Abstract
Purpose
Allergic rhinitis is an immunoglobulin-E (Ig-E)-mediated response driven by type 2 helper T cells. Hesperidin and thymol are biological agents that possess antioxidant and anti-inflammatory characteristics. The purpose of this study was to investigate the effects of hesperidin and thymol in rats with ovalbumin-induced allergic rhinitis.
Methods
Thirty adult Sprague–Dawley rats were randomly assigned into five groups, each containing six animals. The first group constituted the negative control group, while the remaining groups were exposed to an ovalbumin-induced model of allergic rhinitis. In the provocation stage, 4 mL/kg saline was administered to the positive control group, 10 mg/kg desloratadine to the reference group, 100 mg/kg hesperidin to the hesperidin group, and 20 mg/kg thymol to the thymol group, all by gastric lavage for 7 days. Nasal symptoms were scored on day 22. Rats were then sacrificed, and intracardiac blood specimens were collected to measure plasma total Ig-E, IL-5, IL-13, total antioxidant capacity (TAC), and total oxidant status (TOS) levels. Nasal tissues were extracted for histopathological and immunochemical examination.
Results
Nasal symptom scores were highest in the positive control group, while hesperidin and thymol ameliorated these symptoms to the same extent as desloratadine. Ig-E, IL-5, IL-13, and TOS levels increased, while TAC levels decreased significantly in the allergic rhinitis group compared to the other groups. Significant improvement in these parameters was observed in both the hesperidin and thymol groups. At histopathological and immunohistochemical examination of the nasal cavity, severe allergic inflammation and severe TNF-α expression was determined in rats from the allergic rhinitis group. Mild inflammatory changes and mild TNF-α expression were observed in all three treatment groups.
Conclusion
Both hesperidin and thymol were effective in suppressing allergic symptoms and inflammation in the treatment of allergic rhinitis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Allergic rhinitis is characterized by symptoms including sneezing, nasal pruritus, airflow obstruction, and a generally clear nasal discharge, resulting from immunoglobulin E (Ig-E)-mediated reactions to inhaled allergens and mucosal inflammation mediated by type 2 helper T (Th2) cells. Allergic rhinitis has a high prevalence and affects approximately 400 million people worldwide [1]. It has a significant impact on patients’ social and working lives. Diagnosis is generally made through history, endoscopic nasal examination, and measuring serum Ig-E levels. The condition is an immune disease deriving from hypersensitivity to allergens. Recent studies have shown that Th2 cells and mast cells play a particularly critical role in AR. Cytokines released from Th2 cells, such as interleukin (IL)-4, IL-5, and IL-13, and allergic mediators such as histamine produced by mast cells, are of major importance in the development of the inflammatory response [2]. Oxidative stress has also been reported to contribute to the inflammatory response in the pathogenesis of allergic rhinitis [3].
Hesperidin is a flavonoid that is particularly abundant in citrus fruits. It exhibits a range of biological effects on the human body and is known to possess anti-inflammatory, hypolipidemic, vasoprotective, and cholesterol-reducing activities [4, 5]. Anti-allergic properties have also been reported when taken orally [6].
Thymol (2-isopropyl-5-methylphenol) is a monocyclic phenolic compound which occurs naturally and is derived from Thymus vulgaris (Lamiaceae). Its antimicrobial, antiseptic activity against oral bacteria and wound-healing properties mean that is widely used for therapeutic purposes [7]. Thymol has also been reported to exhibit antioxidant, antispasmodic, anti-platelet aggregation and anti-inflammatory properties [8].
The discovery of new treatment strategies aimed at regulating impaired inflammatory cytokine secretion may contribute to the future development of novel modalities for treating allergic rhinitis. The purpose of this study was to investigate the functional, biochemical, and histopathological effects of hesperidin and thymol in an ovalbumin-induced allergic rhinitis model in rats.
Materials and methods
Animals
The study began with 30 female Sprague–Dawley rats aged 10 weeks and weighing 220–280 g. Throughout the study, the rats were housed in special rooms with an automatically adjusted day–night cycle, temperature (24 ± 2 °C), and humidity (40 ± 3%) values, and were treated in accordance with Care and Use of Laboratory Animals principles. Free access was allowed to chow and water.
Study plan
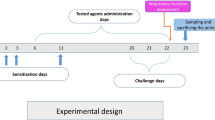
Five groups containing six animals each were established on a random basis. The six rats in the negative control group (group 1) were not exposed to any sensitization or provocation, but 4 mL/kg saline solution was administered by gastric lavage during the provocation stage. The remaining 24 rats were exposed to an ovalbumin-induced allergic rhinitis model. Group 2 represented the positive control group, in which sensitization and provocation were induced with ovalbumin. The rats in this group received saline solution at a dose of 4 mL/kg at the provocation stage. Group 3 was defined as the reference group. Desloratadine, used as a reference drug in previous studies because of its effectiveness against allergic rhinitis, was also adopted as the reference drug in this study. Following sensitization with ovalbumin, desloratadine was administered at the provocation stage in this group. Hesperidin was administered to group 4, and thymol to group 5 at the provocation stages.
Sensitization and provocation with ovalbumin
The ovalbumin was procured from Sigma-Aldrich Chemical Co. Sensitization and provocation were performed with ovalbumin, as described elsewhere in the literature, to establish a model of AR in 24 rats, excluding those in the control group [9]. Ovalbumin 0.3 mg was mixed with 30 mg aluminum hydroxide. The resulting mixture was dissolved in 1 mL saline solution and injected intraperitoneally to the rats every other day for 14 days. The provocation stage commenced following 7th injection. For that purpose, 20 µL of solution containing ovalbumin at a proportion of 10% was administered into both nostrils using a micropipette for 7 days.
Drug administration
Desloratadine (Aerius®, Merck Sharp & Dohme, Istanbul, Turkey), thymol (Sigma-Aldrich Chemical Co.), and hesperidin (Sigma-Aldrich Chemical Co.) were used in this study. All drugs were dissolved in saline solution. Following sensitization with ovalbumin, treatment was administered once daily for 7 days to groups 3, 4, and 5, together with provocation. 1 mL saline solution containing desloratadine at a dose of 10 mg/kg was administered to rats in group 3 by intragastric lavage. 1 mL saline solution containing 100 mg/kg hesperidin was administered to those of group 4 by intragastric lavage, while group 5 received 1 mL saline solution containing 20 mg/kg thymol, also by intragastric lavage.
Evaluation of nasal symptoms
All rats were evaluated for nasal symptoms as described by Wen et al. 24 h following the final drug administration on day 22 [10]. For that purpose, rats were initially placed in individual cages and allowed to adapt to their new surroundings for 10 min. They were then monitored for a further 10 min by blinded observers in terms of sneezing and nasal scratching and discharge. No nasal scratching or sneezing was scored 0, 1–3 behaviors per minute was scored 1, 4–6 per minute was scored 2, and more than six behaviors per minute was scored 3. No nasal discharge was scored 0, discharge from a single nostril 1, discharge from both nostrils 2, and overflowing 3. A total nasal symptom score was calculated for each rat on the basis of these evaluations.
Measurement of total Ig-E and inflammatory parameters
Following nasal symptom scoring, rats were killed under general anesthesia with intraperitoneal administration of ketamine (Ketalar ampoule, Pfizer, Istanbul) at a dose of 40 mg/kg and xylazine at a dose of 10 mg/kg. Intracardiac blood specimens were collected and centrifuged for 10 min at 1000 g at + 4 °C. The separated plasma was stored in a deep freeze at − 80 °C until the day of study.
Spesific ELISA kits produced for rats (Sunredbio, China) were used for measuring plasma total Ig-E, IL-5, and IL-13 values in line with the manufacturer’s instructions. Total Ig-E, IL-5, and IL-13 levels were expressed as KU/L, pg/mL, and ng/mL, respectively.
Measurement of oxidant–antioxidant status
Plasma total oxidant status (TOS) was measured using the technique described elsewhere by Erel et al. [11]. That method is based on the conversion of ferrous ion into ferric ion by oxidants in plasma and on spectrophotometric measurement of the colored complex produced by the ferric ion with xylenol orange in an acidic environment. TOS values were expressed as µmol/L.
Plasma total antioxidant capacity (TAC) was also measured following Erel et al.’s method [12]. This method is based on the measurement of the color change occurring as a result of the oxidization reaction initiated by hydroxyl radical in the reaction compound being suppressed by antioxidants in the specimen. TAC values were expressed as mmol/L.
Histopathological examination
Once rats had been killed, and intracardiac blood specimens had been collected, nasal tissues were also taken for histopathological examination and were fixed in 10% formalin solution for 48 h. Following routine procedures and decalcification, the tissues were embedded in paraffin blocks, from which 4-µm sections were taken. Preparates produced for histopathological examination were stained with hematoxylin-eosin (HE) and studied under a light microscope. A scale of none (–), mild (+), moderate (++), and severe (+++) was used for histopathological examination as defined in Table 1.
Immunohistochemistry
All sections were passed through xylol and alcohol series following fixation to adhesive (poly-l-lysin) slides for immunoperoxidase. After washing with PBS, endogenous peroxidase inactivation was performed by storing the sections in 3% H2O2 for 10 min. Sections were then treated in a microwave at 500 Watts for 2 × 5 min with antigen retrieval solution to reveal the antigen in the tissues and were finally left to cool. The procedure was carried out in line with the immunohistochemistry kit (AbcamHRP/DAB Detection IHC kit) instructions. Tissues washed with PBS were subsequently incubated with tumor necrosis factor alpha (TNF-α) antibody (Catalog no: sc52746, dilution 1/50; Santa Cruz, USA) at 37 °C for 10 min to determine inflammation. 3-3′ Diaminobenzidine was employed as the chromogen. Tissues were washed with distilled water. The classification scale for immunopositivity was defined in Table 1.
Statistical analysis
SPSS 17.0 software was used for statistical analysis. Distributions of biochemical results and nasal symptom scores were evaluated using the Shapiro–Wilk test. Since normal distribution was determined, one-way ANOVA and the post-hoc Tukey test were used for subsequent analysis. The Kruskal–Wallis test was used for analyzing semi-quantitative histopathological data. If significant differences were revealed, the Mann Whitney U test was used for two-way comparisons. p values lower than 0.05 were considered statistically significant.
Results
Nasal symptom findings
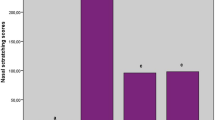
Nasal symptom scores (NSS), assessing nasal pruritus, sneezing, and nasal discharge were significantly higher in the positive control group than in the negative control group (p < 0.001). Hesperidin and thymol reduced the high NSS values in the positive control group (p < 0.001) and were as effective as desloratadine (p > 0.05) (Fig. 1).
Nasal symptom score outcomes. Data are expressed as mean values, with standard deviation values shown above each bar. Statistical comparison was performed with one-way ANOVA and post-hoc Tukey test. p < 0.05 was accepted as statistically significant. The letters above each bar show statistical comparisons. Different letters indicate significance at the p < 0.05 level, while the same letters indicate no significant difference. Group 2 was significantly higher (b) and group 1 was significantly lower (a) than other groups while there was no significant difference between groups 3,4, and 5 (c)
Total Ig-E and inflammatory parameters
Plasma total Ig-E levels of 1.14 ± 0.06 KU/L in the negative control group rose to 2.12 ± 0.14 KU/L in group 2 (p < 0.001). Ig-E levels in the groups treated with hesperidin and thymol were 1.55 ± 0.07 KU/L and 1.69 ± 0.1 KU/L, respectively, significantly lower than those in group 2 (p < 0.001, p < 0.001 respectively). Ig-E level was 1.34 ± 0.07 KU/L in reference group which was significantly lower than those of hesperidin and thymol groups (p < 0.01, p < 0.001, respectively). We observed no significant difference in Ig-E levels between the hesperidin and thymol groups (p > 0.05) (Fig. 2).
Comparison of biochemical parameters. Data are expressed as mean values, with standard deviation values shown above each bar. Statistical comparison was performed with one-way ANOVA and post-hoc Tukey test. p < 0.05 was accepted as statistically significant. The letters above each bar show statistical comparisons. Different letters indicate significance at the p < 0.05 level, while the same letters indicate no significant difference. A Comparison of total Ig-E results among the groups. Group 2 was significantly higher (b) and group 1 was significantly lower (a) than other groups. In treatment groups, while there was no significant difference between groups 4 and 5 (d), group 3 was significantly lower than group 4 and 5 (c). B Comparison of IL-13 results among the groups. Group 2 was significantly higher (b) and group 1 was significantly lower (a) than other groups. In treatment groups, there was no significant difference between groups 3 and 4 (c), and groups 4 and 5 (d). Group 3 was significantly lower than group 5 (c). C Comparison of IL-5 results among the groups. Group 2 was significantly higher (b) and group 1 was significantly lower (a) than other groups. In treatment groups, all three groups were significantly different from each other. Group 3 was the lowest (c) and group 5 was the highest group (e)
Both IL-5 and IL-13 levels were significantly higher in the positive group compared to the negative control group (p < 0.001, p < 0.001 respectively). Hesperidin and thymol were determined to effectively reduce these levels (p < 0.001). When compared to reference group, desloratadine was more effective than both hesperidin (p < 0.05) and thymol (p < 0.05) in terms of IL-5 levels but hesperidin was as effective on IL-13 as desloratadine (p > 0.05) (Fig. 2).
Oxidative parameters
The TAC levels were 1.72 ± 0.1 mmol/L in negative control group and 0.95 ± 0.09 mmol/L in positive control group (p < 0.001). Administration of hesperidin and thymol significantly raised these decreased TAC levels (p < 0.001 compared to group 2). When compared to reference group, there was no difference in terms of TAC levels between desloratadine and both hesperidin and thymol (p > 0.05).
In contrast to TAC, we observed high TOS values in the positive control group (p < 0.001) and the administration of hesperidin and thymol effectively reduced oxidant levels (p < 0.001, compared to group 2). When compared to reference group, both hesperidin and thymol were as effective on plasma TOS levels as desloratadine (p > 0.05) (Fig. 3).
Comparison of oxidative–antioxidative parameters. Data are expressed as mean values, with standard deviation values shown above each bar. Statistical comparison was performed with one-way ANOVA and post-hoc Tukey test. p < 0.05 was accepted as statistically significant. The letters above each bar show statistical comparisons. Different letters indicate significance at the p < 0.05 level, while the same letters indicate no significant difference. A Comparison of TAC results among the groups. Group 2 was significantly lower (b) and group 1 was significantly higher (a) than other groups while there was no significant difference between groups 3,4, and 5 (c). B Comparison of TOS results among the groups. Group 2 was significantly higher (b) than other groups. There was no significant difference between groups 1 and 2 (a). In treatment groups, there was no significant difference between groups 3, 4, and 5 (c)
Histopathological findings
Hematoxylin–eosin staining findings
Histopathological findings are summarized in Table 2 and the histopathological appearances of the nasal cavity are given in Fig. 4. Normal histological appearance was seen in the rats from group 1 (Fig. 4a). Histopathological examination of the rats from group 2 revealed desquamation and severe erosion, epithelial degeneration, and severe proliferation in goblet cells in the lamina epithelialis of the nasal cavity. In the lamina propria; eosinophilic infiltration, severe edema, and congestion and dilatation in vessels were observed. In the lumen; epithelial debris and eosinophilic mass were determined (Fig. 4b). Histopathological examination of group 3 revealed mild desquamation and erosion in the lamina epithelialis, very mild edema, eosinophil and leukocyte infiltration in the lamina propria, and mild proliferation in goblet cells (Fig. 4c). Group 4 exhibited mild degeneration and ciliary desquamation in the lamina epithelialis, mild edema and occasional eosinophil infiltration in the lamina propria, and mild proliferation in goblet cells (Fig. 4d). The nasal cavity in group 5 exhibited moderate degeneration in the lamina epithelialis, moderate proliferation in goblet cells, moderate edema in the lamina propria, dilatation and congestion in vessels, and eosinophilic cell infiltration in the lumen (Fig. 4e).
Histopathological findings. H&E, bar: 20 µm. a Group 1 (negative control group): nasal cavity, normal histological appearance, H&E, bar: 20 µm. b Group 2 (positive control group): nasal cavity, showing desquamation in the lamina epithelialis (arrow heads), proliferation and erosion in goblet cells, eosinophilic cell infiltration and a necrotic mass in the lumen (stars), and eosinophilic cell infiltration in the lamina propria (arrows), H&E, bar: 20 µm. c Group 3 (reference group): nasal cavity, showing mild desquamation and erosion in the lamina epithelialis, very mild edema in the lamina propria (stars), mild proliferation in goblet cells (arrow heads), and eosinophilic leukocyte infiltration, H&E, bar: 20 µm. d Group 4 (hesperidin group): nasal cavity, showing very mild degeneration in the lamina epithelialis (arrows), ciliary desquamation, moderate edema in the lamina propria (stars) and minimal eosinophil infiltration, mild proliferation in goblet cells (arrow heads), and dilatation and congestion in vessels, H&E, bar: 20 µm. e Group 5 (thymol group): nasal cavity, showing moderate desquamation in the lamina epithelialis, moderate edema in the lamina propria, and proliferation in goblet cells, eosinophilic cell infiltration, and a necrotic eosinophilic cell infiltration in the lumen (stars), H&E, bar: 20 µm
Immunohistochemical staining findings
Immunohistochemical examination of group 1 identified negative TNF-α expression in the nasal cavity (Fig. 5a). Group 2 exhibited severe TNF-α expression in mononuclear cells in the lamina epithelialis, lamina propria, and the eosinophilic mass in the lumen (Fig. 5b). Immunohistochemical examination of group 3, 4, and 5 revealed mild TNF-α expressions in the mononuclear cells in lamina epithelialis and lamina propria (Fig. 5c–e). Immunohistochemical findings are summarized in Table 2.
Immunohistochemical findings (IP-IHC, bar: 20 µm). a Group 1 (negative control group): nasal cavity, TNF-α expression negative, IP-IHC, bar: 20 µm. b Group 2 (positive control group): nasal cavity showing severe TNF-α expression in the eosinophilic mass in the lumen, and in the regions of mononuclear cell infiltration in the lamina epithelialis and lamina propria (arrow heads), IP-IHC, bar: 20 µm. c Group 3 (reference group): nasal cavity showing mild TNF-α expression in the lamina epithelialis and lamina propria (arrow heads), IP-IHC, bar: 20 µm. d Group 4 (hesperidin group): nasal cavity showing mild TNF-α expression in the lamina epithelialis and lamina propria (arrow heads), IP-IHC, bar: 20 µm. e Group 5 (thymol group): nasal cavity showing moderate TNF-α expression in the lamina epithelialis and lamina propria (arrow heads), IP-IHC, bar: 20 µm
Discussion
Allergic rhinitis is an inflammatory response caused by inhaled allergens and mediated by Ig-E in the nasal mucosa. Various pharmacological agents, including antihistaminics, leukotriene inhibitors, systemic and topical steroids, decongestants, and cromolyn sodium are used in treatment. However, these agents may be inadequate in some patients. Novel, safe, and effective therapeutic agents, therefore, need to be developed. Various substances, such as cyclosporine, bosentan, quercetin, and pycnogenol have been used for that purpose in the treatment of allergic rhinitis in animal studies [9, 13,14,15]. To the best of our knowledge, no previous studies have examined the use of hesperidin and thymol in experimental models of allergic rhinitis. Our study, therefore, investigated the effectiveness in allergic rhinitis of hesperidin and thymol, both with powerful antioxidant and anti-inflammatory activities.
Allergic rhinitis gives rise to symptoms such as nasal discharge, sneezing, and nasal pruritus. NSS have been used in various studies to reveal the severity of these symptoms in functional evaluations of the disease [14]. Sagit et al. investigated nasal symptoms in rats with allergic rhinitis and reported higher scores in the allergic rhinitis group than in the control group [13]. NSS were also calculated for all rats on day 22 of our study, and significantly higher scores were determined in rats receiving ovalbumin compared to the control group. NSS values improved close to those of the control group following hesperidin and thymol administration, and these medications were identified as being as effective as desloratadine.
One of the most important markers of allergic rhinitis is Ig-E level elevation. The presence of Ig-E has been reported to be a specific diagnostic marker in allergic diseases [16]. Greiner et al. reported the need to study Ig-E levels to confirm diagnosis of allergic rhinitis [1]. In an ovalbumin-induced allergic rhinitis model, Senturk et al. reported significantly higher Ig-E levels in the allergic rhinitis group than in other groups [9]. We also observed significantly higher Ig-E levels in the rat groups in which we induced allergic rhinitis compared to the other groups. Ig-E levels also significantly decreased in the groups treated with hesperidin and thymol.
Cytokines are low molecular weight proteins that play a role in intercellular communication. Cytokines play a regulatory role in various events, including cell life and differentiation, immune cell activation, cell migration, and cell death, and are also involved in the pathogenesis of allergic rhinitis [17]. Levels of cytokines released from Th2 cells, such as IL-4, IL-5, and IL-13, have been shown to increase in an ovalbumin-induced allergic rhinitis model in rats [18]. TNF-α is a cell signaling cytokine with proven involvement in systemic inflammation. TNF-α plays a critical role in the pathogenesis of allergic rhinitis. In our study, plasma IL-5 and IL-13 levels in rats with allergic rhinitis increased significantly compared to those in the control group. Also, immunohistochemical staining of the nasal mucosa of the rats in allergy group showed severe TNF-α expression. Treatment with both hesperidin and thymol significantly decreased the TNF-α expression and plasma levels of IL-5 and IL-13. Especially hesperidin was as effective as desloratadine in terms of lowering the TNF-α expression.
In allergic rhinitis, antigens cause the production of reactive oxygen species (ROS) from nasal epithelial cells. Increasing ROS production triggers the production of cytokines such as TNF-α, IL-6, and cyclooxygenase (COX)-2 by activating the NF-κB pathway. This results in mucosal dysfunction and histopathological changes by damaging the nasal mucosa through direct and indirect pathways [19]. The principal disadvantages inherent in the use of oxidative stress markers are the complexity and cost of the techniques involved and the equipment required. Measurement of individual antioxidant molecules is impracticable, since these molecules exhibit cumulative antioxidant effects. We, therefore, measured TOS and TAC following the method described by Erel et al. [11, 12]. In their study of children with allergic rhinitis, Emin et al. showed a decrease in plasma TAC levels and an increase in TOS values [20]. We also observed a significant decrease in TAC levels and a significant increase in TOS values in rats with allergic rhinitis. Hesperidin and thymol administration regulated the oxidative balance by increasing TAC levels and reducing TOS values. The effectiveness of both hesperidin and thymol against TAC and TOS levels was similar to that of desloratadine.
Hesperidin is a citrus flavonoid with various biological activities. Its best-known characteristics include its anti-cancer, anti-inflammatory, and antioxidant effects. Hesperidin also manifests significant immunomodulatory effects and is thought to be capable of exhibiting antiallergic activity [21]. There are only limited studies in the literature examining the relationship between hesperidin and allergic diseases. Kim et al. reported increased IL-5, IL-17, and Ig-E levels in mice using an induced asthma model, while these cytokines decreased in the groups receiving hesperidin [22]. Similarly, in their study of allergic asthma induced in mice, Wei et al. showed that hesperidin significantly improved ovalbumin-induced airway hyper-responsivity by reducing inflammatory cells and Th2 cytokines such as IL-4, IL-5, and IL-13, and by lowering Ig-E levels [23]. Studies have also shown the effectiveness of hesperidin in the oxidant–antioxidant balance. Kandemir et al. reported that hesperidin reduced oxidant levels and increased antioxidant levels in their animal toxicity study [24]. Selmi et al. reported that hesperidin regulated oxidant–antioxidant status by exhibiting antioxidant effects and that it can, therefore, be useful in chronic diseases [25]. In our study, we observed significant decreases in serum IL-5, IL-13, and Ig-E levels in rats receiving hesperidin compared to the allergy group, and hesperidin also regulated the oxidative balance. Moreover, hesperidin was as effective as desloratadine in terms of NSS, and histopathological and immunohistochemical findings. Our results suggest that on the basis of these properties, hesperidin may represent a new therapeutic approach to allergic airway diseases.
Thymol is a monoterpene phenol derivative with antimicrobial, antioxidant, local anesthetic, and powerful anti-inflammatory properties [26]. It has been reported to exhibit this powerful anti-inflammatory activity by inhibiting the NF-κB signal pathway, and thus TNF-α and IL-6 production, by regulating Th1/Th2 cytokine production [27, 28]. In their study of rats with induced toxicity, Nagoor et al. reported that thymol exhibited anti-inflammatory effects by reducing the expression of pro-inflammatory cytokines such as TNF-α, IL-6, and IL-1β [29]. Güvenc et al. also demonstrated the effectiveness of thymol against chronic inflammation through its antioxidant properties in their experimental animal model study [30]. In another study, Zhou et al. investigated the effect of thymol on Ig-E, IL-4, IL-5, and IL-13 levels in an ovalbumin-induced asthma model in mice and reported that thymol reduced these levels and inhibited the migration of inflammatory cells to the airway [31]. In our study, thymol significantly reduced serum IL-5, IL-13, and Ig-E levels, increased TAC values, and reduced TOS values, while also producing significant improvement in NSS and histopathological findings in rats with induced allergy. These findings appear to show that thymol may be suitable for therapeutic, oral use in allergic airway diseases.
Our study has some limitations. First, we used a single dose of hesperidin and thymol. Using different doses of these drugs would be useful for investigating their effectiveness in a dose-dependent manner. Second, by adding steroids and leukotriene antagonists, other effective drugs for the treatment of allergic diseases, to the study would be helpful for comparing the effects of hesperidin and thymol.
Conclusion
Both hesperidin and thymol produced significant improvement in nasal symptom scores, the biochemical parameters investigated, and histopathological values in an experimentally induced model of allergic rhinitis.
References
Greiner AN, Hellings PW, Rotiroti G, Scadding GK (2011) Allergic rhinitis. Lancet 378:2112–2122
Li C, Fu Y, Wang Y et al (2017) Mesenchymal stromal cells ameliorate acute allergic rhinitis in rats. Cell Biochem Funct 35:420–425
Ulusoy S, Ayan NN, Dinc ME, Is A, Bicer C, Erel O (2016) A new oxidative stress marker for thiol-disulphide homeostasis in seasonal allergic rhinitis. Am J Rhinol Allergy 30:53–57
Garg A, Garg S, Zaneveld LJ, Singla AK (2001) Chemistry and pharmacology of the citrus bioflavonoid hesperidin. Phytother Res 15:655–669
Yeh MH, Kao ST, Hung CM, Liu CJ, Lee KH, Yeh CC (2009) Hesperidin inhibited acetaldehyde-induced matrix metalloproteinase-9 gene expression in human hepatocellular carcinoma cells. Toxicol Lett 184:204–210
Park SH, Park EK, Kim DH (2005) Passive cutaneous anaphylaxis-inhibitory activity of flavanones from Citrus unshiu and Poncirus trifoliata. Planta Med 71:24–27
Aeschbach R, Loliger J, Scott BC et al (1994) Antioxidant actions of thymol, carvacrol, 6-gingerol, zingerone and hydroxytyrosol. Food Chem Toxicol 32:31–36
Kwon HI, Jeong NH, Jun SH et al (2018) Thymol attenuates the worsening of atopic dermatitis induced by Staphylococcus aureus membrane vesicles. Int Immunopharmacol 59:301–309
Senturk E, Yildirim YS, Dogan R et al (2018) Assessment of the effectiveness of cyclosporine nasal spray in an animal model of allergic rhinitis. Eur Arch Otorhinolaryngol 275:117–124
Wen WD, Yuan F, Wang JL, Hou YP (2007) Botulinum toxin therapy in the ovalbumin-sensitized rat. Neuroimmunomodulation 14:78–83
Erel O (2005) A new automated colorimetric method for measuring total oxidant status. Clin Biochem 38:1103–1111
Erel O (2004) A novel automated direct measurement method for total antioxidant capacity using a new generation, more stable ABTS radical cation. Clin Biochem 37:277–285
Sagit M, Polat H, Gurgen SG, Berk E, Guler S, Yasar M (2017) Effectiveness of quercetin in an experimental rat model of allergic rhinitis. Eur Arch Otorhinolaryngol 274:3087–3095
Tatar A, Yayla M, Kose D, Halici Z, Yoruk O, Polat E (2016) The role of endothelin-1 and endothelin receptor antagonists in allergic rhinitis inflammation:ovalbumin-induced rat model. Rhinology 54:266–272
Günel C, Demirci B, Eryılmaz A et al (2016) Inhibitory effect of Pycnogenol(®) on airway inflammation in ovalbumin-induced allergic rhinitis. Balkan Med J 33:620–626
Yman L (2001) Standardization of in vitro methods. Allergy 56:70–74
Caglayan C, Temel Y, Kandemir FM, Yildirim S, Kucukler S (2018) Naringin protects against cyclophosphamide-induced hepatotoxicity and nephrotoxicity through modulation of oxidative stress, inflammation, apoptosis, autophagy, and DNA damage. Environ Sci Pollut Res Int. https://doi.org/10.1007/s11356-018-2242-5 (Epub ahead of print)
Chen B, Qu S, Li M et al (2017) Effects of 1,25-dihydroxyvitamin D3 in an ovalbumin-induced allergic rhinitis model. Int Immunopharmacol 47:182–189
Yu S, Zhao C, Che N, Jing L, Ge R (2017) Hydrogen-rich saline attenuates eosinophil activation in a guinea pig model of allergic rhinitis via reducing oxidative stress. J Inflamm (Lond) 14:1
Emin O, Hasan A, Aysegul D, Rusen D (2012) Total antioxidant status and oxidative stress and their relationship to total IgE levels and eosinophil counts in children with allergic rhinitis. J Investig Allergol Clin Immunol 22:188–192
Parhiz H, Roohbakhsh A, Soltani F, Rezaee R, Iranshahi M (2015) Antioxidant and anti-inflammatory properties of the citrus flavonoids hesperidin and hesperetin: an updated review of their molecular mechanisms and experimental models. Phytother Res 29:323–331
Kim SH, Kim BK, Lee YC (2011) Antiasthmatic effects of hesperidin, a potential Th2 cytokine antagonist, in a mouse model of allergic asthma. Mediat Inflamm 2011:485402
Wei D, Ci X, Chu X, Wei M, Hua S, Deng X (2012) Hesperidin suppresses ovalbumin-induced airway inflammation in a mouse allergic asthma model. Inflammation 35:114–121
Kandemir FM, Ozkaraca M, Küçükler S, Caglayan C, Hanedan B (2017) Preventive effects of hesperidin on diabetic nephropathy induced by streptozotocin via modulating TGF-β1 and oxidative DNA damage. Toxin Rev. https://doi.org/10.1080/15569543.2017.1364268
Selmi S, Rtibi K, Grami D, Sebai H, Marzouki L (2017) Protective effects of orange (Citrus sinensis L.) peel aqueous extract and hesperidin on oxidative stress and peptic ulcer induced by alcohol in rat. Lipids Health Dis 16:152
Riella KR, Marinho RR, Santos JS, Pereira-Filho RN, Cardoso JC, Albuquerque-Junior RL et al. (2012) Anti-inflammatory and cicatrizing activities of thymol, a monoterpene of the essential oil from Lippia gracilis, in rodents. J Ethnopharmacol 143:656–663
El-Sayed el-SM, Mansour AM, Abdul-Hameed MS (2016) Thymol and carvacrol prevent doxorubicin-induced cardiotoxicity by abrogation of oxidative stress, inflammation, and apoptosis in rats. J Biochem Mol Toxicol 30:37–44
Ku CM, Lin JY (2013) Anti-inflammatory effects of 27 selected terpenoid compounds tested through modulating Th1/Th2 cytokine secretion profiles using murine primary splenocytes. Food Chem 141:1104–1113
Nagoor Meeran MF, Jagadeesh GS, Selvaraj P (2015) Thymol attenuates inflammation in isoproterenol induced myocardial infarcted rats by inhibiting the release of lysosomal enzymes and downregulating the expressions of proinflammatory cytokines. Eur J Pharmacol 754:153–161
Güvenç M, Cellat M, Gökçek İ, Yavaş İ, Yurdagül Özsoy Ş (2018) Effects of thymol and carvacrol on sperm quality and oxidant/antioxidant balance in rats. Arch Physiol Biochem 25:1–8
Zhou E, Fu Y, Wei Z, Yu Y, Zhang X, Yang Z (2014) Thymol attenuates allergic airway inflammation in ovalbumin (OVA)-induced mouse asthma. Fitoterapia 96:131–137
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors declare that they have no conflict of interest.
Ethical approval
This study was performed in accordance with the PHS Policy on Human Care and Use of Laboratory Animals, the NIH Guide for the Care and Use of Laboratory Animals, and the Animal Welfare Act (7 U.S.C. et seq.); the animal use protocol was approved by the local Animal Experiments ethical committee.
Rights and permissions
About this article
Cite this article
Kilic, K., Sakat, M.S., Yildirim, S. et al. The amendatory effect of hesperidin and thymol in allergic rhinitis: an ovalbumin-induced rat model. Eur Arch Otorhinolaryngol 276, 407–415 (2019). https://doi.org/10.1007/s00405-018-5222-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00405-018-5222-y