Abstract
Continuous positive airway pressure (CPAP) is the standard treatment for obstructive sleep apnea syndrome (OSAS). However, the impact of CPAP on quality of life (QOL) is controversial. The aim of this study was to systematically review and determine whether CPAP improves QOL in patients with OSAS. We performed a comprehensive literature search to identify studies published between 1966 and 2007comparing values of CPAP with control. Weighted mean difference (WMD) was used to analyze the data. The pooled WMD was calculated by using a fixed or random-effect model. The outcomes for 1,256 patients from 16 studies, of whom 656 patients underwent CPAP and 600 were controls, were included. CPAP led to significant improvements in the Nottingham health profile part 2 (WMD = 1.657; 95% CI = 3.005, −0.308; p = 0.016), but there was no difference in other general QOL scores. Patients undergoing CPAP scored better in physical function (WMD = 3.457; 95% CI = 0.144, 6.771; p = 0.041), body pain (WMD = 4.017; 95% CI = −0.008, 8.042; p = 0.05), energy vitality (WMD = 6.984; 95% CI = 0.557, 13.411; p = 0.033) and physical component summary (PCS) (WMD = 2.040; 95% CI = 0.045, 4.035; p = 0.045) using the SF-36 tool. This meta-analysis shows that CPAP does not improve general QOL scores but does improve physical domains and vitality. Study design and QOL questionnaire tools are important to capture and evaluate information efficiently. However, generic QOL instruments may not be adequate in detecting important changes in quality of life in patients with OSAS.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Obstructive sleep apnea affects nearly one in four men and one in ten women between the ages of 30 and 60 years in the United States [1]. OSAS (obstructive sleep apnea syndrome) is present when the number of apneic and hypopneic episodes, longer than 10 s, per hour of sleep (referred to as the apnea-hypopnea index, AHI) is five or more and the patient has excessive daytime sleepiness [2, 3]. Health consequences that may result from chronic sleep disruption or recurrent hypoxemia include neuropsychiatric and cardiovascular sequelae. Neuropsychiatric effects may include depression and cognitive dysfunction that can disrupt professional, family, and social life and increase risks for automobile and industrial accidents. Cardiovascular sequelae can include pulmonary and systemic hypertension, congestive heart failure, arrhythmia, myocardial infarction, and stroke [4–6].
The standard treatment for OSAS is continuous positive airway pressure (CPAP); it has become the treatment of first choice for patients with substantial disease. The effect of CPAP on quality of life (QOL) is unclear [7, 8]. The present meta-analysis reviewed the available QOL evidence from CPAP randomized controlled trials to summarize the best evidence of the effect of CPAP in the treatment of OSAS on QOL.
Methods
Search Strategy and Selection Criteria
Randomized controlled clinical trials were identified via MEDLINE (source PubMed, 1966 to June 2007), EMBASE (1966 to June 2007), the Cochrane Controlled Clinical Trials Register Database (through 2nd quarter 2007), and the ClinicalTrials.gov website. All searches included the keywords and corresponding MeSH terms for Sleep Apnea Syndromes, “Sleep Apnea, Obstructive,” “quality of life,” “Continuous Positive Airway Pressure,” and “randomized controlled trial.” Manual reference checking of the bibliographies of all retrieved articles was also done.
Data Extraction
Data extraction was conducted independently by the two reviewers (J.Y. Jing and T.C. Huang). The following information was extracted from each study: first author, year of publication, study population characteristics, study design (parallel or pilot, crossover study), inclusion and exclusion criteria, number of subjects in each group [CPAP and control treatment including conservative treatment (CT), sham CPAP, oral placebo], quality of study, QOL tool used, domains of QOL, treatment duration, and severity of OSAS.
Inclusion Criteria
Prospective randomized controlled trials that were done in adults and published in English were considered for inclusion in this meta-analysis. The following criteria were used to select studies for analysis: (1) studies comparing CPAP treatment with control treatment, and (2) studies that used validated tools for QOL measurements.
Exclusion Criteria
Studies were excluded from the analysis for the following reasons: (1) outcomes of comparison were not reported or it was not possible to extract the data from the published results; (2) the study that did not use validated tools for QOL measures; and (3) more than one article reported outcomes on the same patient group (in that case either the more recent article or the one of higher quality was included).
Measures of Outcomes of Interest
The tools identified as providing validated measures of QOL following OSAS were Short Form 36 (SF-36), Nottingham Health Profile (NHF), Functional Outcomes of Sleep Questionnaire (FOSQ), European Quality of Life Questionnaire (EuroQOL), and Sleep Apnea Quality of Life Index (SAQLI) [9]. All five systems measure QOL within a range of domains and provide an overall indication of QOL.
SF-36
SF-36 was developed in the United States and is a generic measure of health status and can be used to measure health outcomes of clinical interventions [10]. It has been validated and tested for use in 13 countries [11]. The scoring method for SF-36 uses an algorithm to transform dichotomous and continuous variables into a scale from 0 to 100, with higher scores indicating best possible health.
NHP
The NHP is another generic health-related QOL measure, widely used in Europe. It was designed to reflect the perceived effects of ill health on everyday life from the perspective of a layperson rather than that of the health professional. Part 1 includes 38 yes/no items in six domains: physical abilities, pain, sleep, social isolation, emotional reactions, and energy level. Part 2 includes seven aspects of life affected by health: occupation, ability to do jobs around the house, social life, home relationships, sexual life, hobbies, and holidays [12, 13].
FOSQ
The FOSQ is a sleep-specific questionnaire developed to reflect the impact of sleep disorders and excessive sleepiness on activities of daily life. FOSQ contains 30 items divided into five scales: activity level, vigilance, intimacy and sexual relationships, general productivity, and social outcome. Scale scores were added to compute a global score ranging from 0 (maximal dysfunction) to 120 [14].
EuroQOL
The EuroQOL has been developed with cross-cultural applications in mind. The instrument was developed by the international European Quality of Life Group, which has since grown to include members from the United States, Canada, and Japan. The EuroQOL covers five dimensions of health: mobility, self-care, usual activities, pain/discomfort, and anxiety/depression.
SAQLI
The SAQLI was designed as a comprehensive health-related QOL measure for use in clinical trials with patients experiencing sleep apnea. It was based on broad-based input from sleep apnea patients and their partners as well as expert clinicians and the research literature. The first 35 questions measure four domains: daily function, social interactions, emotional functioning, and symptoms. The fifth domain on treatment-related symptoms is a unique feature, capturing the potential negative QOL impact of a treatment’s side effects [15, 16].
Quality Assessment
The quality of each fully published trial was assessed by means of an established standard of methodologic quality [17, 18]. The quality of each study was evaluated by examining patient selection methods, comparability of the study groups, and assessment of outcome. Total methodologic quality scores were then used to rank the studies. Studies given six or more stars were considered to be of high quality. Methodologic quality assessment was independently performed by two independent reviewers (J.Y. Jing and W. Cui).
Statistical Analysis
The meta-analysis was performed in line with recommendations from the Cochrane Collaboration and the Quality of Reporting of Meta-analyses (QUORUM) guidelines [19, 20]. Statistical analyses of continuous variables such as domain outcome for the QOL scores were analyzed using the weighted mean difference (WMD) and were reported with 95% confidence intervals (CI). The WMD summarizes the differences between the two groups with respect to continuous variables, accounting for sample size. For studies that presented continuous data as means and range values, the standard deviations (SD) were calculated using statistical algorithms and checked by using “bootstrap” resampling techniques. Thus, all continuous data were standardized for analysis. In the tabulation of results, squares indicate the point estimates of the effect of disease (WMD), with 95% confidence intervals indicated by horizontal bars. The diamond represents the summary estimate from pooled studies with 95% confidence intervals.
We used the χ2 and Fisher exact tests to detect significant statistical heterogeneity. Heterogeneity was assessed using two methods. First, graphical exploration with funnel plots and the Egger test were used to evaluate publication bias [21]. Second, sensitivity analysis was undertaken for each of these groups of data. We also analyzed the effects of other covariates on the QOL (i.e., quality score, control type, time of treatment, study design, severity of OSAS ). Meta-regression analyses were conducted to reveal potential sources of heterogeneity. The covariates in the regression analyses included study duration, quality score, mean AHI, and mean ESS. Analysis was conducted using STATA v8.2 (StataCorp., College Station, TX).
Literature Search
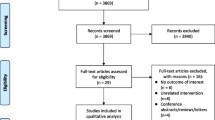
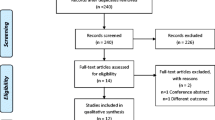
Of the original 237 studies identified, 216 were excluded because they did not compare QOL between CPAP and control treatment. We carefully read the full text of remaining 21 studies. Only 16 studies remained, published between 1994 and 2007, that matched the selection criteria and were included in this meta-analysis [7, 22–37]. Analysis was performed on a total of 1256 patients, which included 656 CPAP patients and 600 control patients. The characteristics of these studies are listed in Table 1.
Meta-analysis
General QOL Score
Seven studies gave a general (or global) health score for FOSQ (including 4 [28, 29, 31, 34] for absolute scores and 3 [7, 27, 35] for total score), five [7, 22–25] for NHP part 2 (energy domain only), one [27] for SF-36, one [30] for EuroQOL, and one [36] for SAQLI (Table 2). CPAP led to significant improvements in the Nottingham health profile part 2 (WMD = 1.657; 95% CI = −3.005, −0.308; p = 0.016) and in SAQLI (WMD = 0.900; 95% CI = 0.625, 1.175; p = 0.000), but there was no difference in general QOL scores when using other measurement tools. The general QOL score expresses the patient’s personal health evaluation and is not a cumulative total of other domain scores.
Individual Domains and Subgroup Analysis
In eight studies [25, 28, 32–37], the SF-36 was used (Table 3). Patients undergoing CPAP scored better in physical function (WMD = 3.457; 95% CI = 0.144, 6.771; p = 0.041), body pain (WMD = 4.017; 95% CI = −0.008, 8.042; p = 0.05), energy vitality (WMD = 6.984; 95% CI = 0.557, 13.411; p = 0.033), and physical component score (PCS) (WMD = 2.040; 95% CI = 0.045, 4.035; p = 0.045) using the SF-36 tool.
In stratified analyses, the following were found: In parallel studies, physical function (WMD = 7.612; 95% CI = 2.573; 12.65; p = 0.003; four studies) physical problems (WMD = 13.906; 95% CI = 5.413, 22.40; p = 0.001; four studies), body pain (WMD = 7.174; 95% CI = 2.099 = 12.249; p = 0.006; four studies), general health (WMD = 5.671; 95% CI = 0.729, 10.614; p = 0.025; four studies), mental health (WMD = 5.659; 95% CI = 1.371, 9.946; p = 0.01; four studies), and PCS (WMD = 2.237; 95% CI 0.178, 4.296; p = 0.033; three studies), components of SF-36, indicated consistent and significant improvements in health status in favor of CPAP. There was no difference in all domains in crossover studies. There was significant statistical heterogeneity between different study designs for physical function, body pain, vitality, physical problems, emotional problems, and mental health domains (Table 3, Fig. 1, 2). In long-term treatment studies, a significant improvement was demonstrated in physical function (WMD = 6.706; 95% CI = 2.573, 12.65; p = 0.027; three studies).
Meta-regression analyses showed that Epworth Sleepiness Scale (ESS), AHI, treatment duration, and study quality score were all not the sources of heterogeneity in most of the SF-36 domain. Only study design and AHI were the sources of heterogeneity in physical problems and body pain domain (Table 4).
Using the FOSQ tool, domain scores did not show differences between the CPAP group and the control group in four studies [28, 31, 34, 35]. There also was no significant statistical heterogeneity between subgroups (Table 5).
Ballester [26] reported no difference using NHP. Lam [36] reported CPAP patients had a significantly better score in the symptoms, emotional, and daily function than the control patients using the SAQLI (Table 6). Further studies using these tools could help to derive a more reliable overview.
Discussion
According to Schipper et al. [38, 39], “Quality of life (QOL) in clinical medicine represents the functional effect of an illness and its consequent therapy upon a patient, as perceived by the patient.” Improving the quality of life of the OSAS patient is one of the main targets of treatment [40–44]. When comparing CPAP treatment with control, there was no difference in general health perception and total QOL score, except for Nottingham health profile part 2 (energy domain only).
In individual domains, the results of the present meta-analysis found that there was no difference between CPAP and CT or placebo in improving emotional function, MCS, or mental health domain. However, CPAP improves physical function, energy vitality, and PCS domains of the SF-36. Pichel et al. found that the QOL score and symptom improvement of long-term treatment were better than short-term one [7, 44], and there was significant statistical heterogeneity in physical function improvement between study designs. The decrease in physical function was influenced by objective indices of sleep discontinuity and subjective sleepiness. The physical function and the physical role were related to nocturnal parameters indicating sleep disruption, i.e., amount of stage 1 and slow wave sleep, with additional influence from indices of daytime sleepiness and BMI [45]. Vitality improvement also could be found using NHP part 2 and was not related with control types or the length of treatment period. There was significant statistical heterogeneity in improvement of energy vitality and severity of OSAS between study designs. Hypoxemia, AHI, sleep disruption, and sleep fragmentation appeared to have an impact on physical function and energy vitality.
The improvement in physical scores, especially energy vitality, with CPAP is consistent with studies demonstrating significant improvements in sleepiness with CPAP.
The present meta-analysis facilitated the aggregation of data from a variety of sources using standardized QOL assessment tools, the results of which provided greater statistical power to detect significant differences, with subsequent sensitivity analysis demonstrating the robustness of the pooled analysis. The heterogeneity of the studies was analyzed and the results can be seen in Tables 2–5 and in the funnel plot in Figure 2. There was significant heterogeneity in some of the outcomes of the overall analysis. Sensitivity and publication bias were analyzed and the results are shown in the funnel plots in Figures 3 and 4. The results show that the design of the study affects the result of QOL significantly, as does the control type. However, ESS, the severity of OSAS, the quality of score, and the duration of treatment do not affect the QOL scores, except that the physical function of SF-36 indicated significant improvements in long-term trials but not in short-term ones and mean AHI was the source of heterogeneity in physical problems and body pain.
The influence of individual studies on the summary WMD. The vertical axis at 3.46 indicates the overall WMD and the two vertical axes at 0.14 and 6.77 indicate its 95% confidence interval (CI). Every open circle indicates the pooled WMD when the left study was omitted in a meta-analysis with a random model. The two ends of every broken line represent the respective 95% CI
Funnel graph for the assessment of potential publication bias in physical function for CPAP compared with control. This is a scatterplot of the incidence estimated from individual studies plotted on the horizontal line (SE of the WMD) and the vertical axis (WMD). The result of the Egger test for publication bias was not significant (p = 0.805)
Study design is important in understanding the relationship between patient characteristics and adherence [46]. The crossover study design reduces the efficiency of capturing the QOL effects of CPAP because the washout period is too short to eliminate the effects of pretreatment. In the included studies [22–25, 28, 29, 31, 34, 37] the washout period ranged from 0 to 2 weeks. Long-term parallel-group trials may be more efficient at capturing the important information regarding the persistence of benefits from CPAP treatment, convenience of continued usage, loss to follow-up, and cardiovascular outcome of CPAP treatment.
Control types include CT, oral placebo, and sham CPAP. The selection of control type is controversial. Engleman et al. [22–25] deemed sham CPAP therapy is not possible because a nasal mask without effective CPAP would make both sleep and gas exchange worse. However, the meta-analysis results do not demonstrate that.
The present meta-analysis results demonstrate that there are some different results among QOL questionnaire tools. As a generic measure, SF-36 does not include questions specific for OSAS. The vitality dimension is the closest proxy for sleep-related disturbances [47]. Thus, SF-36 may successfully discriminate between patients with and without OSAS and be sensitive to treatment-induced changes, but it should be accompanied by an OSAS-specific instrument if the researcher is interested in more besides the eight dimensions and two subscales included in SF-36. For specific health-related QOL instruments, preliminary evidence suggests that the SAQLI and the FOSQ are both potentially useful [9].
Although generic questionnaires are designed to measure all important aspects of QOL, they are less likely to detect change in QOL than a disease-specific questionnaire that focuses on specific areas of QOL [48]. Such a disease-specific questionnaire is clearly needed in OSAS research [49], but the number of randomized controlled trial studies that used the disease-specific questionnaire such as SAQLI was small, so further studies are needed to confirm.
In conclusion, when comparing CPAP with control treatment, our meta-analysis shows little impact of CPAP on general QOL. However, CPAP improves physical domains and vitality. Study designs and validated QOL questionnaire tools are important to capture and evaluate information efficiently. However, generic QOL instruments may not be adequate to detect important changes in the quality of life in patients with OSAS. Future randomized controlled trials in this area should concentrate on a disease-specific questionnaire or on large long-term parallel-group trials.
References
Young T, Palta M, Dempsey J, Skatrud J, Weber S, Badr S (1993) The occurrence of sleep-disordered breathing among middle-aged adults. N Engl J Med 328:1230–1235
Deegan PC, McNicholas WT (1996) Predictive value of clinical features for the obstructive sleep apnoea syndrome. Eur Respir J 9:117–124
White DP (1995) Sleep-related breathing disorders. 2. Pathophysiology of obstructive sleep apnoea. Thorax 50:797–804
Ferguson KA, Fleetham JA (1995) Sleep-related breathing disorders. 4. Consequences of sleep disordered breathing. Thorax 50:998–1004
Peter JH, Koehler U, Grote L, Podszus T (1995) Manifestations and consequences of obstructive sleep apnoea. Eur Respir J 8:1572–1583
Flemons WW (2000) Measuring health related quality of life in sleep apnea. Sleep 23(Suppl 4):S109–S114
Monasterio C, Vidal S, Duran J, Ferrer M, Carmona C, Barbe F, Mayos M, Gonzalez-Mangado N, Juncadella M, Navarro A, Barreira R, Capote F, Mayoralas LR, Peces-Barba G, Alonso J, Montserrat JM (2001) Effectiveness of continuous positive airway pressure in mild sleep apnea-hypopnea syndrome. Am J Respir Crit Care Med 164:939–943
Profant J, Ancoli-Israel S, Dimsdale JE (2003) A randomized, controlled trial of 1 week of continuous positive airway pressure treatment on quality of life. Heart Lung 32:52–58
Moyer CA, Sonnad SS, Garetz SL, Helman JI, Chervin RD (2001) Quality of life in obstructive sleep apnea: a systematic review of the literature. Sleep Med 2:477–491
Bowling A, Bond M, Jenkinson C, Lamping DL (1999) Short Form 36 (SF-36) Health Survey questionnaire: which normative data should be used? Comparisons between the norms provided by the Omnibus Survey in Britain, the Health Survey for England and the Oxford Healthy Life Survey. J Public Health Med 21:255–270
Gandek B, Ware JE Jr (1998) Methods for validating and norming translations of health status questionnaires: the IQOLA Project approach. International quality of life assessment. J Clin Epidemiol 51:953–959
Hunt SM, McKenna SP, McEwen J, Williams J, Papp E (1981) The Nottingham health profile: subjective health status and medical consultations. Soc Sci Med A 15:221–229
Alonso J, Anto JM, Moreno C (1990) Spanish version of the Nottingham health profile: translation and preliminary validity. Am J Public Health 80:704–708
Weaver TE, Laizner AM, Evans LK, Maislin G, Chugh DK, Lyon K, Smith PL, Schwartz AR, Redline S, Pack AI, Dinges DF (1997) An instrument to measure functional status outcomes for disorders of excessive sleepiness. Sleep 20:835–843
Flemons WW, Reimer MA (1998) Development of a disease-specific health-related quality of life questionnaire for sleep apnea. Am J Respir Crit Care Med 158:494–503
Flemons WW, Tsai W (1997) Quality of life consequences of sleep-disordered breathing. J Allergy Clin Immunol 99:S750–S756
Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJ, Gavaghan DJ, McQuay HJ (1996) Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials 17:1–12
Banares R, Albillos A, Rincon D, Alonso S, Gonzalez M, Ruiz-del-Arbol L, Salcedo M, Molinero LM (2002) Endoscopic treatment versus endoscopic plus pharmacologic treatment for acute variceal bleeding: a meta-analysis. Hepatology 35:609–615
Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, Moher D, Becker BJ, Sipe TA, Thacker SB (2000) Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis of observational studies in epidemiology (MOOSE) group. JAMA 283:2008–2012
Clarke M, Horton R (2001) Bringing it all together: Lancet-Cochrane collaborate on systematic reviews. Lancet 357:1728
Egger M, Davey Smith G, Schneider M, Minder C (1997) Bias in meta-analysis detected by a simple, graphical test. BMJ 315:629–634
Engleman HM, Martin SE, Deary IJ, Douglas NJ (1994) Effect of continuous positive airway pressure treatment on daytime function in sleep apnoea/hypopnoea syndrome. Lancet 343:572–575
Engleman HM, Martin SE, Deary IJ, Douglas NJ (1997) Effect of CPAP therapy on daytime function in patients with mild sleep apnoea/hypopnoea syndrome. Thorax 52:114–119
Engleman HM, Martin SE, Kingshott RN, Mackay TW, Deary IJ, Douglas NJ (1998) Randomised placebo controlled trial of daytime function after continuous positive airway pressure (CPAP) therapy for the sleep apnoea/hypopnoea syndrome. Thorax 53:341–345
Engleman HM, Kingshott RN, Wraith PK, Mackay TW, Deary IJ, Douglas NJ (1999) Randomized placebo-controlled crossover trial of continuous positive airway pressure for mild sleep apnea/hypopnea syndrome. Am J Respir Crit Care Med 159:461–467
Ballester E, Badia JR, Hernandez L, Carrasco E, de Pablo J, Fornas C, Rodriguez-Roisin R, Montserrat JM (1999) Evidence of the effectiveness of continuous positive airway pressure in the treatment of sleep apnea/hypopnea syndrome. Am J Respir Crit Care Med 159:495–501
Barbe F, Mayoralas LR, Duran J, Masa JF, Maimo A, Montserrat JM, Monasterio C, Bosch M, Ladaria A, Rubio M, Rubio R, Medinas M, Hernandez L, Vidal S, Douglas NJ, Agusti AG (2001) Treatment with continuous positive airway pressure is not effective in patients with sleep apnea but no daytime sleepiness. a randomized, controlled trial. Ann Intern Med 134:1015–1023
Barnes M, Houston D, Worsnop CJ, Neill AM, Mykytyn IJ, Kay A, Trinder J, Saunders NA, Douglas McEvoy R, Pierce RJ (2002) A randomized controlled trial of continuous positive airway pressure in mild obstructive sleep apnea. Am J Respir Crit Care Med 165:773–780
Barnes M, McEvoy RD, Banks S, Tarquinio N, Murray CG, Vowles N, Pierce RJ (2004) Efficacy of positive airway pressure and oral appliance in mild to moderate obstructive sleep apnea. Am J Respir Crit Care Med 170:656–664
Chakravorty I, Cayton RM, Szczepura A (2002) Health utilities in evaluating intervention in the sleep apnoea/hypopnoea syndrome. Eur Respir J 20:1233–1238
Faccenda JF, Mackay TW, Boon NA, Douglas NJ (2001) Randomized placebo-controlled trial of continuous positive airway pressure on blood pressure in the sleep apnea-hypopnea syndrome. Am J Respir Crit Care Med 163:344–348
Jenkinson C, Davies RJ, Mullins R, Stradling JR (1999) Comparison of therapeutic and subtherapeutic nasal continuous positive airway pressure for obstructive sleep apnoea: a randomised prospective parallel trial. Lancet 353:2100–2105
Mansfield DR, Gollogly NC, Kaye DM, Richardson M, Bergin P, Naughton MT (2004) Controlled trial of continuous positive airway pressure in obstructive sleep apnea and heart failure. Am J Respir Crit Care Med 169:361–366
Marshall NS, Neill AM, Campbell AJ, Sheppard DS (2005) Randomised controlled crossover trial of humidified continuous positive airway pressure in mild obstructive sleep apnoea. Thorax 60:427–432
Montserrat JM, Ferrer M, Hernandez L, Farre R, Vilagut G, Navajas D, Badia JR, Carrasco E, De Pablo J, Ballester E (2001) Effectiveness of CPAP treatment in daytime function in sleep apnea syndrome: a randomized controlled study with an optimized placebo. Am J Respir Crit Care Med 164:608–613
Lam B, Sam K, Mok WY, Cheung MT, Fong DY, Lam JC, Lam DC, Yam LY, Lp MS (2007) Randomised study of three non-surgical treatments in mild to moderate obstructive sleep apnoea. Thorax 62:354–359
Smith LA, Vennelle M, Gardner RS, McDonagh TA, Denvir MA, Douglas NJ, Newby DE (2007) Auto-titrating continuous positive airway pressure therapy in patients with chronic heart failure and obstructive sleep apnoea: a randomized placebo-controlled trial. Eur Heart J 28:1221–1227
Schipper H (1983) Why measure quality of life? Can Med Assoc J 128:1367–1370
Schipper H (1992) Quality of life: the final common pathway. J Palliat Care 8:5–7
Kawahara S, Akashiba T, Akahoshi T, Horie T (2005) Nasal CPAP improves the quality of life and lessens the depressive symptoms in patients with obstructive sleep apnea syndrome. Intern Med 44:422–427
Sin DD, Mayers I, Man GC, Ghahary A, Pawluk L (2002) Can continuous positive airway pressure therapy improve the general health status of patients with obstructive sleep apnea? a clinical effectiveness study. Chest 122:1679–1685
Blondet MC, Perez J, Rodriguez W (2001) Continuous positive airway pressure and obstructive sleep apnea in an Hispanic population. Sleep Breath 5:109–114
Guilleminault C, Lin CM, Goncalves MA, Ramos E (2004) A prospective study of nocturia and the quality of life of elderly patients with obstructive sleep apnea or sleep onset insomnia. J Psychosom Res 56:511–515
Pichel F, Zamarron C, Magan F, del Campo F, Alvarez-Sala R, Suarez JR (2004) Health-related quality of life in patients with obstructive sleep apnea: effects of long-term positive airway pressure treatment. Respir Med 98:968–976
Sforza E, Janssens JP, Rochat T, Ibanez V (2003) Determinants of altered quality of life in patients with sleep-related breathing disorders. Eur Respir J 21:682–687
Giles TL, Lasserson TJ, Smith BJ, White J, Wright J, Cates CJ (2007) Continuous positive airways pressure for obstructive sleep apnoea in adults. Cochrane Database Syst Rev 1:CD001106 [Review]
Jenkinson C, Stradling J, Petersen S (1997) Comparison of three measures of quality of life outcome in the evaluation of continuous positive airways pressure therapy for sleep apnoea. J Sleep Res 6:199–204
Guyatt GH, Feeny DH, Patrick DL (1993) Measuring health-related quality of life. Ann Intern Med 118:622–629
American Thoracic Society/American Sleep Disorders Association (1998) Statement on health outcomes research in sleep apnea. Am J Respir Crit Care Med 157:335–341
Author information
Authors and Affiliations
Corresponding author
Additional information
Jiyong Jing and Tiancha Huang contributed equally to this work.
Rights and permissions
About this article
Cite this article
Jing, J., Huang, T., Cui, W. et al. Effect on Quality of Life of Continuous Positive Airway Pressure in Patients with Obstructive Sleep Apnea Syndrome: A Meta-analysis. Lung 186, 131–144 (2008). https://doi.org/10.1007/s00408-008-9079-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00408-008-9079-5