Anti-malarial drug artesunate protects against cigarette smoke-induced lung injury in mice. Phytomedicine 21: 1638–1644.
Zhao, D., J. Zhang, G. Xu, and Q. Wang. 2017. Artesunate protects LPS-induced acute lung injury by inhibiting TLR4 expression and inducing Nrf2 activation. Inflammation 40: 798–805.
Oztay, F., B. Kara-Kisla, N. Orhan, R. Yanardag, and S. Bolkent. 2016. The protective effects of prostaglandin E1 on lung injury following renal ischemia-reperfusion in rats. Toxicology and Industrial Health 32: 1684–1692.
Leung, W.S., M.L. Yang, S.S. Lee, C.W. Kuo, Y.C. Ho, R. Huang-Liu, H.W. Lin, and Y.H. Kuan. 2017. Protective effect of zerumbone reduces lipopolysaccharide-induced acute lung injury via antioxidative enzymes and Nrf2/HO-1 pathway. International Immunopharmacology 46: 194–200.
Shen, X., B. Hu, G. Xu, F. Chen, R. Ma, N. Zhang, J. Liu, X. Ma, J. Zhu, Y. Wu, and R. Shen. 2017. Activation of Nrf2/HO-1 pathway by glycogen synthase kinase-3beta inhibition attenuates renal ischemia/reperfusion injury in diabetic rats. Kidney & Blood Pressure Research 42: 369–378.
Wu, K.C., S.S. Huang, Y.H. Kuo, Y.L. Ho, C.S. Yang, Y.S. Chang, and G.J. Huang. 2017. Ugonin M, a Helminthostachys zeylanica constituent, prevents LPS-induced acute lung injury through TLR4-mediated MAPK and NF-kappaB signaling pathways. Molecules 22 (4): 573.
Y. Xu, and L. Liu. 2017. Curcumin alleviates macrophage activation and lung inflammation induced by influenza virus infection through inhibiting the NF-kappaB signaling pathway. Influenza and Other Respiratory Viruses 11(5): 457–463.
Lin, Q., X. Qin, M. Shi, Z. Qin, Y. Meng, and S. Guo. 2017. Schisandrin B inhibits LPS-induced inflammatory response in human umbilical vein endothelial cells by activating Nrf2. International Immunopharmacology 49: 142–147.
Liu, Y., M. Song, G. Zhu, X. Xi, K. Li, C. Wu, and L. Huang. 2017. Corynoline attenuates LPS-induced acute lung injury in mice by activating Nrf2. International Immunopharmacology 48: 96–101.
Authors’ Contributions
Zhaohui Liu: conception, design, analysis and interpretation of data; writing the manuscript. Junjie Zhang: conception, design, analysis and interpretation of data; writing the manuscript. Shitong Li: analysis and interpretation of data; writing the manuscript. Jihong Jiang: conception, design, analysis and interpretation of data; writing the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
All procedures were performed in accordance with the Declaration of Helsinki of the World Medical Association. The study was approved by the ethics committee of Shanghai First People’s Hospital, Shanghai, China.
Conflicts of Interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Liu, Z., Zhang, J., Li, S. et al. Artesunate Inhibits Renal Ischemia Reperfusion-Stimulated Lung Inflammation in Rats by Activating HO-1 Pathway. Inflammation 41, 114–121 (2018). https://doi.org/10.1007/s10753-017-0669-3
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10753-017-0669-3