Abstract
Decision making and cognitive flexibility are two components of cognitive control that play a critical role in the emergence, persistence, and relapse of gambling disorder. Transcranial direct current stimulation (tDCS) over the dorsolateral prefrontal cortex (DLPFC) has been reported to enhance decision making and cognitive flexibility in healthy volunteers and individuals with addictive disorders. In this triple-blind randomized sham-controlled parallel study, we aimed to determine whether tDCS over DLPFC would modulate decision making and cognitive flexibility in individuals with gambling disorder. Twenty participants with gambling disorder were administered Iowa Gambling Task (IGT) and Wisconsin Card Sorting Test (WCST). Subsequently, participants were administered three every other day sessions of active right anodal /left cathodal tDCS (20 min, 2 mA) or sham stimulation over bilateral DLPFC. WCST and IGT were readministered following the last session. Baseline clinical severity, depression, impulsivity levels, and cognitive performance were similar between groups. TDCS over the DLPFC resulted in more advantageous decision making (F1,16 = 8.128, p = 0.01, ɳp2 =0.33) and better cognitive flexibility (F1,16 =8.782, p = 0.009, ɳp2 = 0.35), representing large effect sizes. The results suggest for the first time that tDCS enhanced decision making and cognitive flexibility in gambling disorder. Therefore, tDCS may be a promising neuromodulation-based therapeutic approach in gambling disorder.
Trial registration: Clinicaltrials.gov NCT03477799.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Gambling disorder (GD) is defined as a progressive and chronic maladaptive disorder characterized by the failure to control gambling activities despite various aversive outcomes [1]. Among the different diagnostic criteria to define GD, cognitive control over the urge to be involved in gambling activities is considered to be a core feature [1]. Cognitive control requires a complex integration of higher order processes to generate goal-directed flexible and adaptive behavioral responses. Thus, it has been conceptualized as the sum of four components, namely decision making, cognitive flexibility, response inhibition, and conflict monitoring [2]. Decision making and cognitive flexibility are of particular importance in GD as they have a critical role in the emergence and persistence of the disease and are associated with disease severity, gambling frequency, gambling urge intensity, dropout, and relapse rates [3,4,5,6,7].
Decision making is a component of cognitive control that plays a central role in the evaluation of desires and intentions within the context of past experiences and knowledge to modulate behavioral responses in a goal-directed manner [8]. Despite being involved in the neural basis of neuropsychiatric disorders [9, 10], impairment of decision making has particular importance in addictive disorders, as they are characterized by the persistence of the drug use or the addictive behavior, despite multiple undesired consequences [11]. To support this notion, a wealth of literature illustrates that addictive disorders have been reliably associated with deficits of decision making [12,13,14] and cognitive flexibility [14, 15] resulting in the prevention of treatment seeking, passing of time, and worsening of the illness in the addictive disorders [16, 17]. Considering the clinical and neurophysiological similarity [18], it is not surprising that deficits of decision making [5] and cognitive flexibility have also been found in GD [19,20,21], even at a higher level than substance use disorders in a recent study [22]. Moreover, GD might be a pure illustration of pathological decision making than in the absence of exogenous drug effects [23], thus providing an opportunity to examine decision making clearly. Decision making has been commonly assessed with the Iowa Gambling Task (IGT) in GD and IGT is an ecologically valid measure of decision making [5]. Thus, the increment of IGT net scores have been found to be correlated with better decision making in real-life [5]. Moreover, the IGT performance has been found to be correlated with problem gambling severity [24]. The neural substrates of IGT involve different brain areas including the Ventromedial Prefrontal Cortex (VMPFC) and Dorsolateral Prefrontal Cortex (DLPFC) along with dorsal anterior cingulate cortex, insular cortex, and parietal areas [25]. Among them, right DLPFC has been considered to play the general role in the top-down regulation of decision making [26]. Similarly, subjects with GD were reported to have decreased functional Magnetic Resonance Imaging (fMRI) activity in the right VMPFC and DLPFC during deck selection in IGT, as opposed to baseline [27].
Cognitive flexibility is another component of cognitive control [2], responsible for adapting to changing environments [28]. Cognitive flexibility deficits have been shown in addictive disorders [29] and GD [30, 31] and linked to clinical outcomes [7, 29]. Cognitive flexibility has also been reported to be correlated with decision making in healthy subjects, in individuals with HIV-associated neurocognitive disorders and gambling disorder [5, 32, 33]. Similarly, individuals with high cognitive flexibility have also been reported to have better IGT performance [34]. Cognitive flexibility has been measured with the Wisconsin Card Sorting Task (WCST) in GD [30, 31]. In respect of the neural substrates of the WCST, various brain areas have been implicated, including bilateral lateral PFC, anterior cingulate cortex, and inferior parietal lobule [35, 36] indicating a role for both hemispheres. In subjects with GD, a decreased fMRI activation of the right ventrolateral prefrontal cortex was shown during reversal shifting and after monetary losses or gains [37].
Transcranial direct current stimulation (tDCS) is a safe and well-tolerated noninvasive brain stimulation technique [38] that modulates neuroplasticity by applying weak electrical currents passed between two scalp electrodes placed over target cortical locations. Quite a few studies suggest that tDCS over the DLPFC has been reported to enhance decision making [39,40,41,42,43] while different results were achieved in older adults [44]. In addition, participants who received cathodal stimulation over the left DLPFC have been reported to have better cognitive flexibility [45, 46]. Neurostimulation has also been suggested to potentially be beneficial in GD [37] and nascent literature has begun to provide preliminary evidence of this notion [47,48,49]. However, the primary outcomes from these studies did not include measures of decision making and cognitive flexibility. Regarding tDCS, a study applying tDCS in GD showed relationships between prefrontal metabolite levels and levels of risk-taking, impulsivity, and craving, lending support to the potential beneficial effects of tDCS [50]. Thus far, the effect of tDCS on cognitive functions in GD has not been reported yet.
Following the line of research illustrating the enhancing effect of tDCS on decision making and cognitive flexibility, we hypothesized that anodal tDCS over the right DLPFC (coupled with contralateral cathodal stimulation), in comparison to sham, would enhance both decision making and cognitive flexibility in GD. We also evaluated the relationship between decision making and cognitive flexibility in GD.
Materials and methods
Participants
Sample size was calculated using G*Power 3.1.9.2 (Effect size f: 0.6, ɑ: 0.05, Power 0.8, Number of groups: 2. Number of Measurements: 2) for a two-way repeated-measures ANOVA. The total sample size needed was 20. Thus, 20 male right-handed participants satisfying Diagnostic and Statistical Manual of Mental Disorders-5 (DSM-5) criteria for gambling disorder were recruited from the Addiction Outpatient Clinic of the Department of Psychiatry in Istanbul University. No female participants were included due to non-admission to our clinic. Participants were required to be aged between 18 and 65 and to be drug-free. The exclusion criteria were the following: current DSM-5 diagnosis of major depressive disorder, current or previous DSM-5 diagnosis of alcohol and substance use disorders including tobacco use disorder, schizophrenia, bipolar disorder, or other psychotic disorder, use in the past 4 weeks of any medication with known pro-convulsant action or current regular use of any psychotropic medications, any history of any clinically significant neurological disorder, the presence of mental retardation diagnosis (previously identified), any personal or family history (1st degree relatives) of seizures other than febrile childhood seizures, illiteracy, deficient language or refusal to participate. No significant comorbidity was observed. Only one participant had a past history of a major depressive episode and psychotropic drug use.
Baseline clinical gambling severity, depression, and impulsivity were assessed with South Oaks Gambling Screen [SOGS] [51], Canadian Pathological Gambling Severity Index [PGSI] [52], Beck Depression Inventory [BDI] [53], and Barratt Impulsivity Scale-11 [BIS-11] [54].
All participants attended to the procedures in the study and none were excluded after enrollment. The study protocol was approved by both the Institutional Ethical Committee and Turkish Ministry of Health Ethical Committee. The study protocol strictly complied with the Declaration of Helsinki. Written informed consent was obtained from all participants after the details of the procedures had been fully explained.
Experimental design
We conducted a triple-blind randomized sham-controlled parallel study. Participants, interventionist, and the assessor were blinded to the condition. Upon enrollment into the study, participants were randomly assigned with a ratio of 1 to 1 to one of two conditions to receive three every other day 20-min 2 mA sessions of either (i) active anodal right/cathodal left (n = 10) or (ii) sham anodal right/cathodal left (n = 10) tDCS over the DLPFC. The participants were administered the IGT and the Wisconsin Card Sorting Test (WCST) by a trained neuropsychologist in a quiet laboratory. A computerized version of standard IGT was used. The order of the tasks performed in a single session was randomized.
After the psychiatric and neurocognitive assessment, participants received three every other day sessions of 20-min active tDCS or sham tDCS. Predictions of the participants about the stimulation type (active or sham) were recorded to assess the integrity of the blinding. Safety was assessed through open-ended questions based on the tDCS adverse events questionnaire [55]. WCST and IGT were readministered after the last application.
Intervention
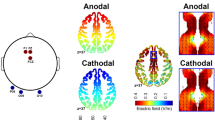
Two 5 × 7 cm electrodes saturated with 0.9% saline solution-soaked sponges were placed using elastic straps [56]. The anode was placed over area F4 and cathode was placed over area F3 using the 10/20 international system. The stimulation was delivered using a battery-driven constant current stimulator (Neuroconn DC-STIMULATOR Plus, neuroConn GmbH, Ilmenau, Germany). Active stimulation involved delivery of 2 mA of direct current for 20 min with a 30-s ramp up and down of current. To deliver sham stimulation, the procedure was identical, except that the current was delivered at 2 mA for 30 s with a 30-s ramp up and ramp down, thus leading to similar initial sensations of tDCS. This method of sham stimulation has been reported to be a reliable application [57]. Predefined codes of the stimulator were used to accomplish the blinding of the investigator and the participant.
Neurocognitive tasks
Iowa gambling task
In the Turkish adaptation of computerized IGT [58], participants are loaned 2000 Turkish Liras of “play money”. They choose cards from four decks of cards, identical in appearance, to maximize the amount of money earned. Participants completed 100 trials during the task. However, participants perform the test without knowing the total number of trials. Decks A and B are considered disadvantageous, whereas the other two decks, decks C and D, are considered advantageous. Each card choice in decks A and B results in a 100 Turkish Liras gain and each card choice in decks C and D result in a 50 Turkish Liras gain. In this respect, it would be advantageous to choose cards from the first two decks. Probabilistic punishments were intermixed amongst rewards in a programmed schedule. Associated with each of block of 10 trials, decks A and B result in a net loss of 250 Turkish Liras, whereas decks C and D result in a net gain of 250 Turkish Liras. Further broken down within each block of 10 trials, decks A and C are associated with more frequent, yet smaller money losses and decks B and D with one large money loss. Participants were expected to learn the contingencies and develop a strategy involving the preference of advantageous decks. Net score was calculated by subtracting the number of disadvantageous choices (decks A’ and B’) from the number of advantageous choices (decks C’ and D’). Net scores were generally associated with overall gain.
Wisconsin card sorting test
WCST [59] is widely used to assess abstraction ability and the ability to shift and maintain cognitive strategies for categorization. Participants were given 128 stimulus cards individually and were required to match each card to one of four key cards that varied on three perceptual dimensions (i.e. color, form, and number). At any given time, the matching rule was to match on one of these dimensions. After each match, the rater informed the participant whether they were correct. After ten consecutive correct sortings, the matching rule changes without warning and the participant must learn the new matching rule for the next set. The test requires strategically planning, conducting organized searches and utilizing environmental feedback to inform decisions, and restriction of impulsive responding. The number of perseverative errors was calculated as a measure of cognitive flexibility.
Data analysis
Data analysis was conducted using SPSS software version 22.0 between two groups: (a) Active group: includes individuals who were delivered 2 mA right anodal/left cathodal stimulation over the DLPFC (b) Sham group: includes individuals who were delivered sham stimulation over the right and left DLPFC. Normality of the data distribution was assessed using the Kolmogorov–Smirnov and the Shapiro–Wilk tests. For the detection of baseline differences between two groups concerning the sociodemographic and clinical characteristics, Chi square or Mann-Whitney-U tests were applied. A repeated measures analysis of variance (RM-ANOVA) was employed with time (pre-tDCS, post-tDCS) as the independent within-subjects variable, tDCS intervention group as the independent between-subjects variable, and the difference in pre-post scores on the neurocognitive tasks as the dependent variable. The outcome measures were pre-tDCS and post-tDCS IGT net scores and the number of perseverative errors in WCST. Effect sizes were calculated as partial eta squared (ηp2). Spearman correlation coefficients, rho, were calculated between the behavioral data and neurocognitive test scores. A Chi square test was used to assess the integrity of blinding.
Results
Baseline characteristics of the sample
Baseline characteristics were summarized in Table 1. The mean age of the sample was 37.2 (± 10.3). Participants had 13.4 (± 3.2) years of education as well as mean scores of BDI, SOGS, PGSI, BIS-11 of 7.6 (± 7.8), 15.8 (± 1.4), 19.4 (± 5.1), 64.1 (± 9.0), respectively. The two groups resembled each other in age, educational level, the age of gambling initiation, engagement frequency in gambling activities, clinical gambling severity, gambling disorder duration, total loss due to gambling, depression level, impulsivity level, and initial neurocognitive test performances (p’s > 0.100), thus indicating that randomization was valid.
Relationship of neurocognitive task scores
Regarding the relationship between neurocognitive test measures, a trend towards negative correlation was found between the Pre-tDCS IGT net score and the number of perseverative errors in the pre-tDCS WCST in the whole sample (r = − 0.430, p = 0.070). Post-tDCS IGT net score correlated negatively with the number of perseverative errors in the post-tDCS WCST in the whole sample (r = − 0.450, p = 0.049) and in the active group (r = − 0.667, p = 0.047, but not in the sham group (p = 0.600).
The effect of tDCS on decision making
RM-ANOVA indicated a main effect of time on IGT net score (F1,16 = 13.571, p = 0.002). A significant tDCS intervention*time interaction was found (F1,16 = 8.128, p = 0.01, ɳp2 =0.330) (Table 2; Fig. 1).
Changes in Iowa Gambling Task Net Score. Mean net score in the Iowa Gambling Task. Active group: Black line, sham group: Gray line. Pre-tDCS shows the baseline scores while post-tDCS shows the score of the Iowa Gambling Task, administered after the third application. Error bars represent standard error. RM-ANOVA indicated a main effect of time on IGT net score (F1,16 = 13.571, p = 0.002). A significant tDCS intervention*time interaction was found (F1,16 = 8.128, p = 0.010, ηp2 =0.330)
The effect of tDCS on cognitive flexibility
No main effect of time on the number of perseverative errors was found (F1,16 = 1.134, p = 0.300). A significant intervention*time interaction was found (Table 2; Fig. 2) (F1,16 =8.782, p < 0.001, ɳp2= 0.350).
Changes in the number of perseverative errors in the Wisconsin Card Sorting Test. The number of perseverative errors in the Wisconsin Card Sorting Test. Active group: Black line, sham group: Gray line. Pre-tDCS shows the baseline values while post-tDCS shows the values of the readministered task (after the third application). Error bars represent standard error. No main effect of time on the number of perseverative errors was found (F1,16 = 1.134, p = 0.300). A significant intervention*time interaction was found (Table 2; Fig. 2) (F1,16 =8.782, p < 0.001, ηp2= 0.350)
Feasibility and blinding efficacy of the procedure
None of the participants reported significant adverse effects during or after the tDCS sessions. Participants were unable to distinguish between active and sham stimulation, thus confirming the validity of blinding (x2 = 0.222, p = 0.500).
Discussion
This triple-blind randomized placebo-controlled parallel study investigated the effect of applying tDCS over the frontal cortices on decision making and cognitive flexibility. Consistent with our hypotheses, we demonstrated that tDCS delivered over bilateral DLPFC (F3/F4) enhanced both decision making and cognitive flexibility in a GD population. We also demonstrated that tDCS is well-tolerated and feasible in GD. To the best of our knowledge, this is the first study assessing the effect of tDCS on cognitive performance in GD.
We found promising results supporting the enhancing effect of tDCS on decision making in GD. The results are consistent with the majority of the studies conducted in healthy populations [39,40,41], in a clinically impulsive sample of veterans [42] and in individuals with addictive disorders [43]. Fecteau et al. were the first to report the effect of right anodal/left cathodal tDCS over DLPFC on risk-taking in healthy subjects [39]. A recent study also [41] showed a similar effect of the right anodal/left cathodal tDCS over the DLPFC under a context of haste in healthy subjects. In a tDCS study comparing cocaine abusers with non-abusers, stimulation over the right DLPFC led to improvements in both Balloon Analog Risk Task (BART) and Game of Dice Task in abusers while stimulation over the left DLPFC only resulted in improvement of BART [43]. In contrast, tDCS over both right and left DLPFC was reported to increase risk propensity in chronic marijuana users [60], possibly due to baseline differences in age and impulsivity or chronic alterations in brain circuits. A study using High Definition-tDCS over the left DLPFC also reported better IGT scores in healthy subjects [61]. Moreover, a recent study reported better IGT scores after 2 mA cathodal stimulation over the right DLPFC in subjects with Parkinson’s Disease [62]. Enhancement of decision making after anodal stimulation of the left DLPFC and cathodal stimulation of the right DLPFC might be due to the bilateral involvement of the DLPFC in IGT performance [63] or alterations of neural circuits among samples (i.e. healthy subjects, subjects with GD or Parkinson’s Disease). Overall, the right anodal/left cathodal montage over the DLPFC was considered to improve the interhemispheric balance of activity during decision making [39]. Thus, we assessed the effect of this particular montage and found the enhancing effect of tDCS on decision making.
The achieved results also support the notion that prefrontal cortices have a significant role in decision making [14, 25, 26, 64]. Given the complexity of the necessary calculations required, it is not surprising that a distributed neural network including many cortical and subcortical areas play a role during decision making [14]. Among them, the importance of both the VMPFC and the DLPFC seems to have a central role [14, 65]. VMPFC activity from fMRI was considered to be associated with encoding of the value signals to direct decision making and the DLPFC activity was considered to be associated with the self-control that modulates these value signals [66]. The DLPFC activity was also reported to be correlated with the VMPFC activity when individuals exercise self-control [66]. Hence, the DLPFC was reported to be required for suppressing the seductive emotional impulses [14]. To sum, both regions might have critical roles in decision making results from the current study.
In respect of cognitive flexibility, the literature shows no consensus on cognitive flexibility deficits in GD. Despite several negative results [20, 21, 67, 68], cognitive flexibility was found to be impaired in GD in other studies [30, 31]. We observed improvement of cognitive flexibility following right anodal/left cathodal stimulation over the DLPFC. Concerning the effect of neurostimulation on cognitive flexibility, significant improvement has been observed in those who received cathodal stimulation over the left DLPFC [45, 46]. Mansouri et al. reported a decrease in post-error slowing indicating better performance in WCST after cathodal stimulation over the dominant hemisphere with larger positive electrode over the contralateral supraorbital area [45]. Accordingly, Luft et al. reported better problem-solving performance using cathodal stimulation over the left DLPFC and explained the results with a better achievement of constraint relaxation [46]. Consistent with previous reports [45, 46], cathodal stimulation over the left DLPFC in our study might result in better disengagement from current task and engagement in the new task.
We also found a correlation between decision making and cognitive flexibility following the tDCS applications. This result is in confirmation with previous research that suggested a possible relationship between these two components of cognitive control [32, 33]. This relationship was considered to be due to the requirement of cognitive flexibility in the long-term decision making to evaluate the consequences of the past choices for better decisions [69]. Hence, it might be argued that the improvement in cognitive flexibility might have played a facilitating role in the improvement of decision making. However, there are also reports of a negative relationship between decision making and cognitive flexibility in healthy people [70,71,72] and in subjects with GD [67, 68] that claimed decision making is irrelevant of general cognitive flexibility. In conclusion, the literature is inconsistent and further research is needed to elucidate this relationship.
Another point to be emphasized here is that the achieved results might not only be due to the modulation of the DLPFC activity. TDCS delivered to F3 and F4 stimulates a majority of frontal cortices. Thus, tDCS delivered over the DLPFC might directly or indirectly modulate the activity of the VMPFC in healthy people [40, 65, 66] and in individuals with addictive disorders [73]. OFC has also been associated with decision making [14] and tDCS over the right OFC also resulted in better decision making and interference inhibition performances in healthy individuals [74]. Therefore, direct or indirect modulation of the right OFC might have contributed to the enhancing effect of tDCS over the right DLPFC on cognitive functions. While stimulation was delivered over DLPFC (F3/F4), it is important to note that these results should not be interpreted as DLPFC specific due to the broad pattern of stimulation delivered by conventional tDCS.
While GD has been considered to be a public health issue [75], there are still various unmet needs for understanding the neural basis and treatment of GD [76]. Moreover, various challenges occur during the treatment of individuals with GD including high dropout and relapse rates [77, 78]. These outcome parameters along with the disease severity were found to be associated with decision making and cognitive flexibility deficits [3, 4, 6, 7]. Therefore, reinforcing the treatment with interventions enhancing cognitive control may be a novel treatment strategy. To this end, psychotherapeutic [79] and pharmacological [80] interventions, as well as cognitive training and repetitive Transcranial Magnetic Stimulation, were explored in addictive disorders [81, 82]. In addition to the above-mentioned strategies, we demonstrated that tDCS over the DLPFC enhanced decision making and cognitive flexibility in GD. Moreover, tDCS over the DLPFC with decision making training may result in better cognitive outcomes even though it has not been tested in GD previously [42].
The achieved results are best viewed in the context of limitations. First, we enrolled a limited number of the participants due to difficulties in recruiting subjects with GD as a result of low admission rate and the exclusion of individuals with other addictive and neuropsychiatric disorders. The study group was restricted to a treatment-seeking population and it was not possible to generalize the present findings to ecological settings as treatment-seeking individuals with GD might be less impulsive [83]. Furthermore, we did not include women due to the non-admission of female individuals during the period of enrollment for the study. This should be taken into account as any sex-based differences in decision making and executive function processes or neural underpinnings may impact response to tDCS in the context of GD [84, 85]. Further research is needed in sex-matched samples. Besides, we assessed the effect of three sessions of tDCS as we considered that long-term protocols might result in high drop-out rates in individuals with GD. Future studies should assess the effect of more sessions of tDCS in individuals with GD to find the most beneficial dose of tDCS. Moreover, we only assessed the short-term effect of tDCS. Future research should replicate our findings with a follow-up period to determine the stability of the achieved results. Finally, our analyses did not include the differences in gambling severity as we considered that detecting changes in gambling severity might require a long-term period.
Conclusions
This study extends findings concerning the enhancing effect of tDCS on cognitive functions to individuals with GD, suggesting for the first time that three sessions of 20-min tDCS over bilateral DLPFC might be a novel intervention to enhance decision making and cognitive flexibility in individuals with GD. Our results also suggest bilateral prefrontal cortices as a potential target of tDCS in conditions associated with deficits of cognitive control. Future research efforts are needed to carefully examine the precise molecular mechanisms involved in the effect of tDCS, short- and long-term effects of different electrode montages, and dosing of tDCS on the clinical variables and cognitive functions in gambling disorder.
Abbreviations
- BART:
-
Balloon analog risk task
- BDI:
-
Beck depression inventory
- BIS-11:
-
Barratt Impulsivity Scale-11
- DLPFC:
-
Dorsolateral prefrontal cortex
- DSM-5:
-
Diagnostic and statistical manual of mental disorders-5
- fMRI:
-
Functional magnetic resonance imaging
- GD:
-
Gambling disorder
- IGT:
-
Iowa gambling task
- PGSI:
-
Pathological Gambling Severity Index
- SOGS:
-
South oaks gambling screen
- tDCS:
-
Transcranial direct current stimulation
- VMPFC:
-
Ventromedial prefrontal cortex
- WCST:
-
Wisconsin card sorting test
References
American Psychiatric Association (2013) Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5)
Morton JB, Ezekiel F, Wilk HA (2011) Cognitive control: easy to identify but hard to define. Top Cogn Sci 3:212–216. https://doi.org/10.1111/j.1756-8765.2011.01139.x
Achab S, Karila L, Khazaal Y (2014) Pathological gambling: update on decision making and neuro-functional studies in clinical samples. Curr Pharm Des 20:4000–4011
Alvarez-Moya EM, Ochoa C, Jiménez-Murcia S et al (2011) Effect of executive functioning, decision-making and self-reported impulsivity on the treatment outcome of pathologic gambling. J Psychiatry Neurosci 36:165–175. https://doi.org/10.1503/jpn.090095
Brevers D, Bechara A, Cleeremans A, Noël X (2013) Iowa Gambling Task (IGT): twenty years after – gambling disorder and IGT. Front Psychol 4:665. https://doi.org/10.3389/fpsyg.2013.00665
Wiehler A, Peters J (2015) Reward-based decision making in pathological gambling: the roles of risk and delay. Neurosci Res 90:3–14. https://doi.org/10.1016/j.neures.2014.09.008
Leppink EW, Redden SA, Chamberlain SR, Grant JE (2016) Cognitive flexibility correlates with gambling severity in young adults. J Psychiatr Res 81:9–15. https://doi.org/10.1016/j.jpsychires.2016.06.010
Coutlee CG, Huettel SA (2012) The functional neuroanatomy of decision making: Prefrontal control of thought and action. Brain Res 1428:3–12. https://doi.org/10.1016/j.brainres.2011.05.053
Lee D (2013) Decision making: from neuroscience to psychiatry. Neuron 78:233–248. https://doi.org/10.1016/j.neuron.2013.04.008
Malloy-Diniz LF, Miranda DM, Grassi-Oliveira R (2017) Editorial: executive functions in psychiatric disorders. Front Psychol 8:1461. https://doi.org/10.3389/fpsyg.2017.01461
Martin CS, Langenbucher JW, Chung T, Sher KJ (2014) Truth or consequences in the diagnosis of substance use disorders. Addiction 109:1773–1778. https://doi.org/10.1111/add.12615
Engel A, Caceda R (2015) Can decision making research provide a better understanding of chemical and behavioral addictions? Curr Drug Abuse Rev 8:75–85. https://doi.org/10.2174/1874473708666150916113131
Amlung M, Vedelago L, Acker J et al (2017) Steep delay discounting and addictive behavior: a meta-analysis of continuous associations. Addiction 112:51–62. https://doi.org/10.1111/add.13535
Fecteau S, Fregni F, Boggio PS et al (2010) Neuromodulation of decision-making in the addictive brain. Subst Use Misuse 45:1766–1786. https://doi.org/10.3109/10826084.2010.482434
Bickel WK, Jarmolowicz DP, Mueller ET et al (2012) Are executive function and impulsivity antipodes? A conceptual reconstruction with special reference to addiction. Psychopharmacology 221:361–387. https://doi.org/10.1007/s00213-012-2689-x
Probst C, Manthey J, Martinez A, Rehm J (2015) Alcohol use disorder severity and reported reasons not to seek treatment: a cross-sectional study in European primary care practices. Subst Abuse Treat Prev Policy 10:32. https://doi.org/10.1186/s13011-015-0028-z
Maremmani AGI, Rovai L, Rugani F et al (2012) Correlations between awareness of illness (insight) and history of addiction in heroin-addicted patients. Front Psychiatry 3:61. https://doi.org/10.3389/fpsyt.2012.00061
Fauth-Bühler M, Mann K, Potenza MN (2017) Pathological gambling: a review of the neurobiological evidence relevant for its classification as an addictive disorder. Addict Biol 22:885–897. https://doi.org/10.1111/adb.12378
Potenza MN (2014) The neural bases of cognitive processes in gambling disorder. Trends Cogn Sci 18:429–438. https://doi.org/10.1016/j.tics.2014.03.007
Kapsomenakis A, Simos PG, Konstantakopoulos G, Kasselimis DS (2018) In search of executive impairment in pathological gambling: a neuropsychological study on non-treatment seeking gamblers. J Gambl Stud. https://doi.org/10.1007/s10899-018-9758-y
Ledgerwood DM, Orr ES, Kaploun KA et al (2012) Executive function in pathological gamblers and healthy controls. J Gambl Stud 28:89–103. https://doi.org/10.1007/s10899-010-9237-6
Kovács I, Richman MJ, Janka Z et al (2017) Decision making measured by the Iowa Gambling Task in alcohol use disorder and gambling disorder: a systematic review and meta-analysis. Drug Alcohol Depend 181:152–161. https://doi.org/10.1016/j.drugalcdep.2017.09.023
Clark L, Averbeck B, Payer D et al (2013) Pathological choice: the neuroscience of gambling and gambling addiction. J Neurosci 33:17617–17623. https://doi.org/10.1523/JNEUROSCI.3231-13.2013
Brevers D, Cleeremans A, Goudriaan AE et al (2012) Decision making under ambiguity but not under risk is related to problem gambling severity. Psychiatry Res 200:568–574. https://doi.org/10.1016/j.psychres.2012.03.053
Li X, Lu Z-L, D’Argembeau A et al (2010) The iowa gambling task in fMRI images. Hum Brain Mapp 31:410–423. https://doi.org/10.1002/hbm.20875
Fleck MS, Daselaar SM, Dobbins IG, Cabeza R (2006) Role of prefrontal and anterior cingulate regions in decision-making processes shared by memory and nonmemory tasks. Cereb Cortex 16:1623–1630. https://doi.org/10.1093/cercor/bhj097
Brevers D, Noël X, He Q et al (2016) Increased ventral-striatal activity during monetary decision making is a marker of problem poker gambling severity. Addict Biol 21:688–699. https://doi.org/10.1111/adb.12239
Jurado MB, Rosselli M (2007) The elusive nature of executive functions: a review of our current understanding. Neuropsychol Rev 17:213–233. https://doi.org/10.1007/s11065-007-9040-z
Domínguez-Salas S, Díaz-Batanero C, Lozano-Rojas OM, Verdejo-García A (2016) Impact of general cognition and executive function deficits on addiction treatment outcomes: Systematic review and discussion of neurocognitive pathways. Neurosci Biobehav Rev 71:772–801. https://doi.org/10.1016/j.neubiorev.2016.09.030
Goudriaan AE, Oosterlaan J, de Beurs E, van den Brink W (2006) Neurocognitive functions in pathological gambling: a comparison with alcohol dependence, Tourette syndrome and normal controls. Addiction 101:534–547. https://doi.org/10.1111/j.1360-0443.2006.01380.x
Ochoa C, Alvarez-Moya EM, Penelo E et al (2013) Decision-making deficits in pathological gambling: the role of executive functions, explicit knowledge and impulsivity in relation to decisions made under ambiguity and risk. Am J Addict 22:492–499. https://doi.org/10.1111/j.1521-0391.2013.12061.x
Brand M, Recknor EC, Grabenhorst F, Bechara A (2007) Decisions under ambiguity and decisions under risk: correlations with executive functions and comparisons of two different gambling tasks with implicit and explicit rules. J Clin Exp Neuropsychol 29:86–99. https://doi.org/10.1080/13803390500507196
Iudicello JE, Woods SP, Cattie JE et al (2013) Risky decision-making in HIV-associated neurocognitive disorders (HAND). Clin Neuropsychol 27:256–275. https://doi.org/10.1080/13854046.2012.740077
Dong X, Du X, Qi B (2016) Conceptual knowledge influences decision making differently in individuals with high or low cognitive flexibility: an ERP study. PLoS One 11:e0158875. https://doi.org/10.1371/journal.pone.0158875
Buchsbaum BR, Greer S, Chang W-L, Berman KF (2005) Meta-analysis of neuroimaging studies of the Wisconsin card-sorting task and component processes. Hum Brain Mapp 25:35–45. https://doi.org/10.1002/hbm.20128
Alvarez JA, Emory E (2006) Executive function and the frontal lobes: a meta-analytic review. Neuropsychol Rev 16:17–42. https://doi.org/10.1007/s11065-006-9002-x
Moccia L, Pettorruso M, De Crescenzo F et al (2017) Neural correlates of cognitive control in gambling disorder: a systematic review of fMRI studies. Neurosci Biobehav Rev 78:104–116. https://doi.org/10.1016/j.neubiorev.2017.04.025
Nitsche MA, Cohen LG, Wassermann EM et al (2008) Transcranial direct current stimulation: State of the art 2008. Brain Stimul 1:206–223. https://doi.org/10.1016/j.brs.2008.06.004
Fecteau S, Knoch D, Fregni F et al (2007) Diminishing risk-taking behavior by modulating activity in the prefrontal cortex: a direct current stimulation study. J Neurosci 27:12500–12505. https://doi.org/10.1523/JNEUROSCI.3283-07.2007
Fecteau S, Pascual-Leone A, Zald DH et al (2007) Activation of prefrontal cortex by transcranial direct current stimulation reduces appetite for risk during ambiguous decision making. J Neurosci 27:6212–6218. https://doi.org/10.1523/JNEUROSCI.0314-07.2007
Cheng GLF, Lee TMC (2016) Altering risky decision-making: Influence of impulsivity on the neuromodulation of prefrontal cortex. Soc Neurosci 11:353–364. https://doi.org/10.1080/17470919.2015.1085895
Gilmore CS, Dickmann PJ, Nelson BG et al (2018) Transcranial Direct Current Stimulation (tDCS) paired with a decision-making task reduces risk-taking in a clinically impulsive sample. Brain Stimul 11:302–309. https://doi.org/10.1016/j.brs.2017.11.011
Gorini A, Lucchiari C, Russell-Edu W, Pravettoni G (2014) Modulation of risky choices in recently abstinent dependent cocaine users: a transcranial direct-current stimulation study. Front Hum Neurosci 8:661. https://doi.org/10.3389/fnhum.2014.00661
Boggio PS, Campanhã C, Valasek CA et al (2010) Modulation of decision-making in a gambling task in older adults with transcranial direct current stimulation. Eur J Neurosci 31:593–597. https://doi.org/10.1111/j.1460-9568.2010.07080.x
Mansouri FA, Fehring DJ, Feizpour A et al (2016) Direct current stimulation of prefrontal cortex modulates error-induced behavioral adjustments. Eur J Neurosci 44:1856–1869. https://doi.org/10.1111/ejn.13281
Luft CDB, Zioga I, Banissy MJ, Bhattacharya J (2017) Relaxing learned constraints through cathodal tDCS on the left dorsolateral prefrontal cortex. Sci Rep 7:2916. https://doi.org/10.1038/s41598-017-03022-2
Zack M, Cho SS, Parlee J et al (2016) Effects of high frequency repeated transcranial magnetic stimulation and continuous theta burst stimulation on gambling reinforcement, delay discounting, and stroop interference in men with pathological gambling. Brain Stimul 9:867–875. https://doi.org/10.1016/j.brs.2016.06.003
Gay A, Boutet C, Sigaud T et al (2017) A single session of repetitive transcranial magnetic stimulation of the prefrontal cortex reduces cue-induced craving in patients with gambling disorder. Eur Psychiatry 41:68–74. https://doi.org/10.1016/j.eurpsy.2016.11.001
Sauvaget A, Bulteau S, Guilleux A et al (2018) Both active and sham low-frequency rTMS single sessions over the right DLPFC decrease cue-induced cravings among pathological gamblers seeking treatment: a randomized, double-blind, sham-controlled crossover trial. J Behav Addict 7:126–136. https://doi.org/10.1556/2006.7.2018.14
Dickler M, Lenglos C, Renauld E et al (2018) Online effects of transcranial direct current stimulation on prefrontal metabolites in gambling disorder. Neuropharmacology 131:51–57. https://doi.org/10.1016/j.neuropharm.2017.12.002
Lesieur HR, Blume SB (1987) The South Oaks Gambling Screen (SOGS): a new instrument for the identification of pathological gamblers. Am J Psychiatry 144:1184–1188. https://doi.org/10.1176/ajp.144.9.1184
Ferris J, Consultants HW, Ladouceur R et al (2001) THE CANADIAN PROBLEM GAMBLING INDEX: FINAL REPORT The Canadian Problem Gambling Index: Final Report Submitted for the Canadian Centre on Substance Abuse (CCSA)
Beck AT, Ward CH, Mendelson M et al (1961) An inventory for measuring depression. Arch Gen Psychiatry 4:561–571
Patton JH, Stanford MS, Barratt ES (1995) Factor structure of the Barratt impulsiveness scale. J Clin Psychol 51:768–774
Brunoni AR, Amadera J, Berbel B et al (2011) A systematic review on reporting and assessment of adverse effects associated with transcranial direct current stimulation. Int J Neuropsychopharmacol 14:1133–1145. https://doi.org/10.1017/S1461145710001690
Woods AJ, Antal A, Bikson M et al (2016) A technical guide to tDCS, and related non-invasive brain stimulation tools. Clin Neurophysiol 127:1031–1048
Gandiga PC, Hummel FC, Cohen LG (2006) Transcranial DC stimulation (tDCS): a tool for double-blind sham-controlled clinical studies in brain stimulation. Clin Neurophysiol 117:845–850. https://doi.org/10.1016/j.clinph.2005.12.003
Icellioglu S (2015) Iowa Kumar Testi: Normatif veriler ve yürütücü işlevlerle ilişkisi. Dusunen Adam J Psychiatry Neurol Sci 222–230. https://doi.org/10.5350/DAJPN2015280305
Heaton SK, Chelune GJ, Talley JL, Kay GG, Curtiss G (1993) Wisconsin Card Sorting Test Manual: Revised and expanded. Odessa, Florida
Boggio PS, Zaghi S, Villani AB et al (2010) Modulation of risk-taking in marijuana users by transcranial direct current stimulation (tDCS) of the dorsolateral prefrontal cortex (DLPFC). Drug Alcohol Depend 112:220–225. https://doi.org/10.1016/j.drugalcdep.2010.06.019
He Q, Chen M, Chen C et al (2016) Anodal stimulation of the left DLPFC increases IGT scores and decreases delay discounting rate in healthy males. Front Psychol 7:1421. https://doi.org/10.3389/fpsyg.2016.01421
Benussi A, Alberici A, Cantoni V et al (2017) Modulating risky decision-making in Parkinson’s disease by transcranial direct current stimulation. Eur J Neurol 24:751–754. https://doi.org/10.1111/ene.13286
Ouerchefani R, Ouerchefani N, Allain P et al (2017) Contribution of different regions of the prefrontal cortex and lesion laterality to deficit of decision-making on the Iowa Gambling Task. Brain Cogn 111:73–85. https://doi.org/10.1016/j.bandc.2016.06.010
Fellows LK, Farah MJ (2005) Different underlying impairments in decision-making following ventromedial and dorsolateral frontal lobe damage in humans. Cereb Cortex 15:58–63. https://doi.org/10.1093/cercor/bhh108
Baumgartner T, Knoch D, Hotz P et al (2011) Dorsolateral and ventromedial prefrontal cortex orchestrate normative choice. Nat Neurosci 14:1468–1474. https://doi.org/10.1038/nn.2933
Hare TA, Camerer CF, Rangel A (2009) Self-control in decision-making involves modulation of the vmPFC valuation system. Science 324:646–648. https://doi.org/10.1126/science.1168450
Boog M, Höppener P, Wetering VD, et al (2014) Cognitive inflexibility in gamblers is primarily present in reward-related decision making. Front Hum Neurosci 8:569. https://doi.org/10.3389/fnhum.2014.00569
Cavedini P, Riboldi G, Keller R et al (2002) Frontal lobe dysfunction in pathological gambling patients. Biol Psychiatry 51:334–341
Gleichgerrcht E, Ibáñez A, Roca M et al (2010) Decision-making cognition in neurodegenerative diseases. Nat Rev Neurol 6:611–623. https://doi.org/10.1038/nrneurol.2010.148
Brand M, Labudda K, Markowitsch HJ (2006) Neuropsychological correlates of decision-making in ambiguous and risky situations. Neural Netw 19:1266–1276. https://doi.org/10.1016/j.neunet.2006.03.001
Toplak ME, Sorge GB, Benoit A et al (2010) Decision-making and cognitive abilities: a review of associations between Iowa Gambling Task performance, executive functions, and intelligence. Clin Psychol Rev 30:562–581. https://doi.org/10.1016/j.cpr.2010.04.002
Overman WH, Frassrand K, Ansel S et al (2004) Performance on the IOWA card task by adolescents and adults. Neuropsychologia 42:1838–1851. https://doi.org/10.1016/j.neuropsychologia.2004.03.014
Nakamura-Palacios EM, Lopes IBC, Souza RA et al (2016) Ventral medial prefrontal cortex (vmPFC) as a target of the dorsolateral prefrontal modulation by transcranial direct current stimulation (tDCS) in drug addiction. J Neural Transm 123:1179–1194. https://doi.org/10.1007/s00702-016-1559-9
Ouellet J, McGirr A, Van den Eynde F et al (2015) Enhancing decision-making and cognitive impulse control with transcranial direct current stimulation (tDCS) applied over the orbitofrontal cortex (OFC): A randomized and sham-controlled exploratory study. J Psychiatr Res 69:27–34. https://doi.org/10.1016/j.jpsychires.2015.07.018
The Lancet (2017) Problem gambling is a public health concern. Lancet 390:913. https://doi.org/10.1016/S0140-6736(17)32333-4
Choi S-W, Shin Y-C, Kim D-J et al (2017) Treatment modalities for patients with gambling disorder. Ann Gen Psychiatry 16:23. https://doi.org/10.1186/s12991-017-0146-2
Ronzitti S, Soldini E, Smith N et al (2018) Are Treatment Outcomes Determined by Type of Gambling? A UK Study. J Gambl Stud, In Press. https://doi.org/10.1007/s10899-018-9752-4
Pfund RA, Peter SC, Whelan JP, Meyers AW (2017) When Does Premature Treatment Termination Occur? Examining Session-by-Session Dropout Among Clients with Gambling Disorder. J Gambl Stud 34:617–630. https://doi.org/10.1007/s10899-017-9733-z
Challet-Bouju G, Bruneau M, Group IGNACE C, et al (2017) Cognitive Remediation Interventions for Gambling Disorder: A Systematic Review. Front Psychol 8:1961. https://doi.org/10.3389/fpsyg.2017.01961
Perkins FN, Freeman KB (2018) Pharmacotherapies for decreasing maladaptive choice in drug addiction: targeting the behavior and the drug. Pharmacol Biochem Behav 164:40–49. https://doi.org/10.1016/j.pbb.2017.06.015
Bickel WK, Quisenberry AJ, Moody L, Wilson AG (2015) Therapeutic opportunities for self-control repair in addiction and related disorders: change and the limits of change in trans-disease processes. Clin Psychol Sci 3:140–153. https://doi.org/10.1177/2167702614541260
Verdejo-Garcia A (2016) Cognitive training for substance use disorders: Neuroscientific mechanisms. Neurosci Biobehav Rev 68:270–281. https://doi.org/10.1016/j.neubiorev.2016.05.018
Leblond J, Ladouceur R, Blaszczynski A (2003) Which pathological gamblers will complete treatment? Br J Clin Psychol 42:205–209. https://doi.org/10.1348/014466503321903607
van den Bos R, Homberg J, de Visser L (2013) A critical review of sex differences in decision-making tasks: focus on the Iowa Gambling Task. Behav Brain Res 238:95–108. https://doi.org/10.1016/j.bbr.2012.10.002
Reber J, Tranel D (2017) Sex differences in the functional lateralization of emotion and decision making in the human brain. J Neurosci Res 95:270–278. https://doi.org/10.1002/jnr.23829
Acknowledgements
The authors would like to thank all the participants who have kindly taken part in this study. The authors gratefully acknowledge the assistance of Mustafa Çetinkaya for referring participants and Göktuğ Aşçı for the preparation of the equipment.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Rights and permissions
About this article
Cite this article
Soyata, A.Z., Aksu, S., Woods, A.J. et al. Effect of transcranial direct current stimulation on decision making and cognitive flexibility in gambling disorder. Eur Arch Psychiatry Clin Neurosci 269, 275–284 (2019). https://doi.org/10.1007/s00406-018-0948-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00406-018-0948-5